ITO Free Electrode Technologies in Medical Device Manufacturing
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
ITO-Free Electrode Technology Background and Objectives
Indium Tin Oxide (ITO) has been the dominant transparent conductive material in medical device manufacturing for decades, particularly in applications requiring both optical transparency and electrical conductivity. The evolution of this technology began in the 1950s with the development of transparent conductive oxides, but has faced increasing challenges in recent years due to indium's limited supply, rising costs, and processing limitations.
The medical device industry has witnessed significant transformation in electrode technologies, moving from traditional metal electrodes to transparent conductive materials that enable advanced functionalities such as simultaneous optical imaging and electrical stimulation. ITO emerged as the standard solution due to its excellent balance of transparency and conductivity, becoming essential in devices like glucose monitors, ECG sensors, and various implantable medical technologies.
However, several factors are driving the search for ITO alternatives. Indium is classified as a critical raw material with supply risks, as it is primarily sourced as a by-product of zinc mining with China controlling approximately 60% of global production. The brittle nature of ITO also limits its application in flexible medical devices, an increasingly important segment of the market. Additionally, the high-temperature deposition process for ITO is incompatible with many temperature-sensitive medical polymers and biomaterials.
The technical objectives for ITO-free electrode technologies in medical devices focus on developing alternative materials that maintain or exceed ITO's performance while addressing its limitations. These objectives include achieving comparable or superior electrical conductivity (less than 100 Ω/sq sheet resistance) while maintaining optical transparency above 85% in the visible spectrum. Flexibility is another critical parameter, with targets for maintaining conductivity after thousands of bending cycles at radii below 5mm.
Biocompatibility represents a paramount objective for medical applications, requiring materials that meet ISO 10993 standards for cytotoxicity, sensitization, and hemocompatibility. Manufacturing scalability is equally important, with processes that can be integrated into existing production lines while reducing environmental impact through lower energy consumption and elimination of rare earth elements.
The trajectory of ITO-free electrode technology development is expected to progress through several phases: initial material discovery and characterization, optimization of deposition techniques, integration with medical device substrates, and finally clinical validation and regulatory approval. This evolution aims to support the growing demand for advanced medical devices including wearable health monitors, point-of-care diagnostic tools, and next-generation implantable technologies that require transparent, flexible, and biocompatible electrodes.
The medical device industry has witnessed significant transformation in electrode technologies, moving from traditional metal electrodes to transparent conductive materials that enable advanced functionalities such as simultaneous optical imaging and electrical stimulation. ITO emerged as the standard solution due to its excellent balance of transparency and conductivity, becoming essential in devices like glucose monitors, ECG sensors, and various implantable medical technologies.
However, several factors are driving the search for ITO alternatives. Indium is classified as a critical raw material with supply risks, as it is primarily sourced as a by-product of zinc mining with China controlling approximately 60% of global production. The brittle nature of ITO also limits its application in flexible medical devices, an increasingly important segment of the market. Additionally, the high-temperature deposition process for ITO is incompatible with many temperature-sensitive medical polymers and biomaterials.
The technical objectives for ITO-free electrode technologies in medical devices focus on developing alternative materials that maintain or exceed ITO's performance while addressing its limitations. These objectives include achieving comparable or superior electrical conductivity (less than 100 Ω/sq sheet resistance) while maintaining optical transparency above 85% in the visible spectrum. Flexibility is another critical parameter, with targets for maintaining conductivity after thousands of bending cycles at radii below 5mm.
Biocompatibility represents a paramount objective for medical applications, requiring materials that meet ISO 10993 standards for cytotoxicity, sensitization, and hemocompatibility. Manufacturing scalability is equally important, with processes that can be integrated into existing production lines while reducing environmental impact through lower energy consumption and elimination of rare earth elements.
The trajectory of ITO-free electrode technology development is expected to progress through several phases: initial material discovery and characterization, optimization of deposition techniques, integration with medical device substrates, and finally clinical validation and regulatory approval. This evolution aims to support the growing demand for advanced medical devices including wearable health monitors, point-of-care diagnostic tools, and next-generation implantable technologies that require transparent, flexible, and biocompatible electrodes.
