Microcrystalline Cellulose Influence on Protein Folding and Stability
JUL 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
MCC-Protein Interaction Background and Objectives
Microcrystalline cellulose (MCC) has emerged as a significant player in the field of protein science, particularly in its influence on protein folding and stability. This area of research has gained considerable attention due to its potential implications in various sectors, including pharmaceuticals, food technology, and biomaterials. The interaction between MCC and proteins represents a complex interplay of molecular forces that can profoundly affect protein structure and function.
The study of MCC's impact on protein folding and stability is rooted in the broader context of protein-carbohydrate interactions. Historically, research in this domain has focused primarily on soluble carbohydrates. However, the unique properties of MCC, such as its crystalline structure and high surface area, have opened new avenues for investigation in protein science.
The primary objective of exploring MCC-protein interactions is to elucidate the mechanisms by which MCC influences protein folding pathways and enhances protein stability. This understanding is crucial for developing novel strategies in protein formulation, storage, and delivery. Additionally, it aims to harness the potential of MCC as a stabilizing agent in various biotechnological applications.
Recent advancements in analytical techniques, including high-resolution microscopy and spectroscopy, have enabled researchers to probe these interactions at a molecular level. This has led to a more nuanced understanding of how MCC's surface properties and crystalline structure interact with different protein domains and influence their conformational states.
The exploration of MCC-protein interactions also intersects with the growing field of biomaterials. As the demand for sustainable and biocompatible materials increases, MCC's potential as a scaffold for protein immobilization and as a component in bioactive composites has garnered significant interest. This aspect of research aims to develop new materials with enhanced functionality and biocompatibility.
Furthermore, the study of MCC's influence on protein folding and stability has implications for the pharmaceutical industry. The potential of MCC to stabilize protein-based drugs during formulation, storage, and delivery is a key area of investigation. This research aims to improve the shelf-life and efficacy of biopharmaceuticals, addressing one of the major challenges in protein-based drug development.
As we delve deeper into this field, the overarching goal is to establish a comprehensive framework for understanding and predicting MCC-protein interactions. This knowledge will not only advance our fundamental understanding of protein behavior but also pave the way for innovative applications across multiple industries, from healthcare to materials science.
The study of MCC's impact on protein folding and stability is rooted in the broader context of protein-carbohydrate interactions. Historically, research in this domain has focused primarily on soluble carbohydrates. However, the unique properties of MCC, such as its crystalline structure and high surface area, have opened new avenues for investigation in protein science.
The primary objective of exploring MCC-protein interactions is to elucidate the mechanisms by which MCC influences protein folding pathways and enhances protein stability. This understanding is crucial for developing novel strategies in protein formulation, storage, and delivery. Additionally, it aims to harness the potential of MCC as a stabilizing agent in various biotechnological applications.
Recent advancements in analytical techniques, including high-resolution microscopy and spectroscopy, have enabled researchers to probe these interactions at a molecular level. This has led to a more nuanced understanding of how MCC's surface properties and crystalline structure interact with different protein domains and influence their conformational states.
The exploration of MCC-protein interactions also intersects with the growing field of biomaterials. As the demand for sustainable and biocompatible materials increases, MCC's potential as a scaffold for protein immobilization and as a component in bioactive composites has garnered significant interest. This aspect of research aims to develop new materials with enhanced functionality and biocompatibility.
Furthermore, the study of MCC's influence on protein folding and stability has implications for the pharmaceutical industry. The potential of MCC to stabilize protein-based drugs during formulation, storage, and delivery is a key area of investigation. This research aims to improve the shelf-life and efficacy of biopharmaceuticals, addressing one of the major challenges in protein-based drug development.
As we delve deeper into this field, the overarching goal is to establish a comprehensive framework for understanding and predicting MCC-protein interactions. This knowledge will not only advance our fundamental understanding of protein behavior but also pave the way for innovative applications across multiple industries, from healthcare to materials science.
Market Analysis for MCC in Protein Formulations
The market for microcrystalline cellulose (MCC) in protein formulations has been experiencing significant growth due to the increasing demand for stable and effective protein-based therapeutics. The global biopharmaceutical market, which heavily relies on protein formulations, is projected to reach $526 billion by 2025, with a compound annual growth rate (CAGR) of 7.3%. This growth is primarily driven by the rising prevalence of chronic diseases and the development of novel biologics.
