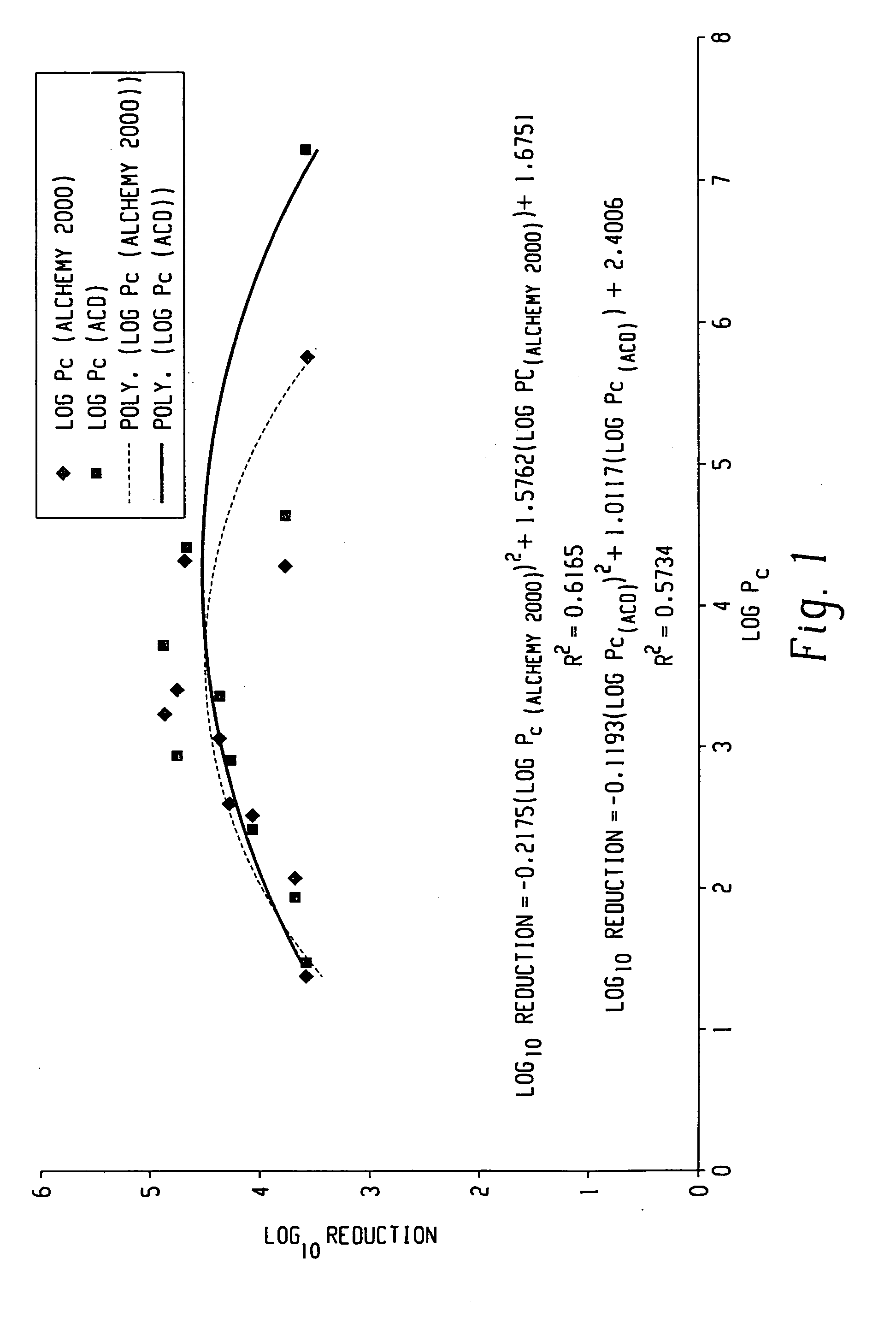
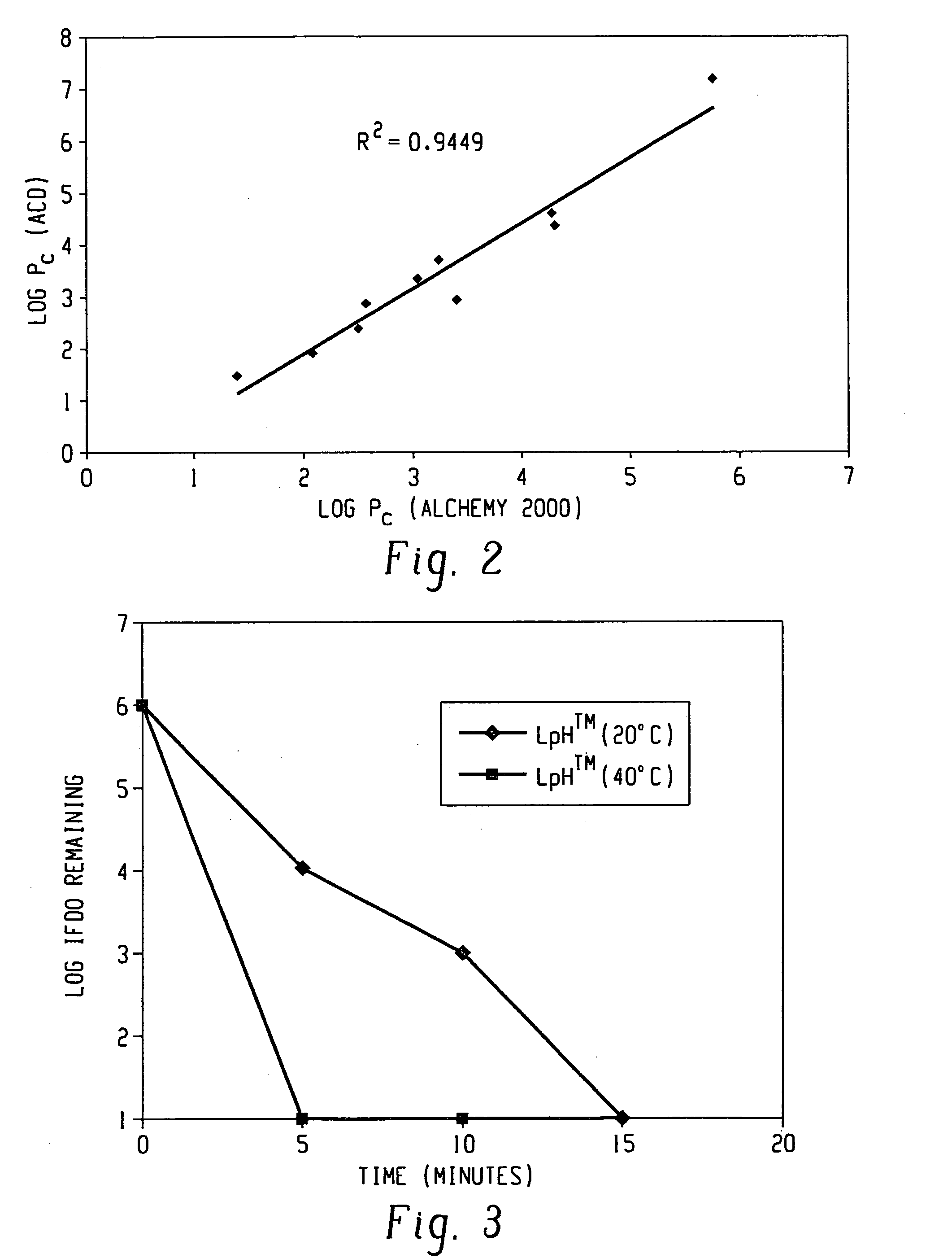
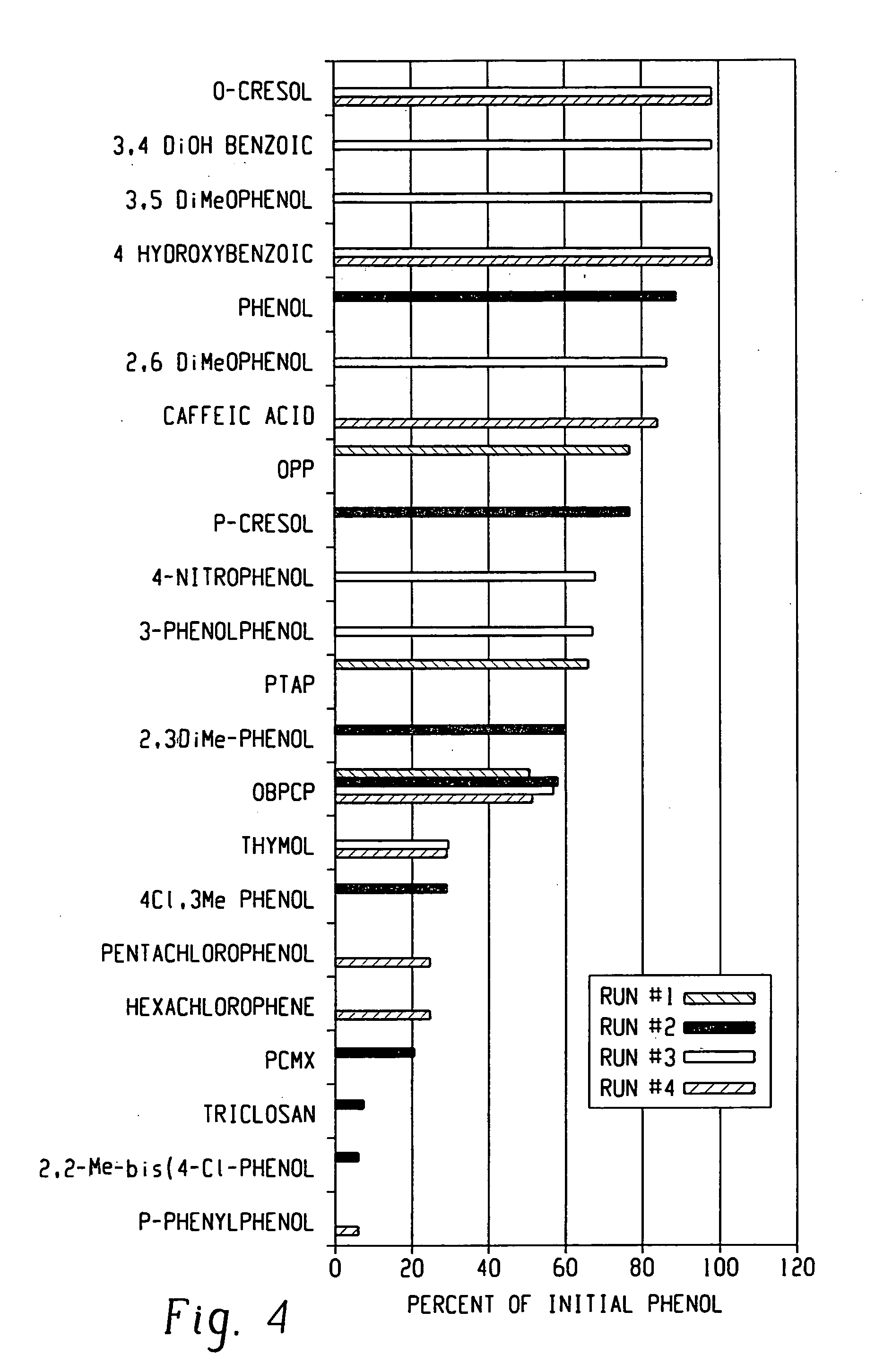
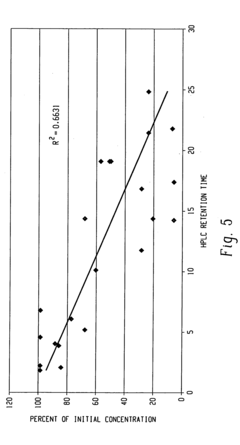
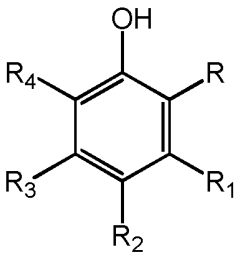
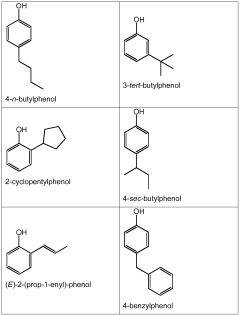
Mechanisms of Carbolic Acid in Industrial Disinfectant Formulations
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbolic Acid Evolution
Carbolic acid, also known as phenol, has undergone significant evolution in its use as an industrial disinfectant. The journey of this compound began in the mid-19th century when it was first isolated from coal tar. Its antiseptic properties were discovered by Joseph Lister in 1865, marking a pivotal moment in the history of surgical sterilization and disinfection.
The early applications of carbolic acid in disinfectants were characterized by its use in its pure form, which was highly effective but also caustic and potentially harmful. As understanding of its