Market Demand Analysis for ITO Alternatives in Medical Devices
The medical device market is experiencing a significant shift away from traditional Indium Tin Oxide (ITO) electrodes, driven by several converging factors. The global medical device market, valued at approximately $476 billion in 2022, is projected to grow at a CAGR of 5.9% through 2030, with touch-enabled and flexible medical devices representing an increasingly important segment. Within this context, the demand for ITO alternatives is accelerating due to both supply chain vulnerabilities and technical limitations of ITO.
Supply chain concerns represent a primary market driver, as indium is classified as a critical raw material with limited global reserves. China controls over 70% of the world's indium production, creating geopolitical vulnerabilities for medical device manufacturers in Western markets. Price volatility of indium has further intensified interest in alternative materials, with historical price fluctuations exceeding 300% during supply shortages.
Technical limitations of ITO are equally driving market demand for alternatives. The inherent brittleness of ITO severely restricts its application in the rapidly growing segment of flexible medical devices, including wearable health monitors and implantable sensors. The medical wearables market alone is expected to reach $30 billion by 2026, growing at 15% annually, creating substantial demand for flexible electrode technologies.
Healthcare industry trends are further amplifying the need for ITO alternatives. The shift toward point-of-care diagnostics, remote patient monitoring, and minimally invasive procedures all require more durable, flexible, and cost-effective electrode solutions. The COVID-19 pandemic has accelerated these trends, with telehealth adoption increasing by over 3,800% since early 2020.
Regulatory considerations are also influencing market demand. Medical device manufacturers face increasing pressure to reduce environmental impact and improve sustainability metrics. The energy-intensive sputtering process used for ITO deposition and the limited recyclability of indium are becoming regulatory liabilities in markets with stringent environmental regulations.
End-user preferences are shifting toward devices with longer battery life, greater durability, and enhanced functionality. Alternative electrode technologies that can deliver improved performance while maintaining or reducing costs are positioned to capture significant market share. Hospitals and healthcare systems are particularly interested in devices with longer operational lifespans to reduce total cost of ownership.
The convergence of these factors has created a robust market opportunity for ITO alternatives in medical devices, with particular demand in cardiac monitoring, glucose sensing, drug delivery systems, and advanced imaging technologies. Market research indicates that manufacturers who successfully implement ITO alternatives could achieve cost reductions of 15-30% while simultaneously improving device performance and reliability.
Supply chain concerns represent a primary market driver, as indium is classified as a critical raw material with limited global reserves. China controls over 70% of the world's indium production, creating geopolitical vulnerabilities for medical device manufacturers in Western markets. Price volatility of indium has further intensified interest in alternative materials, with historical price fluctuations exceeding 300% during supply shortages.
Technical limitations of ITO are equally driving market demand for alternatives. The inherent brittleness of ITO severely restricts its application in the rapidly growing segment of flexible medical devices, including wearable health monitors and implantable sensors. The medical wearables market alone is expected to reach $30 billion by 2026, growing at 15% annually, creating substantial demand for flexible electrode technologies.
Healthcare industry trends are further amplifying the need for ITO alternatives. The shift toward point-of-care diagnostics, remote patient monitoring, and minimally invasive procedures all require more durable, flexible, and cost-effective electrode solutions. The COVID-19 pandemic has accelerated these trends, with telehealth adoption increasing by over 3,800% since early 2020.
Regulatory considerations are also influencing market demand. Medical device manufacturers face increasing pressure to reduce environmental impact and improve sustainability metrics. The energy-intensive sputtering process used for ITO deposition and the limited recyclability of indium are becoming regulatory liabilities in markets with stringent environmental regulations.
End-user preferences are shifting toward devices with longer battery life, greater durability, and enhanced functionality. Alternative electrode technologies that can deliver improved performance while maintaining or reducing costs are positioned to capture significant market share. Hospitals and healthcare systems are particularly interested in devices with longer operational lifespans to reduce total cost of ownership.
The convergence of these factors has created a robust market opportunity for ITO alternatives in medical devices, with particular demand in cardiac monitoring, glucose sensing, drug delivery systems, and advanced imaging technologies. Market research indicates that manufacturers who successfully implement ITO alternatives could achieve cost reductions of 15-30% while simultaneously improving device performance and reliability.