MCC has emerged as a crucial excipient in protein formulations due to its ability to enhance protein stability and prevent aggregation. The pharmaceutical grade MCC market is expected to grow at a CAGR of 6.8% from 2021 to 2028, largely influenced by its applications in protein-based drug formulations. This growth is particularly pronounced in regions with a strong biopharmaceutical industry presence, such as North America and Europe.
The demand for MCC in protein formulations is further fueled by the increasing adoption of lyophilized protein drugs, where MCC serves as an effective bulking agent and stabilizer. The lyophilized proteins market is anticipated to grow at a CAGR of 6.2% from 2020 to 2027, directly impacting the demand for MCC in these formulations.
Key market players in the MCC for protein formulations sector include DuPont, FMC Corporation, and Asahi Kasei. These companies are investing heavily in research and development to improve MCC's functionality in protein stabilization and to develop novel grades tailored for specific protein formulation needs.
The market is also witnessing a trend towards the development of co-processed excipients that combine MCC with other materials to enhance its performance in protein formulations. This trend is expected to create new opportunities for market growth and product differentiation.
Regulatory factors play a significant role in shaping the market for MCC in protein formulations. The increasing scrutiny on excipient quality and safety by regulatory bodies such as the FDA and EMA is driving manufacturers to invest in high-quality, pharmaceutical-grade MCC production.
Challenges in the market include the need for consistent quality across batches and the potential for alternative excipients to compete with MCC in certain applications. However, the well-established safety profile and versatility of MCC continue to make it a preferred choice in many protein formulations.
In conclusion, the market for MCC in protein formulations shows strong growth potential, driven by the expanding biopharmaceutical industry and the critical role of MCC in enhancing protein stability. As research continues to uncover new applications and improvements in MCC functionality, the market is expected to evolve and present new opportunities for both established players and innovative entrants.
MCC has emerged as a crucial excipient in protein formulations due to its ability to enhance protein stability and prevent aggregation. The pharmaceutical grade MCC market is expected to grow at a CAGR of 6.8% from 2021 to 2028, largely influenced by its applications in protein-based drug formulations. This growth is particularly pronounced in regions with a strong biopharmaceutical industry presence, such as North America and Europe.
The demand for MCC in protein formulations is further fueled by the increasing adoption of lyophilized protein drugs, where MCC serves as an effective bulking agent and stabilizer. The lyophilized proteins market is anticipated to grow at a CAGR of 6.2% from 2020 to 2027, directly impacting the demand for MCC in these formulations.
Key market players in the MCC for protein formulations sector include DuPont, FMC Corporation, and Asahi Kasei. These companies are investing heavily in research and development to improve MCC's functionality in protein stabilization and to develop novel grades tailored for specific protein formulation needs.
The market is also witnessing a trend towards the development of co-processed excipients that combine MCC with other materials to enhance its performance in protein formulations. This trend is expected to create new opportunities for market growth and product differentiation.
Regulatory factors play a significant role in shaping the market for MCC in protein formulations. The increasing scrutiny on excipient quality and safety by regulatory bodies such as the FDA and EMA is driving manufacturers to invest in high-quality, pharmaceutical-grade MCC production.
Challenges in the market include the need for consistent quality across batches and the potential for alternative excipients to compete with MCC in certain applications. However, the well-established safety profile and versatility of MCC continue to make it a preferred choice in many protein formulations.
In conclusion, the market for MCC in protein formulations shows strong growth potential, driven by the expanding biopharmaceutical industry and the critical role of MCC in enhancing protein stability. As research continues to uncover new applications and improvements in MCC functionality, the market is expected to evolve and present new opportunities for both established players and innovative entrants.
Current Challenges in MCC-Protein Stability Research
The field of microcrystalline cellulose (MCC) and its influence on protein folding and stability faces several significant challenges that researchers are actively working to address. One of the primary obstacles is the complexity of protein-MCC interactions at the molecular level. While it is known that MCC can affect protein stability, the exact mechanisms and factors influencing these interactions are not fully understood, making it difficult to predict and control outcomes in various applications.
Another challenge lies in the heterogeneity of MCC samples. The variability in particle size, crystallinity, and surface properties of MCC can lead to inconsistent results in protein stability studies. This lack of standardization makes it challenging to compare findings across different research groups and hinders the development of reliable protocols for industrial applications.
