How to Develop Efficient Carboxylic Acid Photocatalysts?
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Photocatalysis Background and Objectives
Photocatalysis has emerged as a promising field in sustainable chemistry, offering environmentally friendly approaches to various chemical transformations. The development of efficient carboxylic acid photocatalysts represents a significant area of research within this domain, aiming to harness light energy for the activation and transformation of carboxylic acids. This technology has the potential to revolutionize synthetic processes in pharmaceutical, agrochemical, and materials industries.
The evolution of photocatalysis can be traced back to the discovery of the Honda-Fujishima effect in 1972, which demonstrated the photocatalytic splitting of water using titanium dioxide. Since then, the field has expanded rapidly, encompassing a wide range of applications from water purification to organic synthesis. The focus on carboxylic acid photocatalysts has gained momentum in recent years due to the ubiquity of carboxylic acids in natural and synthetic compounds.
Current research in carboxylic acid photocatalysis is driven by the need for more efficient, selective, and sustainable methods for chemical synthesis. Traditional approaches often require harsh conditions, toxic reagents, or energy-intensive processes. Photocatalysis offers a greener alternative, utilizing visible light as a renewable energy source to drive chemical reactions under mild conditions.
The primary objectives in developing efficient carboxylic acid photocatalysts include enhancing light absorption across a broader spectrum, improving quantum efficiency, and increasing catalyst stability and recyclability. Researchers aim to design catalysts that can effectively activate carboxylic acids for various transformations, such as decarboxylation, coupling reactions, and functionalization of remote C-H bonds.
Another crucial goal is to expand the scope of substrates and reaction types amenable to photocatalytic processes. This involves developing catalysts capable of activating a wide range of carboxylic acids, including those with diverse functional groups and structural complexities. Additionally, there is a focus on achieving high levels of chemo-, regio-, and stereoselectivity in photocatalytic transformations.
The development of efficient carboxylic acid photocatalysts also aligns with broader trends in green chemistry and sustainable development. By reducing the reliance on stoichiometric reagents and minimizing waste generation, these catalysts contribute to more environmentally benign chemical processes. Furthermore, the ability to harness solar energy for chemical synthesis represents a significant step towards reducing the carbon footprint of the chemical industry.
As research in this field progresses, there is an increasing emphasis on understanding the fundamental mechanisms of photocatalytic processes involving carboxylic acids. This knowledge is crucial for rational catalyst design and optimization. Advanced spectroscopic techniques and computational modeling are being employed to elucidate reaction pathways and identify key intermediates, guiding the development of more efficient catalytic systems.
The evolution of photocatalysis can be traced back to the discovery of the Honda-Fujishima effect in 1972, which demonstrated the photocatalytic splitting of water using titanium dioxide. Since then, the field has expanded rapidly, encompassing a wide range of applications from water purification to organic synthesis. The focus on carboxylic acid photocatalysts has gained momentum in recent years due to the ubiquity of carboxylic acids in natural and synthetic compounds.
Current research in carboxylic acid photocatalysis is driven by the need for more efficient, selective, and sustainable methods for chemical synthesis. Traditional approaches often require harsh conditions, toxic reagents, or energy-intensive processes. Photocatalysis offers a greener alternative, utilizing visible light as a renewable energy source to drive chemical reactions under mild conditions.
The primary objectives in developing efficient carboxylic acid photocatalysts include enhancing light absorption across a broader spectrum, improving quantum efficiency, and increasing catalyst stability and recyclability. Researchers aim to design catalysts that can effectively activate carboxylic acids for various transformations, such as decarboxylation, coupling reactions, and functionalization of remote C-H bonds.
Another crucial goal is to expand the scope of substrates and reaction types amenable to photocatalytic processes. This involves developing catalysts capable of activating a wide range of carboxylic acids, including those with diverse functional groups and structural complexities. Additionally, there is a focus on achieving high levels of chemo-, regio-, and stereoselectivity in photocatalytic transformations.
The development of efficient carboxylic acid photocatalysts also aligns with broader trends in green chemistry and sustainable development. By reducing the reliance on stoichiometric reagents and minimizing waste generation, these catalysts contribute to more environmentally benign chemical processes. Furthermore, the ability to harness solar energy for chemical synthesis represents a significant step towards reducing the carbon footprint of the chemical industry.
As research in this field progresses, there is an increasing emphasis on understanding the fundamental mechanisms of photocatalytic processes involving carboxylic acids. This knowledge is crucial for rational catalyst design and optimization. Advanced spectroscopic techniques and computational modeling are being employed to elucidate reaction pathways and identify key intermediates, guiding the development of more efficient catalytic systems.