Current Status and Challenges in ITO-Free Electrode Development
The global pursuit of ITO-free electrode technologies has gained significant momentum in recent years, driven by the increasing demand for flexible, biocompatible medical devices and the inherent limitations of indium tin oxide (ITO). Currently, several alternative materials and approaches have emerged as potential replacements for ITO in medical device manufacturing, each with distinct advantages and challenges.
Carbon-based materials, particularly graphene and carbon nanotubes (CNTs), represent one of the most promising alternatives. These materials offer exceptional electrical conductivity, mechanical flexibility, and biocompatibility. Research institutions across North America and Europe have demonstrated graphene-based electrodes with transparency comparable to ITO while providing superior flexibility. However, challenges remain in scaling up production methods to achieve consistent quality and reducing manufacturing costs to commercially viable levels.
Metal nanowire networks, especially those based on silver (Ag) and copper (Cu), have also gained traction. These materials can be solution-processed at relatively low temperatures, making them compatible with flexible substrates. Several companies in Asia, particularly in South Korea and Japan, have developed proprietary formulations that achieve conductivity approaching that of ITO. The primary challenges include long-term stability issues, particularly oxidation of copper nanowires, and potential biocompatibility concerns for implantable medical devices.
Conductive polymers, such as PEDOT:PSS, represent another significant category of ITO alternatives. These materials offer excellent flexibility and can be processed using conventional coating techniques. Recent advancements have substantially improved their conductivity, though they still lag behind ITO in this aspect. Their main advantages include low-cost processing and compatibility with roll-to-roll manufacturing.
A major technical hurdle across all alternative materials is achieving the optimal balance between transparency, conductivity, and flexibility—properties that are often in competition with each other. Additionally, ensuring long-term stability under physiological conditions remains challenging, particularly for implantable medical devices that must function reliably for years.
Regulatory barriers present another significant challenge. Novel electrode materials must undergo rigorous biocompatibility testing and meet stringent safety standards before they can be incorporated into medical devices. This process is time-consuming and expensive, potentially slowing the adoption of promising ITO-free technologies.
Manufacturing scalability represents a persistent challenge, as many promising materials have been demonstrated only at laboratory scale. Transitioning from proof-of-concept to mass production requires significant investment in process development and quality control systems, creating barriers to entry for smaller companies and startups in this space.
Carbon-based materials, particularly graphene and carbon nanotubes (CNTs), represent one of the most promising alternatives. These materials offer exceptional electrical conductivity, mechanical flexibility, and biocompatibility. Research institutions across North America and Europe have demonstrated graphene-based electrodes with transparency comparable to ITO while providing superior flexibility. However, challenges remain in scaling up production methods to achieve consistent quality and reducing manufacturing costs to commercially viable levels.
Metal nanowire networks, especially those based on silver (Ag) and copper (Cu), have also gained traction. These materials can be solution-processed at relatively low temperatures, making them compatible with flexible substrates. Several companies in Asia, particularly in South Korea and Japan, have developed proprietary formulations that achieve conductivity approaching that of ITO. The primary challenges include long-term stability issues, particularly oxidation of copper nanowires, and potential biocompatibility concerns for implantable medical devices.
Conductive polymers, such as PEDOT:PSS, represent another significant category of ITO alternatives. These materials offer excellent flexibility and can be processed using conventional coating techniques. Recent advancements have substantially improved their conductivity, though they still lag behind ITO in this aspect. Their main advantages include low-cost processing and compatibility with roll-to-roll manufacturing.
A major technical hurdle across all alternative materials is achieving the optimal balance between transparency, conductivity, and flexibility—properties that are often in competition with each other. Additionally, ensuring long-term stability under physiological conditions remains challenging, particularly for implantable medical devices that must function reliably for years.
Regulatory barriers present another significant challenge. Novel electrode materials must undergo rigorous biocompatibility testing and meet stringent safety standards before they can be incorporated into medical devices. This process is time-consuming and expensive, potentially slowing the adoption of promising ITO-free technologies.
Manufacturing scalability represents a persistent challenge, as many promising materials have been demonstrated only at laboratory scale. Transitioning from proof-of-concept to mass production requires significant investment in process development and quality control systems, creating barriers to entry for smaller companies and startups in this space.