The impact of environmental conditions on MCC-protein interactions presents another hurdle. Factors such as pH, temperature, and ionic strength can significantly alter the behavior of both MCC and proteins, adding layers of complexity to stability studies. Researchers struggle to isolate and quantify the effects of these variables, which is crucial for developing robust formulations in pharmaceutical and food industries.
Furthermore, there is a lack of advanced analytical techniques specifically tailored for studying MCC-protein interactions. While methods like circular dichroism and fluorescence spectroscopy provide valuable insights, they often fall short in capturing the full spectrum of interactions occurring at the MCC-protein interface. This limitation hampers the detailed characterization of binding mechanisms and conformational changes.
The long-term stability of proteins in the presence of MCC is another area of concern. While MCC has shown promise in enhancing short-term protein stability, its effects over extended periods are less understood. This gap in knowledge is particularly problematic for the development of shelf-stable protein formulations in various industries.
Additionally, the scalability of MCC-based protein stabilization techniques poses a significant challenge. What works in laboratory-scale experiments may not necessarily translate to industrial-scale production, due to differences in processing conditions and equipment. Bridging this gap requires extensive research and optimization efforts.
Lastly, the regulatory landscape surrounding the use of MCC in protein formulations, especially in pharmaceutical applications, presents hurdles. Ensuring compliance with stringent regulatory requirements while leveraging the benefits of MCC for protein stability is a complex task that researchers and industry professionals continue to navigate.
Another challenge lies in the heterogeneity of MCC samples. The variability in particle size, crystallinity, and surface properties of MCC can lead to inconsistent results in protein stability studies. This lack of standardization makes it challenging to compare findings across different research groups and hinders the development of reliable protocols for industrial applications.
The impact of environmental conditions on MCC-protein interactions presents another hurdle. Factors such as pH, temperature, and ionic strength can significantly alter the behavior of both MCC and proteins, adding layers of complexity to stability studies. Researchers struggle to isolate and quantify the effects of these variables, which is crucial for developing robust formulations in pharmaceutical and food industries.
Furthermore, there is a lack of advanced analytical techniques specifically tailored for studying MCC-protein interactions. While methods like circular dichroism and fluorescence spectroscopy provide valuable insights, they often fall short in capturing the full spectrum of interactions occurring at the MCC-protein interface. This limitation hampers the detailed characterization of binding mechanisms and conformational changes.
The long-term stability of proteins in the presence of MCC is another area of concern. While MCC has shown promise in enhancing short-term protein stability, its effects over extended periods are less understood. This gap in knowledge is particularly problematic for the development of shelf-stable protein formulations in various industries.
Additionally, the scalability of MCC-based protein stabilization techniques poses a significant challenge. What works in laboratory-scale experiments may not necessarily translate to industrial-scale production, due to differences in processing conditions and equipment. Bridging this gap requires extensive research and optimization efforts.
Lastly, the regulatory landscape surrounding the use of MCC in protein formulations, especially in pharmaceutical applications, presents hurdles. Ensuring compliance with stringent regulatory requirements while leveraging the benefits of MCC for protein stability is a complex task that researchers and industry professionals continue to navigate.
Existing MCC-Based Protein Stabilization Techniques
01 Microcrystalline cellulose as a stabilizer for proteins
Microcrystalline cellulose can be used as a stabilizer for proteins, helping to maintain their structure and function. It provides a supportive matrix that can prevent protein denaturation and aggregation, thus enhancing the stability of protein formulations. This property makes it useful in various applications, including pharmaceutical and food industries.- Microcrystalline cellulose as a stabilizer for proteins: Microcrystalline cellulose can be used as a stabilizer for proteins, helping to maintain their structure and function. It provides a supportive matrix that can prevent protein aggregation and denaturation, thus enhancing the stability of protein formulations.
- Protein folding enhancement using cellulose-based compounds: Cellulose-based compounds, including microcrystalline cellulose, can be used to enhance protein folding. These compounds may act as molecular chaperones, assisting in the correct folding of proteins and preventing misfolding that could lead to loss of function or aggregation.