properties grew, formulations began to evolve. The late 19th and early 20th centuries saw the development of more refined carbolic acid solutions, often combined with soap to create carbolic soap, which became a staple in hospitals and households alike.
The mid-20th century brought about significant advancements in the understanding of carbolic acid's mechanism of action. Researchers discovered that phenol disrupts cell membranes and denatures proteins, leading to its potent antimicrobial effects. This knowledge led to the development of more sophisticated formulations, incorporating carbolic acid derivatives and synergistic compounds to enhance efficacy while reducing toxicity.
In the latter half of the 20th century, environmental and safety concerns began to shape the evolution of carbolic acid in disinfectant formulations. The focus shifted towards creating more environmentally friendly and less toxic alternatives. This led to the development of phenol-based compounds that retained the disinfectant properties of carbolic acid but with improved safety profiles.
The turn of the 21st century saw a renewed interest in carbolic acid and its derivatives due to the emergence of antibiotic-resistant bacteria. Researchers began exploring novel formulations that combined carbolic acid with other antimicrobial agents to create broad-spectrum disinfectants capable of tackling a wide range of pathogens, including resistant strains.
Recent developments in nanotechnology have opened new avenues for carbolic acid formulations. Nanoencapsulation techniques have been employed to create controlled-release disinfectant systems, allowing for prolonged antimicrobial activity and reduced environmental impact. Additionally, the incorporation of carbolic acid into advanced polymer matrices has led to the development of self-disinfecting surfaces, a significant advancement in infection control strategies.