Current Technical Solutions for ITO Replacement
01 Carbon-based electrode materials
Carbon-based materials such as graphene, carbon nanotubes, and carbon composites are being used as alternatives to ITO for transparent electrodes. These materials offer high conductivity, flexibility, and optical transparency. The carbon-based electrodes can be fabricated through various methods including chemical vapor deposition, solution processing, and printing techniques, making them suitable for flexible electronics and display applications.- Carbon-based electrode materials: Carbon-based materials such as carbon nanotubes, graphene, and carbon composites are being used as alternatives to ITO for transparent electrodes. These materials offer good electrical conductivity, flexibility, and can be processed at lower temperatures. Carbon-based electrodes are particularly suitable for flexible electronic devices and can be manufactured using various deposition techniques including printing methods.
- Metal nanowire electrodes: Metal nanowires, particularly silver nanowires, are emerging as a promising ITO alternative due to their excellent conductivity and optical transparency. These nanowires can be deposited in networks to form transparent conductive films. The technology allows for flexible, stretchable electrodes that maintain performance under mechanical stress, making them suitable for wearable electronics and flexible displays.
- Conductive polymer electrodes: Conductive polymers such as PEDOT:PSS and polyaniline are being developed as ITO alternatives. These materials offer advantages including solution processability, flexibility, and compatibility with roll-to-roll manufacturing. Conductive polymers can be modified with additives to enhance conductivity and stability, making them suitable for various applications including solar cells, OLEDs, and touch panels.
- Metal mesh and grid electrodes: Metal mesh and grid structures are being utilized as ITO alternatives, featuring patterned metal lines that are thin enough to maintain transparency while providing electrical conductivity. These structures can be fabricated using various techniques including lithography, printing, and laser patterning. The geometry of the mesh can be optimized to balance optical transparency and electrical conductivity for specific applications.
- Metal oxide composite electrodes: Alternative metal oxide composites are being developed to replace ITO while maintaining similar optical and electrical properties. These include doped zinc oxide, aluminum-doped zinc oxide (AZO), and fluorine-doped tin oxide (FTO). These materials can be deposited using various techniques including sputtering and solution processing, and offer advantages such as earth-abundant materials, lower processing temperatures, and improved mechanical flexibility.
02 Metal nanowire electrode technologies
Metal nanowires, particularly silver nanowires, are emerging as promising ITO alternatives due to their excellent conductivity and transparency. These nanowires can be deposited in networks to form transparent conductive films. The fabrication typically involves solution-based processes followed by post-treatments to enhance conductivity. These electrodes demonstrate superior flexibility compared to ITO and can be manufactured using cost-effective roll-to-roll processes.Expand Specific Solutions03 Conductive polymer electrode systems
Conductive polymers such as PEDOT:PSS and polyaniline are being developed as ITO alternatives for flexible and stretchable electronics. These materials can be solution-processed at low temperatures, making them compatible with plastic substrates. Recent advancements have focused on improving their conductivity and stability through various doping strategies and composite formations with other materials like metal nanowires or carbon-based materials.Expand Specific Solutions04 Metal mesh and grid electrode structures
Metal mesh and grid structures are fabricated using metals like copper, aluminum, or silver to create transparent conductive electrodes. These structures can be produced through various techniques including photolithography, nanoimprint lithography, and laser patterning. The mesh design allows for high conductivity while maintaining optical transparency by optimizing the grid spacing and line width. These electrodes offer better mechanical flexibility than ITO and can be manufactured using established industrial processes.Expand Specific Solutions05 Metal oxide alternatives to ITO
Alternative metal oxide materials such as aluminum-doped zinc oxide (AZO), fluorine-doped tin oxide (FTO), and gallium-doped zinc oxide (GZO) are being developed as ITO replacements. These materials offer similar optical and electrical properties to ITO but can be more abundant and cost-effective. Various deposition techniques including sputtering, sol-gel, and chemical vapor deposition are used to fabricate these alternative transparent conductive oxide films for applications in displays, solar cells, and touch panels.Expand Specific Solutions
Key Industry Players in ITO-Free Electrode Manufacturing
The ITO Free Electrode Technologies in Medical Device Manufacturing market is currently in a growth phase, with increasing demand driven by the need for more flexible, durable, and cost-effective medical devices. The global market size is expanding rapidly, estimated to reach significant value in the coming years as healthcare facilities upgrade their equipment. Technologically, the field is advancing from early-stage development toward commercial maturity, with key players demonstrating varying levels of innovation. Companies like Medtronic and Philips lead with established R&D capabilities, while academic institutions such as King Abdullah University and Columbia University contribute fundamental research. Asian manufacturers including LG Display, China Star Optoelectronics, and Eastman Kodak are leveraging their display technology expertise to develop specialized medical applications, creating a competitive landscape that spans multiple continents and technological approaches.