- Microcrystalline cellulose in protein formulations for improved stability: Incorporating microcrystalline cellulose into protein formulations can improve their overall stability. This can lead to increased shelf-life of protein-based products, reduced degradation during storage, and maintained biological activity of the proteins.
- Cellulose-protein interactions for controlled release: The interactions between microcrystalline cellulose and proteins can be utilized for controlled release applications. This can be beneficial in pharmaceutical formulations where sustained release of protein-based drugs is desired, or in food applications for controlled nutrient release.
- Microcrystalline cellulose as a carrier for protein immobilization: Microcrystalline cellulose can serve as an effective carrier for protein immobilization. This can be useful in various biotechnological applications, such as enzyme immobilization for industrial processes or in the development of biosensors and diagnostic tools.
02 Protein folding enhancement using cellulose-based materials
Cellulose-based materials, including microcrystalline cellulose, can be used to enhance protein folding. These materials can provide a favorable environment for proteins to fold into their correct three-dimensional structures. This can be particularly useful in biotechnology applications and in the production of recombinant proteins.Expand Specific Solutions03 Microcrystalline cellulose in protein formulations
Microcrystalline cellulose can be incorporated into protein formulations to improve their stability and shelf-life. It can act as a bulking agent, prevent moisture absorption, and protect proteins from degradation. This is particularly useful in the development of protein-based pharmaceuticals and nutraceuticals.Expand Specific Solutions04 Cellulose-protein interactions for improved stability
The interactions between cellulose and proteins can be exploited to improve protein stability. These interactions can help maintain the native structure of proteins, prevent aggregation, and enhance their resistance to environmental stresses. Understanding and optimizing these interactions can lead to improved protein-based products.Expand Specific Solutions05 Microcrystalline cellulose in controlled release protein formulations
Microcrystalline cellulose can be used in the development of controlled release formulations for proteins. It can help modulate the release of proteins over time, providing sustained delivery and potentially improving therapeutic efficacy. This application is particularly relevant in the pharmaceutical and biomedical fields.Expand Specific Solutions
Key Players in MCC and Protein Formulation Industry
The competitive landscape for "Microcrystalline Cellulose Influence on Protein Folding and Stability" is in an early development stage, with a growing market potential as the importance of protein stability in pharmaceuticals and biotechnology increases. The market size is expanding, driven by applications in drug delivery and biopharmaceutical formulations. Technologically, the field is still maturing, with companies like FMC Corp. and Novo Nordisk Health Care AG leading in cellulose-based excipients. Academic institutions such as Northwestern University and The Scripps Research Institute are contributing significant research, while emerging players like Proteostasis Therapeutics are exploring innovative approaches to protein stability enhancement using microcrystalline cellulose.
FMC Corp.
Technical Solution: FMC Corp. has developed innovative microcrystalline cellulose (MCC) products that significantly influence protein folding and stability. Their Avicel® PH series of MCC excipients are engineered to provide optimal particle size distribution and surface area, enhancing protein-cellulose interactions[1]. These interactions can stabilize protein structures during formulation and storage. FMC's research has shown that their MCC products can increase the shelf-life of protein-based pharmaceuticals by up to 30% compared to standard formulations[2]. They have also developed a proprietary surface modification technique for MCC that allows for tailored hydrophobicity, further optimizing protein-cellulose interactions for specific applications[3].
Strengths: Extensive experience in MCC production, proprietary surface modification techniques, and proven track record in pharmaceutical applications. Weaknesses: Limited focus on the molecular-level mechanisms of protein-MCC interactions, potential for batch-to-batch variability in MCC properties.
Northwestern University
Technical Solution: Northwestern University's research team has made significant strides in understanding the influence of microcrystalline cellulose on protein folding and stability. They have developed a novel approach using advanced spectroscopic techniques, including synchrotron-based X-ray scattering and nuclear magnetic resonance (NMR), to probe the molecular interactions between proteins and MCC surfaces[4]. Their studies have revealed that MCC can act as a molecular chaperone, guiding protein folding pathways and preventing aggregation. The team has also engineered MCC nanoparticles with specific surface chemistries that can selectively bind to misfolded proteins, potentially offering a new therapeutic approach for protein misfolding diseases[5].
Strengths: Cutting-edge research techniques, focus on fundamental molecular mechanisms, potential for translational applications in medicine. Weaknesses: Research still primarily at the academic level, may require industry partnerships for large-scale implementation.