The ongoing evolution of carbolic acid in industrial disinfectant formulations continues to be driven by the need for more effective, safer, and sustainable solutions. Current research focuses on optimizing the synergistic effects of carbolic acid with other antimicrobial agents, exploring novel delivery systems, and developing formulations that can adapt to specific environmental conditions for maximum efficacy.
The early applications of carbolic acid in disinfectants were characterized by its use in its pure form, which was highly effective but also caustic and potentially harmful. As understanding of its properties grew, formulations began to evolve. The late 19th and early 20th centuries saw the development of more refined carbolic acid solutions, often combined with soap to create carbolic soap, which became a staple in hospitals and households alike.
The mid-20th century brought about significant advancements in the understanding of carbolic acid's mechanism of action. Researchers discovered that phenol disrupts cell membranes and denatures proteins, leading to its potent antimicrobial effects. This knowledge led to the development of more sophisticated formulations, incorporating carbolic acid derivatives and synergistic compounds to enhance efficacy while reducing toxicity.
In the latter half of the 20th century, environmental and safety concerns began to shape the evolution of carbolic acid in disinfectant formulations. The focus shifted towards creating more environmentally friendly and less toxic alternatives. This led to the development of phenol-based compounds that retained the disinfectant properties of carbolic acid but with improved safety profiles.
The turn of the 21st century saw a renewed interest in carbolic acid and its derivatives due to the emergence of antibiotic-resistant bacteria. Researchers began exploring novel formulations that combined carbolic acid with other antimicrobial agents to create broad-spectrum disinfectants capable of tackling a wide range of pathogens, including resistant strains.
Recent developments in nanotechnology have opened new avenues for carbolic acid formulations. Nanoencapsulation techniques have been employed to create controlled-release disinfectant systems, allowing for prolonged antimicrobial activity and reduced environmental impact. Additionally, the incorporation of carbolic acid into advanced polymer matrices has led to the development of self-disinfecting surfaces, a significant advancement in infection control strategies.
The ongoing evolution of carbolic acid in industrial disinfectant formulations continues to be driven by the need for more effective, safer, and sustainable solutions. Current research focuses on optimizing the synergistic effects of carbolic acid with other antimicrobial agents, exploring novel delivery systems, and developing formulations that can adapt to specific environmental conditions for maximum efficacy.
Disinfectant Market Trends
The global disinfectant market has experienced significant growth in recent years, driven by increasing awareness of hygiene and sanitation, particularly in healthcare settings and public spaces. The COVID-19 pandemic has further accelerated this trend, with a surge in demand for disinfectant products across various sectors. The market is expected to continue its upward trajectory, with projections indicating sustained growth over the next decade.
One of the key trends in the disinfectant market is the shift towards more environmentally friendly and sustainable formulations. Consumers and businesses alike are seeking products that are effective against pathogens while minimizing environmental impact. This has led to the development of eco-friendly disinfectants, often based on natural ingredients or biodegradable compounds.
Another notable trend is the increasing adoption of advanced disinfection technologies. These include electrostatic sprayers, UV-C light disinfection systems, and hydrogen peroxide vapor systems. These technologies offer more efficient and thorough disinfection, particularly in large spaces or high-traffic areas.
The healthcare sector remains a primary driver of the disinfectant market, with hospitals, clinics, and long-term care facilities maintaining stringent hygiene protocols. However, other sectors such as hospitality, education, and food service are also contributing significantly to market growth as they implement enhanced cleaning and disinfection practices.
In terms of product types, surface disinfectants continue to dominate the market, followed by air disinfectants and water disinfectants. There is a growing demand for ready-to-use disinfectant wipes and sprays, which offer convenience and ease of use for both professional and consumer applications.
Geographically, North America and Europe lead the disinfectant market, owing to stringent regulations and high healthcare expenditure. However, the Asia-Pacific region is expected to witness the fastest growth, driven by increasing healthcare infrastructure, rising disposable incomes, and growing awareness of hygiene practices.
The market is characterized by intense competition among key players, leading to continuous innovation in product formulations and delivery methods. Companies are investing heavily in research and development to create more effective, faster-acting, and longer-lasting disinfectant solutions. Additionally, there is a trend towards the development of multi-purpose disinfectants that can address a broader spectrum of pathogens while being safe for various surfaces.
One of the key trends in the disinfectant market is the shift towards more environmentally friendly and sustainable formulations. Consumers and businesses alike are seeking products that are effective against pathogens while minimizing environmental impact. This has led to the development of eco-friendly disinfectants, often based on natural ingredients or biodegradable compounds.
Another notable trend is the increasing adoption of advanced disinfection technologies. These include electrostatic sprayers, UV-C light disinfection systems, and hydrogen peroxide vapor systems. These technologies offer more efficient and thorough disinfection, particularly in large spaces or high-traffic areas.
The healthcare sector remains a primary driver of the disinfectant market, with hospitals, clinics, and long-term care facilities maintaining stringent hygiene protocols. However, other sectors such as hospitality, education, and food service are also contributing significantly to market growth as they implement enhanced cleaning and disinfection practices.