Koninklijke Philips NV
Technical Solution: Philips has pioneered ITO-free electrode technology for medical imaging and patient monitoring devices using carbon nanotube (CNT) networks and graphene-based transparent conductors. Their approach involves spray-coating techniques to create uniform conductive layers on flexible substrates, enabling the production of conformable electrodes for ECG monitoring and ultrasound transducers. The company has developed a proprietary process for creating patterned graphene electrodes that maintain transparency while offering conductivity comparable to ITO but with superior mechanical flexibility. These electrodes are integrated into their latest generation of portable ultrasound devices and patient monitoring systems. Philips has also created hybrid materials combining silver nanowires with conductive polymers to achieve optimal balance between transparency, conductivity, and flexibility. Their manufacturing process incorporates roll-to-roll techniques that significantly reduce production costs while enabling large-scale fabrication of medical-grade electrodes with consistent performance characteristics.
Strengths: Excellent mechanical durability with resistance to repeated bending and flexing; compatibility with existing medical device manufacturing processes; reduced environmental impact compared to ITO-based alternatives. Weaknesses: Higher initial investment costs for manufacturing equipment; some variability in electrical performance across large area electrodes; challenges in achieving uniform coating thickness at industrial scale.
LG Chem Ltd.
Technical Solution: LG Chem has developed advanced ITO-free electrode technologies applicable to medical device manufacturing using their expertise in materials science. Their approach centers on silver nanowire (AgNW) networks embedded in biocompatible polymers, creating transparent conductive films with excellent flexibility and durability. The company has optimized the nanowire density and junction resistance to achieve conductivity comparable to ITO while maintaining over 90% transparency in the visible spectrum. Their manufacturing process involves solution-based coating techniques compatible with roll-to-roll production, significantly reducing costs compared to vacuum deposition methods required for ITO. LG Chem has also developed hybrid materials combining AgNWs with conductive polymers like PEDOT:PSS to enhance stability and uniformity. These materials have been successfully implemented in flexible biosensors, wearable health monitors, and touch-sensitive medical interfaces. The company's proprietary surface treatment processes ensure strong adhesion between the conductive layer and substrate, addressing a common failure point in flexible electrode applications.
Strengths: Excellent combination of optical transparency and electrical conductivity; superior mechanical flexibility allowing for conformable medical devices; compatibility with high-volume manufacturing processes. Weaknesses: Potential for silver migration in certain biological environments; higher material costs compared to some alternatives; challenges in achieving uniform performance over large areas.
Core Patents and Innovations in ITO-Free Electrode Technologies
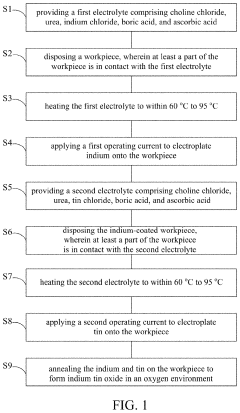
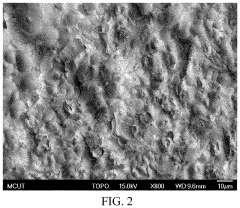
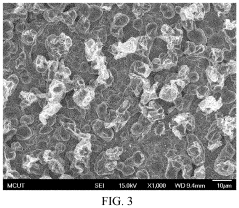
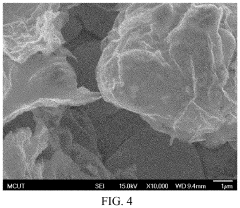
Manufacturing method of indium tin oxide
PatentActiveUS11359299B2
Innovation
- A manufacturing method involving electroplating indium and tin using specific electrolytes composed of choline chloride, urea, indium chloride, boric acid, and ascorbic acid, followed by annealing in an oxygen environment to form indium tin oxide, which is simpler and applicable to large-area production, using safe and reusable electrolytes.
