Microcrystalline Cellulose in Antibacterial Surface Coatings for Medical Devices
JUL 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
MCC Antibacterial Coatings: Background and Objectives
Microcrystalline cellulose (MCC) has emerged as a promising material in the development of antibacterial surface coatings for medical devices. This research area has gained significant attention due to the increasing need for effective infection control in healthcare settings. The evolution of MCC-based antibacterial coatings can be traced back to the broader field of biomaterials and nanotechnology, which has seen rapid advancements in recent decades.
The primary objective of this research is to explore the potential of MCC in creating innovative antibacterial surface coatings that can effectively prevent microbial colonization on medical devices. This goal aligns with the growing emphasis on reducing healthcare-associated infections and improving patient outcomes. The unique properties of MCC, including its biocompatibility, biodegradability, and ability to be functionalized, make it an attractive candidate for such applications.
Recent technological trends in this field have focused on enhancing the antibacterial efficacy of MCC-based coatings through various strategies. These include the incorporation of antimicrobial agents, such as silver nanoparticles or quaternary ammonium compounds, into the MCC matrix. Additionally, researchers are exploring surface modification techniques to improve the adhesion and durability of these coatings on different medical device materials.
The development of MCC antibacterial coatings is driven by several factors, including the rise of antibiotic-resistant bacteria, the need for long-lasting and environmentally friendly solutions, and the increasing use of implantable medical devices. As such, this research aims to address these challenges by developing coatings that offer broad-spectrum antimicrobial activity, prolonged efficacy, and minimal toxicity to human cells.
Another key objective of this research is to optimize the processing and application methods for MCC-based coatings. This involves investigating various techniques such as spray coating, dip coating, and electrospinning to achieve uniform and stable coatings on diverse medical device surfaces. The goal is to develop scalable and cost-effective manufacturing processes that can be readily adopted by the medical device industry.
Furthermore, this research seeks to elucidate the mechanisms of action of MCC-based antibacterial coatings. Understanding how these coatings interact with bacterial cells and prevent biofilm formation is crucial for designing more effective and targeted solutions. This knowledge will also contribute to the broader field of antimicrobial materials and surface engineering.
In conclusion, the research on MCC in antibacterial surface coatings for medical devices represents a convergence of materials science, microbiology, and medical technology. By leveraging the unique properties of MCC and addressing current healthcare challenges, this research aims to develop next-generation antibacterial coatings that can significantly improve the safety and performance of medical devices.
The primary objective of this research is to explore the potential of MCC in creating innovative antibacterial surface coatings that can effectively prevent microbial colonization on medical devices. This goal aligns with the growing emphasis on reducing healthcare-associated infections and improving patient outcomes. The unique properties of MCC, including its biocompatibility, biodegradability, and ability to be functionalized, make it an attractive candidate for such applications.
Recent technological trends in this field have focused on enhancing the antibacterial efficacy of MCC-based coatings through various strategies. These include the incorporation of antimicrobial agents, such as silver nanoparticles or quaternary ammonium compounds, into the MCC matrix. Additionally, researchers are exploring surface modification techniques to improve the adhesion and durability of these coatings on different medical device materials.
The development of MCC antibacterial coatings is driven by several factors, including the rise of antibiotic-resistant bacteria, the need for long-lasting and environmentally friendly solutions, and the increasing use of implantable medical devices. As such, this research aims to address these challenges by developing coatings that offer broad-spectrum antimicrobial activity, prolonged efficacy, and minimal toxicity to human cells.
Another key objective of this research is to optimize the processing and application methods for MCC-based coatings. This involves investigating various techniques such as spray coating, dip coating, and electrospinning to achieve uniform and stable coatings on diverse medical device surfaces. The goal is to develop scalable and cost-effective manufacturing processes that can be readily adopted by the medical device industry.
Furthermore, this research seeks to elucidate the mechanisms of action of MCC-based antibacterial coatings. Understanding how these coatings interact with bacterial cells and prevent biofilm formation is crucial for designing more effective and targeted solutions. This knowledge will also contribute to the broader field of antimicrobial materials and surface engineering.
In conclusion, the research on MCC in antibacterial surface coatings for medical devices represents a convergence of materials science, microbiology, and medical technology. By leveraging the unique properties of MCC and addressing current healthcare challenges, this research aims to develop next-generation antibacterial coatings that can significantly improve the safety and performance of medical devices.