In terms of product types, surface disinfectants continue to dominate the market, followed by air disinfectants and water disinfectants. There is a growing demand for ready-to-use disinfectant wipes and sprays, which offer convenience and ease of use for both professional and consumer applications.
Geographically, North America and Europe lead the disinfectant market, owing to stringent regulations and high healthcare expenditure. However, the Asia-Pacific region is expected to witness the fastest growth, driven by increasing healthcare infrastructure, rising disposable incomes, and growing awareness of hygiene practices.
The market is characterized by intense competition among key players, leading to continuous innovation in product formulations and delivery methods. Companies are investing heavily in research and development to create more effective, faster-acting, and longer-lasting disinfectant solutions. Additionally, there is a trend towards the development of multi-purpose disinfectants that can address a broader spectrum of pathogens while being safe for various surfaces.
Carbolic Acid Challenges
Carbolic acid, also known as phenol, presents several challenges in its application as an industrial disinfectant. One of the primary concerns is its high toxicity to humans and the environment. Prolonged exposure or high concentrations can lead to severe health issues, including skin burns, respiratory problems, and potential organ damage. This necessitates stringent safety protocols and protective measures during handling and application, which can increase operational costs and complexity.
The corrosive nature of carbolic acid poses another significant challenge. It can damage various materials, including metals, plastics, and rubber, limiting its use in certain industrial settings and requiring careful selection of compatible equipment and storage containers. This corrosiveness also contributes to potential environmental hazards if not properly contained or disposed of.
Stability issues present further complications in formulating carbolic acid-based disinfectants. The compound is sensitive to light and air, potentially degrading over time and losing its effectiveness. This instability necessitates careful formulation techniques and appropriate packaging to maintain potency throughout the product's shelf life.
The strong, persistent odor of carbolic acid is another challenge, particularly in enclosed spaces or areas requiring frequent disinfection. This characteristic can lead to user discomfort and potential respiratory irritation, limiting its acceptability in certain applications, especially those involving prolonged human presence.
Regulatory compliance presents an ongoing challenge for manufacturers and users of carbolic acid disinfectants. Stringent regulations govern its use, storage, and disposal due to its hazardous nature. Compliance with these regulations often requires significant investment in safety equipment, training, and documentation, adding to the overall cost and complexity of using carbolic acid-based products.
The development of resistance in microorganisms to carbolic acid is an emerging concern. While less common than with other disinfectants, some bacterial strains have shown increased tolerance to phenolic compounds, potentially reducing the long-term efficacy of carbolic acid-based formulations in certain applications.
Balancing efficacy and safety in formulations remains a persistent challenge. While higher concentrations of carbolic acid generally provide greater antimicrobial activity, they also increase the associated risks and potential for adverse effects. Formulators must carefully optimize the concentration to achieve effective disinfection while minimizing hazards, a task that often requires extensive testing and validation.
The corrosive nature of carbolic acid poses another significant challenge. It can damage various materials, including metals, plastics, and rubber, limiting its use in certain industrial settings and requiring careful selection of compatible equipment and storage containers. This corrosiveness also contributes to potential environmental hazards if not properly contained or disposed of.
Stability issues present further complications in formulating carbolic acid-based disinfectants. The compound is sensitive to light and air, potentially degrading over time and losing its effectiveness. This instability necessitates careful formulation techniques and appropriate packaging to maintain potency throughout the product's shelf life.
The strong, persistent odor of carbolic acid is another challenge, particularly in enclosed spaces or areas requiring frequent disinfection. This characteristic can lead to user discomfort and potential respiratory irritation, limiting its acceptability in certain applications, especially those involving prolonged human presence.
Regulatory compliance presents an ongoing challenge for manufacturers and users of carbolic acid disinfectants. Stringent regulations govern its use, storage, and disposal due to its hazardous nature. Compliance with these regulations often requires significant investment in safety equipment, training, and documentation, adding to the overall cost and complexity of using carbolic acid-based products.
The development of resistance in microorganisms to carbolic acid is an emerging concern. While less common than with other disinfectants, some bacterial strains have shown increased tolerance to phenolic compounds, potentially reducing the long-term efficacy of carbolic acid-based formulations in certain applications.
Balancing efficacy and safety in formulations remains a persistent challenge. While higher concentrations of carbolic acid generally provide greater antimicrobial activity, they also increase the associated risks and potential for adverse effects. Formulators must carefully optimize the concentration to achieve effective disinfection while minimizing hazards, a task that often requires extensive testing and validation.