Aqueous solution method for manufacturing palladium doped electrode
PatentInactiveUS20190085474A1
Innovation
- A method involving immersion of a metal oxide conducting electrode in an aqueous solution of a palladium precursor followed by reduction with a borohydride compound to form palladium nanoparticles with controlled size and density, enhancing electrocatalytic performance.
Biocompatibility and Safety Considerations
Biocompatibility remains a critical concern when implementing ITO-free electrode technologies in medical devices. Traditional indium tin oxide (ITO) electrodes have established biocompatibility profiles, whereas newer alternative materials require rigorous evaluation to ensure patient safety. Materials such as carbon nanotubes (CNTs), graphene, PEDOT:PSS, and silver nanowires must undergo comprehensive testing according to ISO 10993 standards to assess cytotoxicity, sensitization, irritation, and systemic toxicity.
The surface properties of these alternative electrode materials significantly influence their interaction with biological tissues. Factors including surface roughness, hydrophilicity/hydrophobicity, and chemical stability determine protein adsorption patterns and subsequent cellular responses. For instance, PEDOT:PSS demonstrates excellent biocompatibility in neural interfaces but may require surface modifications to optimize long-term stability in implantable applications.
Leaching of components presents another safety consideration for ITO-free technologies. While ITO electrodes are relatively stable, some alternative materials may release particles or ions during extended use. Silver nanowires, despite their excellent conductivity, pose potential risks due to silver ion release, necessitating appropriate encapsulation strategies or surface treatments to mitigate these concerns.
Sterilization compatibility represents a crucial aspect of medical device manufacturing that directly impacts material selection. ITO-free materials must maintain their electrical, mechanical, and biological properties when subjected to standard sterilization methods including ethylene oxide, gamma irradiation, and autoclave procedures. Research indicates that some conductive polymers may degrade under certain sterilization conditions, requiring careful validation of sterilization protocols for each specific material composition.
Long-term implantation safety requires extensive investigation for novel electrode materials. While acute biocompatibility testing provides initial safety data, chronic inflammatory responses, fibrotic encapsulation, and material degradation over extended periods remain critical concerns. Accelerated aging studies and animal models with extended implantation periods are essential to predict the long-term performance of ITO-free electrodes in clinical applications.
Regulatory considerations for ITO-free technologies have evolved significantly, with agencies including the FDA and EMA establishing specific guidance for novel biomaterials. Manufacturers must develop comprehensive biological evaluation plans that address both known and potential risks associated with these materials. The regulatory pathway typically requires comparative studies against predicate devices, with particular emphasis on demonstrating equivalent or superior safety profiles compared to traditional ITO-based technologies.
The surface properties of these alternative electrode materials significantly influence their interaction with biological tissues. Factors including surface roughness, hydrophilicity/hydrophobicity, and chemical stability determine protein adsorption patterns and subsequent cellular responses. For instance, PEDOT:PSS demonstrates excellent biocompatibility in neural interfaces but may require surface modifications to optimize long-term stability in implantable applications.
Leaching of components presents another safety consideration for ITO-free technologies. While ITO electrodes are relatively stable, some alternative materials may release particles or ions during extended use. Silver nanowires, despite their excellent conductivity, pose potential risks due to silver ion release, necessitating appropriate encapsulation strategies or surface treatments to mitigate these concerns.
Sterilization compatibility represents a crucial aspect of medical device manufacturing that directly impacts material selection. ITO-free materials must maintain their electrical, mechanical, and biological properties when subjected to standard sterilization methods including ethylene oxide, gamma irradiation, and autoclave procedures. Research indicates that some conductive polymers may degrade under certain sterilization conditions, requiring careful validation of sterilization protocols for each specific material composition.