Market Analysis for Carboxylic Acid Photocatalysts
The market for carboxylic acid photocatalysts is experiencing significant growth driven by increasing demand for sustainable and efficient chemical synthesis methods. This technology offers a promising alternative to traditional catalytic processes, particularly in the pharmaceutical and fine chemical industries where selective and environmentally friendly reactions are highly valued.
The global market for photocatalysts is projected to expand rapidly in the coming years, with carboxylic acid photocatalysts representing a key segment within this broader market. The pharmaceutical sector, in particular, is showing strong interest in these catalysts due to their potential to enable greener and more cost-effective drug synthesis pathways.
Environmental regulations and sustainability initiatives are major drivers for the adoption of carboxylic acid photocatalysts. As governments worldwide implement stricter environmental policies, industries are increasingly seeking cleaner production methods. Photocatalytic processes offer reduced energy consumption and waste generation compared to traditional catalytic reactions, aligning well with these regulatory trends.
The fine chemicals industry is another significant market for carboxylic acid photocatalysts. Manufacturers in this sector are exploring photocatalytic reactions to develop novel compounds and improve existing synthesis routes. The ability of these catalysts to facilitate selective transformations under mild conditions is particularly attractive for producing high-value specialty chemicals.
Geographically, North America and Europe currently lead in the adoption of carboxylic acid photocatalysts, primarily due to their strong pharmaceutical and chemical industries, as well as stringent environmental regulations. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by rapid industrialization and increasing investments in green technologies.
Despite the promising outlook, several factors are influencing market dynamics. The high initial cost of developing and implementing photocatalytic systems remains a barrier for some potential users, particularly smaller companies. Additionally, the need for specialized equipment and expertise in photochemistry can slow adoption rates in certain sectors.
Competition in the carboxylic acid photocatalyst market is intensifying, with both established chemical companies and innovative startups vying for market share. This competition is driving research and development efforts, leading to continuous improvements in catalyst efficiency and applicability.
Looking ahead, the market for carboxylic acid photocatalysts is expected to benefit from ongoing technological advancements, such as the development of more efficient light sources and reactor designs. These improvements are likely to expand the range of applications and increase the economic viability of photocatalytic processes across various industries.
The global market for photocatalysts is projected to expand rapidly in the coming years, with carboxylic acid photocatalysts representing a key segment within this broader market. The pharmaceutical sector, in particular, is showing strong interest in these catalysts due to their potential to enable greener and more cost-effective drug synthesis pathways.
Environmental regulations and sustainability initiatives are major drivers for the adoption of carboxylic acid photocatalysts. As governments worldwide implement stricter environmental policies, industries are increasingly seeking cleaner production methods. Photocatalytic processes offer reduced energy consumption and waste generation compared to traditional catalytic reactions, aligning well with these regulatory trends.
The fine chemicals industry is another significant market for carboxylic acid photocatalysts. Manufacturers in this sector are exploring photocatalytic reactions to develop novel compounds and improve existing synthesis routes. The ability of these catalysts to facilitate selective transformations under mild conditions is particularly attractive for producing high-value specialty chemicals.
Geographically, North America and Europe currently lead in the adoption of carboxylic acid photocatalysts, primarily due to their strong pharmaceutical and chemical industries, as well as stringent environmental regulations. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by rapid industrialization and increasing investments in green technologies.
Despite the promising outlook, several factors are influencing market dynamics. The high initial cost of developing and implementing photocatalytic systems remains a barrier for some potential users, particularly smaller companies. Additionally, the need for specialized equipment and expertise in photochemistry can slow adoption rates in certain sectors.
Competition in the carboxylic acid photocatalyst market is intensifying, with both established chemical companies and innovative startups vying for market share. This competition is driving research and development efforts, leading to continuous improvements in catalyst efficiency and applicability.
Looking ahead, the market for carboxylic acid photocatalysts is expected to benefit from ongoing technological advancements, such as the development of more efficient light sources and reactor designs. These improvements are likely to expand the range of applications and increase the economic viability of photocatalytic processes across various industries.
Current Challenges in Carboxylic Acid Photocatalysis
Despite significant advancements in carboxylic acid photocatalysis, several challenges persist in developing efficient catalysts for this process. One of the primary obstacles is the limited absorption range of many photocatalysts, which often fail to utilize the full spectrum of visible light. This limitation reduces the overall efficiency of the catalytic process and restricts its applicability in various environmental conditions.