Current Carbolic Solutions
01 Carbolic acid as an effective disinfectant
Carbolic acid, also known as phenol, has been widely used as a disinfectant due to its strong antimicrobial properties. It is effective against a broad spectrum of microorganisms, including bacteria, fungi, and some viruses. The efficacy of carbolic acid as a disinfectant is attributed to its ability to denature proteins and disrupt cell membranes of microorganisms.- Carbolic acid as an effective disinfectant: Carbolic acid, also known as phenol, has been widely used as a disinfectant due to its strong antimicrobial properties. It is effective against a broad spectrum of microorganisms, including bacteria, fungi, and some viruses. The efficacy of carbolic acid as a disinfectant is attributed to its ability to denature proteins and disrupt cell membranes of microorganisms.
- Formulations enhancing carbolic acid efficacy: Various formulations have been developed to enhance the disinfectant efficacy of carbolic acid. These may include combinations with other active ingredients, surfactants, or stabilizers to improve its performance and broaden its spectrum of activity. Such formulations can increase the overall effectiveness of carbolic acid-based disinfectants.
- Application methods for carbolic acid disinfectants: The efficacy of carbolic acid disinfectants can be influenced by the method of application. Different techniques such as spraying, fogging, or surface wiping may be employed depending on the target area and required level of disinfection. Proper application methods ensure optimal contact time and coverage, maximizing the disinfectant's effectiveness.
- Concentration and contact time for optimal efficacy: The disinfectant efficacy of carbolic acid is highly dependent on its concentration and contact time with the target surface or microorganisms. Higher concentrations and longer contact times generally result in greater efficacy. However, optimal concentrations and exposure durations may vary depending on the specific application and target pathogens.
- Safety considerations and alternatives: While carbolic acid is an effective disinfectant, its use may be limited due to safety concerns and potential toxicity. As a result, research has been conducted on safer alternatives or modified formulations that maintain disinfectant efficacy while reducing associated risks. These alternatives may include less toxic phenolic compounds or entirely different classes of disinfectants.
02 Concentration and application methods
The efficacy of carbolic acid as a disinfectant depends on its concentration and application method. Different concentrations are used for various purposes, with higher concentrations typically used for more resistant microorganisms or heavily contaminated surfaces. Proper application methods, such as spraying, wiping, or immersion, ensure optimal disinfection results.Expand Specific Solutions03 Synergistic effects with other disinfectants
Carbolic acid can be combined with other disinfectants to enhance its efficacy. These combinations may exhibit synergistic effects, improving the overall disinfection power and broadening the spectrum of antimicrobial activity. Such combinations can be particularly useful in healthcare settings or other environments requiring high-level disinfection.Expand Specific Solutions04 Safety considerations and alternatives
While carbolic acid is an effective disinfectant, it can be toxic and corrosive at high concentrations. Safety precautions must be taken when handling and using carbolic acid-based disinfectants. Due to these concerns, alternative disinfectants with similar efficacy but improved safety profiles have been developed and are increasingly being used in various applications.Expand Specific Solutions05 Environmental impact and biodegradability
The environmental impact of carbolic acid disinfectants is a growing concern. Research has been conducted to assess the biodegradability of carbolic acid and its derivatives, as well as their potential effects on ecosystems. Efforts are being made to develop more environmentally friendly formulations that maintain high disinfection efficacy while reducing ecological risks.Expand Specific Solutions
Key Disinfectant Players
The market for carbolic acid in industrial disinfectant formulations is in a mature stage, with established players and well-developed applications. The global market size is estimated to be in the billions of dollars, driven by increasing demand for hygiene and sanitation products across various industries. Technologically, the field is relatively stable, with incremental improvements rather than disruptive innovations. Key players like DuPont, Henkel, and Unilever have extensive R&D capabilities and product portfolios, while specialized companies such as Schülke & Mayr and Bode Chemie focus on niche applications. Academic institutions like Sichuan University and East China University of Science & Technology contribute to fundamental research, potentially leading to future advancements in formulation efficiency and environmental sustainability.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed advanced carbolic acid-based disinfectant formulations utilizing their proprietary VIRKON technology. This technology incorporates a synergistic blend of organic acids, surfactants, and inorganic buffers with carbolic acid to enhance its disinfectant properties. The formulation achieves a broad-spectrum antimicrobial efficacy against bacteria, viruses, and fungi, while maintaining a favorable environmental profile. DuPont's research has shown that their carbolic acid formulations can achieve up to 99.99% pathogen reduction within 5 minutes of contact time [1][3]. The company has also implemented nanotechnology to create slow-release carbolic acid particles, extending the duration of antimicrobial activity in industrial settings.
Strengths: Broad-spectrum efficacy, rapid action, and extended antimicrobial activity. Weaknesses: Potential for skin and eye irritation, strong odor, and higher cost compared to basic carbolic acid formulations.