Long-term implantation safety requires extensive investigation for novel electrode materials. While acute biocompatibility testing provides initial safety data, chronic inflammatory responses, fibrotic encapsulation, and material degradation over extended periods remain critical concerns. Accelerated aging studies and animal models with extended implantation periods are essential to predict the long-term performance of ITO-free electrodes in clinical applications.
Regulatory considerations for ITO-free technologies have evolved significantly, with agencies including the FDA and EMA establishing specific guidance for novel biomaterials. Manufacturers must develop comprehensive biological evaluation plans that address both known and potential risks associated with these materials. The regulatory pathway typically requires comparative studies against predicate devices, with particular emphasis on demonstrating equivalent or superior safety profiles compared to traditional ITO-based technologies.
Supply Chain Resilience and Material Sustainability
The global supply chain for ITO (Indium Tin Oxide) has faced significant vulnerabilities in recent years, highlighting the critical need for alternative electrode technologies in medical device manufacturing. Indium, a key component of ITO, is classified as a critical raw material due to its limited geographical availability, with China controlling approximately 57% of global production. This concentration creates substantial supply chain risks, as evidenced during the COVID-19 pandemic when medical device manufacturers experienced severe disruptions in ITO availability.
Material sustainability concerns further compound these challenges. Indium mining and processing generate considerable environmental impacts, including habitat destruction, water pollution, and high energy consumption. The carbon footprint associated with ITO production is estimated to be 150-200 kg CO2 equivalent per kilogram of material produced, significantly higher than many alternative conductive materials being developed.
Medical device manufacturers are increasingly adopting risk mitigation strategies to address these vulnerabilities. Diversification of suppliers represents one approach, with companies establishing relationships with multiple ITO providers across different geographical regions. However, this strategy remains limited by the fundamental scarcity and concentrated production of indium.
Material stockpiling has emerged as another common practice, with manufacturers maintaining 6-18 month reserves of critical materials like ITO. While effective as a short-term buffer against supply disruptions, this approach ties up significant capital and warehouse space, and does not address the fundamental sustainability concerns.
The most promising long-term solution involves transitioning to ITO-free alternatives that utilize more abundant and sustainable materials. Silver nanowire networks, carbon-based electrodes, and metal mesh technologies not only reduce supply chain vulnerabilities but also offer improved sustainability profiles. For instance, carbon-based alternatives typically demonstrate 40-60% lower environmental impact across their lifecycle compared to traditional ITO.
Regulatory frameworks are evolving to support this transition, with the EU's Medical Device Regulation and the FDA's recent guidance on sustainable materials in medical devices encouraging manufacturers to consider supply chain resilience and environmental impact in material selection. Companies demonstrating leadership in sustainable material sourcing are increasingly gaining competitive advantages in both regulatory approval processes and market positioning.
Material sustainability concerns further compound these challenges. Indium mining and processing generate considerable environmental impacts, including habitat destruction, water pollution, and high energy consumption. The carbon footprint associated with ITO production is estimated to be 150-200 kg CO2 equivalent per kilogram of material produced, significantly higher than many alternative conductive materials being developed.
Medical device manufacturers are increasingly adopting risk mitigation strategies to address these vulnerabilities. Diversification of suppliers represents one approach, with companies establishing relationships with multiple ITO providers across different geographical regions. However, this strategy remains limited by the fundamental scarcity and concentrated production of indium.
Material stockpiling has emerged as another common practice, with manufacturers maintaining 6-18 month reserves of critical materials like ITO. While effective as a short-term buffer against supply disruptions, this approach ties up significant capital and warehouse space, and does not address the fundamental sustainability concerns.
The most promising long-term solution involves transitioning to ITO-free alternatives that utilize more abundant and sustainable materials. Silver nanowire networks, carbon-based electrodes, and metal mesh technologies not only reduce supply chain vulnerabilities but also offer improved sustainability profiles. For instance, carbon-based alternatives typically demonstrate 40-60% lower environmental impact across their lifecycle compared to traditional ITO.
Regulatory frameworks are evolving to support this transition, with the EU's Medical Device Regulation and the FDA's recent guidance on sustainable materials in medical devices encouraging manufacturers to consider supply chain resilience and environmental impact in material selection. Companies demonstrating leadership in sustainable material sourcing are increasingly gaining competitive advantages in both regulatory approval processes and market positioning.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!