Another critical challenge is the stability of photocatalysts under prolonged exposure to light and reactive species. Many catalysts suffer from photodegradation or deactivation over time, leading to decreased performance and the need for frequent replacement. This not only increases operational costs but also limits the scalability of photocatalytic processes for industrial applications.
The selectivity of carboxylic acid photocatalysts remains a significant hurdle. Achieving high selectivity towards desired products while minimizing unwanted side reactions is crucial for efficient and economical processes. Current catalysts often lack the precision required to selectively target specific functional groups or produce desired isomers, resulting in complex product mixtures that require extensive purification.
Heterogeneous catalysis, while advantageous for easy separation and reuse, faces challenges in terms of mass transfer limitations and reduced active site accessibility. The development of catalysts with optimized surface area, porosity, and dispersion of active sites is essential to overcome these limitations and enhance overall catalytic efficiency.
The mechanism of carboxylic acid photocatalysis is not fully understood in many cases, hindering rational catalyst design. Elucidating the complex interplay between light absorption, charge separation, and surface reactions is crucial for developing more efficient catalysts. This lack of mechanistic insight often leads to empirical approaches in catalyst development, which can be time-consuming and less effective.
Scalability and cost-effectiveness present significant challenges in the practical implementation of carboxylic acid photocatalysts. Many high-performance catalysts rely on expensive or rare materials, limiting their large-scale application. Developing catalysts based on earth-abundant elements without compromising efficiency is a key challenge that needs to be addressed.
Environmental concerns and sustainability issues also pose challenges in catalyst development. The use of toxic or environmentally harmful materials in catalyst synthesis or the generation of hazardous by-products during the catalytic process are significant drawbacks that need to be overcome to ensure the widespread adoption of these technologies.
Another critical challenge is the stability of photocatalysts under prolonged exposure to light and reactive species. Many catalysts suffer from photodegradation or deactivation over time, leading to decreased performance and the need for frequent replacement. This not only increases operational costs but also limits the scalability of photocatalytic processes for industrial applications.
The selectivity of carboxylic acid photocatalysts remains a significant hurdle. Achieving high selectivity towards desired products while minimizing unwanted side reactions is crucial for efficient and economical processes. Current catalysts often lack the precision required to selectively target specific functional groups or produce desired isomers, resulting in complex product mixtures that require extensive purification.
Heterogeneous catalysis, while advantageous for easy separation and reuse, faces challenges in terms of mass transfer limitations and reduced active site accessibility. The development of catalysts with optimized surface area, porosity, and dispersion of active sites is essential to overcome these limitations and enhance overall catalytic efficiency.
The mechanism of carboxylic acid photocatalysis is not fully understood in many cases, hindering rational catalyst design. Elucidating the complex interplay between light absorption, charge separation, and surface reactions is crucial for developing more efficient catalysts. This lack of mechanistic insight often leads to empirical approaches in catalyst development, which can be time-consuming and less effective.
Scalability and cost-effectiveness present significant challenges in the practical implementation of carboxylic acid photocatalysts. Many high-performance catalysts rely on expensive or rare materials, limiting their large-scale application. Developing catalysts based on earth-abundant elements without compromising efficiency is a key challenge that needs to be addressed.
Environmental concerns and sustainability issues also pose challenges in catalyst development. The use of toxic or environmentally harmful materials in catalyst synthesis or the generation of hazardous by-products during the catalytic process are significant drawbacks that need to be overcome to ensure the widespread adoption of these technologies.
Existing Carboxylic Acid Photocatalyst Solutions
01 Carboxylic acid derivatives as photocatalysts
Certain carboxylic acid derivatives have been found to be effective photocatalysts. These compounds can be used in various photochemical reactions, improving the efficiency of light-driven processes. The specific structure of these derivatives plays a crucial role in their photocatalytic activity.- Carboxylic acid derivatives as photocatalysts: Various carboxylic acid derivatives have been explored as efficient photocatalysts. These compounds can absorb light and initiate photochemical reactions, making them useful in organic synthesis and other applications. The efficiency of these photocatalysts depends on their molecular structure and electronic properties.
- Optimization of reaction conditions for carboxylic acid photocatalysts: The efficiency of carboxylic acid photocatalysts can be improved by optimizing reaction conditions such as solvent choice, temperature, and light source. These factors influence the photocatalytic activity and selectivity of the reactions, leading to enhanced overall efficiency.