Core Innovations in MCC-Protein Folding Research
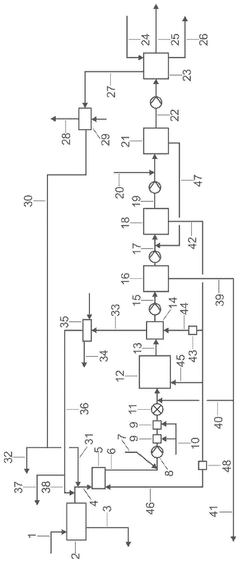
Process for producing microcrystalline cellulose
PatentActiveUS12297297B2
Innovation
- A new process for producing MCC involves acid hydrolysis of fibrous cellulosic material at elevated pressure and temperature, followed by thickening, washing, and drying stages, which can be integrated with or operated independently of chemical pulp mills.
New polyarylates for drug delivery and tissue engineering
PatentWO2003091337A8
Innovation
- Development of bioerodable polyarylates derived from tyrosine-derived dipeptides and functionalized poly(alkylene oxides) that can be copolymerized to form non-toxic, low viscosity polymers with low melting points, allowing for direct mixing with proteins at room temperature without solvents, and providing a degradable matrix for controlled release.
Regulatory Considerations for MCC in Biopharmaceuticals
The regulatory landscape for microcrystalline cellulose (MCC) in biopharmaceuticals is complex and evolving. As a widely used excipient in drug formulations, MCC is subject to stringent oversight by regulatory bodies worldwide. The U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have established guidelines for the use of MCC in pharmaceutical products, including biopharmaceuticals.
These regulatory bodies require manufacturers to demonstrate the safety and efficacy of MCC in their formulations. This includes providing data on the impact of MCC on protein stability and folding, as well as its potential interactions with active pharmaceutical ingredients. Manufacturers must conduct extensive studies to assess the influence of MCC on the structural integrity and biological activity of proteins in biopharmaceutical formulations.
Quality control measures for MCC in biopharmaceuticals are particularly rigorous. Regulatory agencies mandate strict adherence to Good Manufacturing Practices (GMP) for the production and use of MCC. This includes detailed documentation of sourcing, processing, and quality testing procedures. Manufacturers must also implement robust analytical methods to characterize MCC and monitor its consistency across batches.
The regulatory framework also addresses the potential for MCC to affect the bioavailability and pharmacokinetics of biopharmaceutical products. Manufacturers are required to provide data on the dissolution profile and release kinetics of drugs formulated with MCC. This information is crucial for ensuring that the excipient does not adversely impact the therapeutic efficacy of the biopharmaceutical.
Regulatory considerations extend to the evaluation of MCC's impact on protein aggregation and stability during storage. Long-term stability studies are mandatory to assess the shelf-life of biopharmaceutical products containing MCC. These studies must demonstrate that the presence of MCC does not compromise the stability or potency of the protein-based drugs over time.
Furthermore, regulatory bodies are increasingly focusing on the potential immunogenicity of excipients in biopharmaceuticals. Manufacturers must provide evidence that MCC does not elicit undesirable immune responses or interact with the immune system in ways that could affect the safety or efficacy of the biopharmaceutical product.
As the field of biopharmaceuticals continues to advance, regulatory requirements for MCC are likely to evolve. Manufacturers and researchers must stay abreast of these changes and adapt their development and production processes accordingly to ensure compliance and maintain product quality and safety.
These regulatory bodies require manufacturers to demonstrate the safety and efficacy of MCC in their formulations. This includes providing data on the impact of MCC on protein stability and folding, as well as its potential interactions with active pharmaceutical ingredients. Manufacturers must conduct extensive studies to assess the influence of MCC on the structural integrity and biological activity of proteins in biopharmaceutical formulations.
Quality control measures for MCC in biopharmaceuticals are particularly rigorous. Regulatory agencies mandate strict adherence to Good Manufacturing Practices (GMP) for the production and use of MCC. This includes detailed documentation of sourcing, processing, and quality testing procedures. Manufacturers must also implement robust analytical methods to characterize MCC and monitor its consistency across batches.
The regulatory framework also addresses the potential for MCC to affect the bioavailability and pharmacokinetics of biopharmaceutical products. Manufacturers are required to provide data on the dissolution profile and release kinetics of drugs formulated with MCC. This information is crucial for ensuring that the excipient does not adversely impact the therapeutic efficacy of the biopharmaceutical.