Schülke & Mayr AG
Technical Solution: Schülke & Mayr AG has pioneered a novel approach to carbolic acid-based disinfectants by developing a microemulsion technology. This technology allows for the creation of stable, clear formulations with enhanced penetration and surface coverage. Their research has demonstrated that microemulsion-based carbolic acid formulations can achieve a 30% increase in surface coverage compared to traditional solutions [2]. The company has also incorporated natural plant extracts with synergistic antimicrobial properties to reduce the overall concentration of carbolic acid required, thereby minimizing potential environmental impact. Schülke & Mayr's formulations have shown particular efficacy in biofilm removal, with studies indicating up to 90% reduction in biofilm mass within 24 hours of application [4].
Strengths: Enhanced surface coverage, reduced environmental impact, and effective biofilm removal. Weaknesses: Higher production costs and potential compatibility issues with certain materials.
Carbolic Acid Innovations
Decontamination of prion-contaminated surfaces with phenols
PatentInactiveUS20050032913A1
Innovation
- A phenol-based disinfectant composition is used, comprising phenols such as p-tertiary-amylphenol, o-benzyl-p-chlorophenol, and 2-phenyl phenol, which effectively inactivates prions by forming complexes with the prion protein, rendering them harmless without breaking them down, and is compatible with a wide range of materials.
Antimicrobial composition
PatentWO2013083581A1
Innovation
- A composition comprising monosubstituted phenols and terpineol, which provides synergistic antimicrobial action, achieving complete microbial inactivation in 15 seconds without relying on halogenated phenols, and offering a more acceptable sensory profile and reduced active ingredient amounts.
Environmental Impact
The environmental impact of carbolic acid, also known as phenol, in industrial disinfectant formulations is a critical consideration for manufacturers, regulators, and end-users. Carbolic acid's effectiveness as a disinfectant has led to its widespread use in various industrial applications. However, its potential environmental consequences require careful examination and mitigation strategies.
When released into the environment, carbolic acid can have significant effects on aquatic ecosystems. It is highly soluble in water and can persist for extended periods, potentially accumulating in sediments and aquatic organisms. The compound's toxicity to aquatic life is well-documented, with adverse effects observed on fish, invertebrates, and algae at relatively low concentrations. This toxicity can lead to disruptions in aquatic food chains and overall ecosystem balance.
In soil environments, carbolic acid can exhibit varying degrees of mobility and persistence depending on soil composition and environmental conditions. While it can be biodegraded by certain microorganisms, high concentrations may inhibit microbial activity, potentially affecting soil fertility and nutrient cycling processes. The compound's ability to leach through soil layers also raises concerns about potential groundwater contamination.
Atmospheric release of carbolic acid, though less common, can occur through volatilization from industrial processes or improper disposal. In the atmosphere, it undergoes photochemical reactions, contributing to the formation of secondary air pollutants. While its atmospheric half-life is relatively short, localized air quality impacts near point sources of emission remain a concern.
The bioaccumulation potential of carbolic acid in the food chain is generally considered low to moderate. However, continuous exposure in contaminated environments can lead to elevated levels in certain organisms, potentially affecting higher trophic levels and posing risks to human health through consumption of contaminated food sources.
Efforts to mitigate the environmental impact of carbolic acid in industrial disinfectant formulations focus on several key areas. These include improving wastewater treatment processes to enhance removal efficiency, developing more environmentally friendly formulations with reduced carbolic acid concentrations or alternative active ingredients, and implementing stricter handling and disposal protocols to prevent environmental releases.
Regulatory frameworks worldwide have established guidelines and limits for carbolic acid in various environmental compartments. Ongoing research aims to refine these standards based on emerging ecotoxicological data and improved understanding of the compound's environmental fate and behavior. Additionally, there is growing interest in developing green chemistry alternatives that can provide comparable disinfectant efficacy while minimizing environmental risks.
When released into the environment, carbolic acid can have significant effects on aquatic ecosystems. It is highly soluble in water and can persist for extended periods, potentially accumulating in sediments and aquatic organisms. The compound's toxicity to aquatic life is well-documented, with adverse effects observed on fish, invertebrates, and algae at relatively low concentrations. This toxicity can lead to disruptions in aquatic food chains and overall ecosystem balance.
In soil environments, carbolic acid can exhibit varying degrees of mobility and persistence depending on soil composition and environmental conditions. While it can be biodegraded by certain microorganisms, high concentrations may inhibit microbial activity, potentially affecting soil fertility and nutrient cycling processes. The compound's ability to leach through soil layers also raises concerns about potential groundwater contamination.
Atmospheric release of carbolic acid, though less common, can occur through volatilization from industrial processes or improper disposal. In the atmosphere, it undergoes photochemical reactions, contributing to the formation of secondary air pollutants. While its atmospheric half-life is relatively short, localized air quality impacts near point sources of emission remain a concern.
The bioaccumulation potential of carbolic acid in the food chain is generally considered low to moderate. However, continuous exposure in contaminated environments can lead to elevated levels in certain organisms, potentially affecting higher trophic levels and posing risks to human health through consumption of contaminated food sources.