- Structural modifications to enhance photocatalytic efficiency: Researchers have explored various structural modifications of carboxylic acid photocatalysts to enhance their efficiency. These modifications include the introduction of electron-donating or electron-withdrawing groups, extending conjugation, and incorporating metal complexes to improve light absorption and catalytic activity.
- Application of carboxylic acid photocatalysts in organic synthesis: Carboxylic acid photocatalysts have shown promising results in various organic synthesis reactions, including C-C bond formation, oxidation, and reduction processes. Their efficiency in these applications has been demonstrated through improved yields, selectivity, and milder reaction conditions compared to traditional methods.
- Sustainable and green chemistry applications: Carboxylic acid photocatalysts have been investigated for their potential in sustainable and green chemistry applications. These catalysts can enable more environmentally friendly processes by reducing the use of toxic reagents, lowering energy consumption, and facilitating the use of renewable resources in chemical transformations.
02 Efficiency enhancement through structural modifications
The efficiency of carboxylic acid photocatalysts can be improved through structural modifications. This includes the addition of specific functional groups or alterations to the carbon chain length. These modifications can enhance light absorption, electron transfer, or stability of the photocatalyst.Expand Specific Solutions03 Application in organic synthesis
Carboxylic acid photocatalysts have shown promising results in organic synthesis reactions. They can facilitate various transformations, including oxidations, reductions, and carbon-carbon bond formations, under mild conditions using light as the energy source. This approach offers a more environmentally friendly alternative to traditional synthetic methods.Expand Specific Solutions04 Photocatalytic degradation of pollutants
Some carboxylic acid-based photocatalysts have demonstrated efficiency in the degradation of environmental pollutants. When exposed to light, these catalysts can generate reactive species that break down organic contaminants in water or air. This application shows potential for water and air purification technologies.Expand Specific Solutions05 Factors affecting photocatalytic efficiency
The efficiency of carboxylic acid photocatalysts is influenced by various factors, including pH, temperature, light intensity, and the presence of other chemical species. Understanding and optimizing these parameters is crucial for maximizing the performance of these photocatalysts in different applications.Expand Specific Solutions
Key Players in Photocatalysis Research
The development of efficient carboxylic acid photocatalysts is currently in an emerging phase, with significant potential for growth. The market size is expanding as industries recognize the value of sustainable and energy-efficient chemical processes. Technologically, the field is rapidly evolving, with varying degrees of maturity among key players. Companies like BASF Corp. and Mitsubishi Gas Chemical Co., Inc. are leveraging their extensive chemical expertise to advance photocatalyst development. Academic institutions such as the University of Strasbourg and the University of Science & Technology of China are contributing fundamental research. Collaborations between industry and academia, exemplified by partnerships involving the Centre National de la Recherche Scientifique, are accelerating progress in this promising field.
Centre National de la Recherche Scientifique
Technical Solution: CNRS has developed innovative carboxylic acid photocatalysts using visible light-driven approaches. Their research focuses on the use of organic dyes as photocatalysts for the selective oxidation of alcohols to carboxylic acids. They have demonstrated the effectiveness of 9-mesityl-10-methylacridinium perchlorate as a photocatalyst, achieving high yields and selectivity under mild conditions[1]. CNRS has also explored the use of metal-organic frameworks (MOFs) as heterogeneous photocatalysts for carboxylic acid synthesis, showing improved recyclability and stability compared to homogeneous systems[2]. Additionally, they have investigated the application of photoredox catalysis for the direct C-H functionalization of carboxylic acids, enabling the synthesis of complex molecules with high atom economy[3].
Strengths: High selectivity, mild reaction conditions, and potential for green chemistry applications. Weaknesses: Some systems may require specialized equipment or have limited substrate scope.
IFP Energies Nouvelles
Technical Solution: IFP Energies Nouvelles has developed efficient carboxylic acid photocatalysts focusing on sustainable and environmentally friendly processes. Their research includes the development of biomass-derived photocatalysts for the oxidation of alcohols to carboxylic acids. They have demonstrated the use of carbon quantum dots (CQDs) derived from lignin as effective visible-light photocatalysts, achieving high yields and selectivity for various substrates[7]. IFP has also explored the application of metal-free organic photocatalysts, such as benzothiadiazole derivatives, for the aerobic oxidation of aldehydes to carboxylic acids under visible light irradiation[8]. Additionally, they have investigated the use of photoelectrochemical cells for the simultaneous production of carboxylic acids and hydrogen, offering a potential route for value-added chemicals from renewable resources[9].