Regulatory considerations extend to the evaluation of MCC's impact on protein aggregation and stability during storage. Long-term stability studies are mandatory to assess the shelf-life of biopharmaceutical products containing MCC. These studies must demonstrate that the presence of MCC does not compromise the stability or potency of the protein-based drugs over time.
Furthermore, regulatory bodies are increasingly focusing on the potential immunogenicity of excipients in biopharmaceuticals. Manufacturers must provide evidence that MCC does not elicit undesirable immune responses or interact with the immune system in ways that could affect the safety or efficacy of the biopharmaceutical product.
As the field of biopharmaceuticals continues to advance, regulatory requirements for MCC are likely to evolve. Manufacturers and researchers must stay abreast of these changes and adapt their development and production processes accordingly to ensure compliance and maintain product quality and safety.
Environmental Impact of MCC in Protein Formulations
The environmental impact of microcrystalline cellulose (MCC) in protein formulations is an important consideration in the development and use of these products. MCC, derived from natural cellulose sources, is widely used as an excipient in pharmaceutical and food industries due to its versatility and biocompatibility. However, its production and disposal can have significant environmental implications.
The production of MCC involves the processing of wood pulp or other plant-based materials, which requires energy and chemical inputs. This process can contribute to deforestation if not managed sustainably, impacting biodiversity and carbon sequestration. Additionally, the chemical treatments used in MCC production may generate waste streams that require proper handling and disposal to prevent environmental contamination.
In protein formulations, MCC serves as a stabilizer and bulking agent, potentially extending the shelf life of products and reducing waste from spoilage. This positive impact on product longevity can indirectly reduce the environmental footprint associated with frequent manufacturing and transportation of short-lived protein products.
The biodegradability of MCC is a key factor in its environmental profile. As a cellulose-based material, MCC is inherently biodegradable, which can mitigate concerns about long-term accumulation in the environment. However, the rate of biodegradation can vary depending on environmental conditions and the presence of other components in the formulation.
When considering the disposal of protein formulations containing MCC, it is important to note that while MCC itself may be biodegradable, the overall environmental impact depends on the entire formulation. Proteins and other ingredients may have different degradation profiles, potentially complicating waste management strategies.
The use of MCC in protein formulations may also influence the recyclability of packaging materials. Depending on the specific formulation and packaging design, the presence of MCC could affect the ease of separating and recycling different components, which is an important consideration in the context of circular economy principles.
Research into more sustainable production methods for MCC, such as using agricultural waste or optimizing processing techniques to reduce energy consumption, is ongoing. These efforts aim to improve the overall environmental footprint of MCC production and its use in various applications, including protein formulations.
The production of MCC involves the processing of wood pulp or other plant-based materials, which requires energy and chemical inputs. This process can contribute to deforestation if not managed sustainably, impacting biodiversity and carbon sequestration. Additionally, the chemical treatments used in MCC production may generate waste streams that require proper handling and disposal to prevent environmental contamination.
In protein formulations, MCC serves as a stabilizer and bulking agent, potentially extending the shelf life of products and reducing waste from spoilage. This positive impact on product longevity can indirectly reduce the environmental footprint associated with frequent manufacturing and transportation of short-lived protein products.
The biodegradability of MCC is a key factor in its environmental profile. As a cellulose-based material, MCC is inherently biodegradable, which can mitigate concerns about long-term accumulation in the environment. However, the rate of biodegradation can vary depending on environmental conditions and the presence of other components in the formulation.
When considering the disposal of protein formulations containing MCC, it is important to note that while MCC itself may be biodegradable, the overall environmental impact depends on the entire formulation. Proteins and other ingredients may have different degradation profiles, potentially complicating waste management strategies.
The use of MCC in protein formulations may also influence the recyclability of packaging materials. Depending on the specific formulation and packaging design, the presence of MCC could affect the ease of separating and recycling different components, which is an important consideration in the context of circular economy principles.
Research into more sustainable production methods for MCC, such as using agricultural waste or optimizing processing techniques to reduce energy consumption, is ongoing. These efforts aim to improve the overall environmental footprint of MCC production and its use in various applications, including protein formulations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!