Efforts to mitigate the environmental impact of carbolic acid in industrial disinfectant formulations focus on several key areas. These include improving wastewater treatment processes to enhance removal efficiency, developing more environmentally friendly formulations with reduced carbolic acid concentrations or alternative active ingredients, and implementing stricter handling and disposal protocols to prevent environmental releases.
Regulatory frameworks worldwide have established guidelines and limits for carbolic acid in various environmental compartments. Ongoing research aims to refine these standards based on emerging ecotoxicological data and improved understanding of the compound's environmental fate and behavior. Additionally, there is growing interest in developing green chemistry alternatives that can provide comparable disinfectant efficacy while minimizing environmental risks.
Safety Regulations
The use of carbolic acid (phenol) in industrial disinfectant formulations is subject to stringent safety regulations due to its potential health and environmental hazards. Regulatory bodies worldwide have established comprehensive guidelines to ensure the safe handling, storage, and application of carbolic acid-based disinfectants.
In the United States, the Environmental Protection Agency (EPA) regulates the registration and use of disinfectants containing carbolic acid under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA). Manufacturers must provide extensive data on product efficacy, toxicity, and environmental impact to obtain EPA approval. The Occupational Safety and Health Administration (OSHA) sets permissible exposure limits (PELs) for carbolic acid in the workplace, currently at 5 parts per million (ppm) for an 8-hour time-weighted average.
The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation governs the use of carbolic acid in disinfectant formulations. Under REACH, manufacturers must register carbolic acid and provide detailed safety information. The EU has also classified carbolic acid as a priority substance under the Water Framework Directive, necessitating strict controls on its release into the environment.
Safety Data Sheets (SDS) for carbolic acid-based disinfectants must comply with the Globally Harmonized System of Classification and Labelling of Chemicals (GHS). These documents provide crucial information on hazards, handling procedures, and emergency measures. Proper labeling of products containing carbolic acid is mandatory, including hazard pictograms, signal words, and precautionary statements.
Transportation of carbolic acid and its formulations is regulated by international agreements such as the European Agreement concerning the International Carriage of Dangerous Goods by Road (ADR) and the International Maritime Dangerous Goods (IMDG) Code. These regulations specify packaging requirements, documentation, and handling procedures to ensure safe transport.
Many countries have implemented restrictions on the concentration of carbolic acid in consumer products due to its corrosive nature. For example, the European Commission's Cosmetic Regulation prohibits the use of carbolic acid in cosmetic products, except for specific authorized uses at low concentrations.
Waste management regulations also apply to carbolic acid-containing disinfectants. In the US, the Resource Conservation and Recovery Act (RCRA) classifies certain phenol-containing wastes as hazardous, requiring special handling and disposal procedures. Similar regulations exist in other countries to prevent environmental contamination.
Compliance with these safety regulations is crucial for manufacturers, distributors, and end-users of carbolic acid-based disinfectants. Regular audits, employee training, and up-to-date documentation are essential to ensure adherence to these standards and maintain a safe working environment.
In the United States, the Environmental Protection Agency (EPA) regulates the registration and use of disinfectants containing carbolic acid under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA). Manufacturers must provide extensive data on product efficacy, toxicity, and environmental impact to obtain EPA approval. The Occupational Safety and Health Administration (OSHA) sets permissible exposure limits (PELs) for carbolic acid in the workplace, currently at 5 parts per million (ppm) for an 8-hour time-weighted average.
The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation governs the use of carbolic acid in disinfectant formulations. Under REACH, manufacturers must register carbolic acid and provide detailed safety information. The EU has also classified carbolic acid as a priority substance under the Water Framework Directive, necessitating strict controls on its release into the environment.
Safety Data Sheets (SDS) for carbolic acid-based disinfectants must comply with the Globally Harmonized System of Classification and Labelling of Chemicals (GHS). These documents provide crucial information on hazards, handling procedures, and emergency measures. Proper labeling of products containing carbolic acid is mandatory, including hazard pictograms, signal words, and precautionary statements.
Transportation of carbolic acid and its formulations is regulated by international agreements such as the European Agreement concerning the International Carriage of Dangerous Goods by Road (ADR) and the International Maritime Dangerous Goods (IMDG) Code. These regulations specify packaging requirements, documentation, and handling procedures to ensure safe transport.
Many countries have implemented restrictions on the concentration of carbolic acid in consumer products due to its corrosive nature. For example, the European Commission's Cosmetic Regulation prohibits the use of carbolic acid in cosmetic products, except for specific authorized uses at low concentrations.
Waste management regulations also apply to carbolic acid-containing disinfectants. In the US, the Resource Conservation and Recovery Act (RCRA) classifies certain phenol-containing wastes as hazardous, requiring special handling and disposal procedures. Similar regulations exist in other countries to prevent environmental contamination.
Compliance with these safety regulations is crucial for manufacturers, distributors, and end-users of carbolic acid-based disinfectants. Regular audits, employee training, and up-to-date documentation are essential to ensure adherence to these standards and maintain a safe working environment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!