Strengths: Sustainable approach, utilization of renewable resources, and potential for integrated energy-chemical processes. Weaknesses: Some systems may have lower efficiency compared to traditional metal-based catalysts.
Core Innovations in Photocatalyst Design
Photocatalytic carboxylation of substrates with carbon oxide
PatentWO2025059098A2
Innovation
- A light-driven photocatalytic process using carbon dioxide to produce carboxylic acids from various substrates, such as alkanes, alkenes, alkynes, ethers, esters, and amines, at lower temperatures and with lower-cost feedstocks compared to traditional methods.
Method for producing optically active carboxylic acid
PatentInactiveUS20060211882A1
Innovation
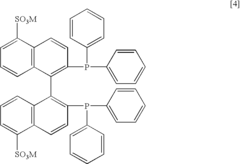
- The use of a sulfonated BINAP—Ru complex in an aqueous solvent for asymmetric hydrogenation of α,β-unsaturated carboxylic acids, allowing for the recovery and recycling of the catalyst while maintaining high optical purity and catalytic activity.
Environmental Impact of Photocatalytic Processes
The environmental impact of photocatalytic processes involving carboxylic acid catalysts is a crucial consideration in the development of efficient and sustainable chemical technologies. These processes offer significant potential for green chemistry applications, but their environmental implications must be carefully evaluated.
Photocatalytic reactions utilizing carboxylic acid catalysts generally operate under mild conditions, often at room temperature and atmospheric pressure. This inherently reduces energy consumption compared to traditional thermal catalytic processes, leading to a lower carbon footprint. Additionally, the use of light as an energy source aligns with the principles of green chemistry, promoting the utilization of renewable energy in chemical transformations.
The selectivity of carboxylic acid photocatalysts can contribute to reduced waste generation. By enabling targeted reactions and minimizing unwanted side products, these catalysts can improve atom economy and reduce the environmental burden associated with byproduct disposal. Furthermore, the potential for catalyst recycling and reuse in some photocatalytic systems can decrease the overall material consumption and waste production in chemical processes.
Water-based reaction media are often employed in carboxylic acid photocatalysis, reducing the reliance on harmful organic solvents. This shift towards aqueous systems not only minimizes the risk of volatile organic compound (VOC) emissions but also reduces the environmental impact associated with solvent production and disposal. However, it is essential to consider the potential release of trace amounts of catalysts or reaction intermediates into aqueous waste streams and implement appropriate treatment measures.
The long-term environmental effects of carboxylic acid photocatalysts must be carefully assessed. While these catalysts are generally considered less toxic than many traditional metal-based catalysts, their potential accumulation in the environment and effects on ecosystems require thorough investigation. Biodegradability and bioaccumulation studies of both the catalysts and their degradation products are crucial for ensuring environmental safety.
Life cycle assessment (LCA) studies are essential for comprehensively evaluating the environmental impact of carboxylic acid photocatalytic processes. These assessments should consider factors such as raw material sourcing, energy consumption during catalyst synthesis and use, and end-of-life disposal or recycling options. LCA results can guide the optimization of photocatalytic systems to minimize their overall environmental footprint.
The scalability of carboxylic acid photocatalytic processes also influences their environmental impact. As these technologies transition from laboratory scale to industrial applications, considerations such as light penetration in large-scale reactors and the energy efficiency of artificial light sources become increasingly important. Innovations in reactor design and light delivery systems are crucial for maintaining the environmental benefits of these processes at scale.
Photocatalytic reactions utilizing carboxylic acid catalysts generally operate under mild conditions, often at room temperature and atmospheric pressure. This inherently reduces energy consumption compared to traditional thermal catalytic processes, leading to a lower carbon footprint. Additionally, the use of light as an energy source aligns with the principles of green chemistry, promoting the utilization of renewable energy in chemical transformations.
The selectivity of carboxylic acid photocatalysts can contribute to reduced waste generation. By enabling targeted reactions and minimizing unwanted side products, these catalysts can improve atom economy and reduce the environmental burden associated with byproduct disposal. Furthermore, the potential for catalyst recycling and reuse in some photocatalytic systems can decrease the overall material consumption and waste production in chemical processes.
Water-based reaction media are often employed in carboxylic acid photocatalysis, reducing the reliance on harmful organic solvents. This shift towards aqueous systems not only minimizes the risk of volatile organic compound (VOC) emissions but also reduces the environmental impact associated with solvent production and disposal. However, it is essential to consider the potential release of trace amounts of catalysts or reaction intermediates into aqueous waste streams and implement appropriate treatment measures.
The long-term environmental effects of carboxylic acid photocatalysts must be carefully assessed. While these catalysts are generally considered less toxic than many traditional metal-based catalysts, their potential accumulation in the environment and effects on ecosystems require thorough investigation. Biodegradability and bioaccumulation studies of both the catalysts and their degradation products are crucial for ensuring environmental safety.
Life cycle assessment (LCA) studies are essential for comprehensively evaluating the environmental impact of carboxylic acid photocatalytic processes. These assessments should consider factors such as raw material sourcing, energy consumption during catalyst synthesis and use, and end-of-life disposal or recycling options. LCA results can guide the optimization of photocatalytic systems to minimize their overall environmental footprint.
The scalability of carboxylic acid photocatalytic processes also influences their environmental impact. As these technologies transition from laboratory scale to industrial applications, considerations such as light penetration in large-scale reactors and the energy efficiency of artificial light sources become increasingly important. Innovations in reactor design and light delivery systems are crucial for maintaining the environmental benefits of these processes at scale.
Scalability and Industrial Applications
The scalability and industrial applications of carboxylic acid photocatalysts represent crucial aspects for their widespread adoption in various sectors. As research progresses, the potential for large-scale implementation of these catalysts in industrial processes becomes increasingly apparent. One of the primary advantages of carboxylic acid photocatalysts is their ability to operate under mild conditions, which translates to reduced energy consumption and operational costs in industrial settings.
In the chemical manufacturing industry, the scalability of carboxylic acid photocatalysts offers promising opportunities for more sustainable and efficient production processes. These catalysts can be integrated into existing production lines, potentially replacing traditional energy-intensive methods. For instance, in the pharmaceutical sector, the synthesis of complex drug molecules often involves multiple steps that require harsh conditions. Carboxylic acid photocatalysts could streamline these processes, reducing the environmental impact and improving overall yield.
The scalability of these photocatalysts also extends to the field of environmental remediation. Large-scale water treatment facilities could benefit from the incorporation of carboxylic acid photocatalysts to degrade persistent organic pollutants. This application holds particular promise for addressing water pollution issues in industrial areas, where conventional treatment methods may fall short.
In the realm of renewable energy, the industrial application of carboxylic acid photocatalysts shows potential in artificial photosynthesis systems. As research advances, these catalysts could play a crucial role in the development of large-scale solar fuel production facilities, contributing to the global transition towards cleaner energy sources.
However, scaling up carboxylic acid photocatalysts for industrial use presents several challenges. One major hurdle is the need for efficient light penetration in large-scale reactors. Innovative reactor designs and advanced light distribution systems are being explored to address this issue. Additionally, the long-term stability and recyclability of these catalysts in industrial settings require further investigation to ensure their economic viability.
The industrial application of carboxylic acid photocatalysts also necessitates the development of standardized production methods and quality control measures. As the technology matures, establishing industry-wide standards will be crucial for ensuring consistent performance and facilitating widespread adoption across different sectors.
In the chemical manufacturing industry, the scalability of carboxylic acid photocatalysts offers promising opportunities for more sustainable and efficient production processes. These catalysts can be integrated into existing production lines, potentially replacing traditional energy-intensive methods. For instance, in the pharmaceutical sector, the synthesis of complex drug molecules often involves multiple steps that require harsh conditions. Carboxylic acid photocatalysts could streamline these processes, reducing the environmental impact and improving overall yield.
The scalability of these photocatalysts also extends to the field of environmental remediation. Large-scale water treatment facilities could benefit from the incorporation of carboxylic acid photocatalysts to degrade persistent organic pollutants. This application holds particular promise for addressing water pollution issues in industrial areas, where conventional treatment methods may fall short.
In the realm of renewable energy, the industrial application of carboxylic acid photocatalysts shows potential in artificial photosynthesis systems. As research advances, these catalysts could play a crucial role in the development of large-scale solar fuel production facilities, contributing to the global transition towards cleaner energy sources.
However, scaling up carboxylic acid photocatalysts for industrial use presents several challenges. One major hurdle is the need for efficient light penetration in large-scale reactors. Innovative reactor designs and advanced light distribution systems are being explored to address this issue. Additionally, the long-term stability and recyclability of these catalysts in industrial settings require further investigation to ensure their economic viability.
The industrial application of carboxylic acid photocatalysts also necessitates the development of standardized production methods and quality control measures. As the technology matures, establishing industry-wide standards will be crucial for ensuring consistent performance and facilitating widespread adoption across different sectors.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!