Market Analysis for Medical Device Coatings
The global market for medical device coatings is experiencing significant growth, driven by the increasing demand for advanced medical devices and the growing emphasis on infection prevention in healthcare settings. The market is expected to reach substantial value in the coming years, with a compound annual growth rate (CAGR) that reflects the sector's robust expansion.
A key factor propelling this market is the rising incidence of healthcare-associated infections (HAIs), which has led to a greater focus on developing antimicrobial coatings for medical devices. The integration of microcrystalline cellulose in antibacterial surface coatings represents a promising avenue within this market segment, offering potential advantages in terms of biocompatibility and effectiveness.
The medical device coatings market is segmented based on product type, with hydrophilic coatings, antimicrobial coatings, and drug-eluting coatings being among the primary categories. Antimicrobial coatings, which include those incorporating microcrystalline cellulose, are witnessing particularly strong growth due to their ability to reduce the risk of infections associated with medical devices.
Geographically, North America currently holds the largest share of the medical device coatings market, followed by Europe and Asia-Pacific. The Asia-Pacific region is expected to exhibit the highest growth rate in the coming years, driven by improving healthcare infrastructure and increasing healthcare expenditure in countries like China and India.
The market is characterized by intense competition among key players, including major chemical companies and specialized coating manufacturers. These companies are investing heavily in research and development to innovate new coating technologies, with a particular focus on enhancing the antimicrobial properties of their products.
The adoption of microcrystalline cellulose in antibacterial surface coatings for medical devices is influenced by several market trends. There is a growing preference for eco-friendly and sustainable materials in medical device manufacturing, which aligns well with the natural origin of microcrystalline cellulose. Additionally, the increasing use of minimally invasive surgical procedures is driving demand for specialized coatings that can improve the performance of intricate medical devices.
Regulatory factors play a crucial role in shaping the market landscape for medical device coatings. Stringent regulations regarding the safety and efficacy of medical devices, particularly in developed markets, are influencing product development and market entry strategies. This regulatory environment is expected to drive further innovation in coating technologies, including those utilizing microcrystalline cellulose, to meet evolving safety standards and performance requirements.
A key factor propelling this market is the rising incidence of healthcare-associated infections (HAIs), which has led to a greater focus on developing antimicrobial coatings for medical devices. The integration of microcrystalline cellulose in antibacterial surface coatings represents a promising avenue within this market segment, offering potential advantages in terms of biocompatibility and effectiveness.
The medical device coatings market is segmented based on product type, with hydrophilic coatings, antimicrobial coatings, and drug-eluting coatings being among the primary categories. Antimicrobial coatings, which include those incorporating microcrystalline cellulose, are witnessing particularly strong growth due to their ability to reduce the risk of infections associated with medical devices.
Geographically, North America currently holds the largest share of the medical device coatings market, followed by Europe and Asia-Pacific. The Asia-Pacific region is expected to exhibit the highest growth rate in the coming years, driven by improving healthcare infrastructure and increasing healthcare expenditure in countries like China and India.
The market is characterized by intense competition among key players, including major chemical companies and specialized coating manufacturers. These companies are investing heavily in research and development to innovate new coating technologies, with a particular focus on enhancing the antimicrobial properties of their products.
The adoption of microcrystalline cellulose in antibacterial surface coatings for medical devices is influenced by several market trends. There is a growing preference for eco-friendly and sustainable materials in medical device manufacturing, which aligns well with the natural origin of microcrystalline cellulose. Additionally, the increasing use of minimally invasive surgical procedures is driving demand for specialized coatings that can improve the performance of intricate medical devices.
Regulatory factors play a crucial role in shaping the market landscape for medical device coatings. Stringent regulations regarding the safety and efficacy of medical devices, particularly in developed markets, are influencing product development and market entry strategies. This regulatory environment is expected to drive further innovation in coating technologies, including those utilizing microcrystalline cellulose, to meet evolving safety standards and performance requirements.
Current Challenges in Antibacterial Surface Technologies
Despite significant advancements in antibacterial surface technologies, several challenges persist in developing effective and sustainable solutions for medical devices. One of the primary obstacles is the emergence of antibiotic-resistant bacteria, which render many traditional antibacterial coatings ineffective. This necessitates the development of novel approaches that can combat resistant strains without promoting further resistance.
Another critical challenge is maintaining long-term efficacy of antibacterial coatings. Many current solutions exhibit diminishing effectiveness over time due to wear, degradation, or depletion of active agents. This is particularly problematic for implantable medical devices that require prolonged antibacterial protection.
Biocompatibility remains a significant concern in antibacterial surface technologies. While a coating may effectively eliminate bacteria, it must also be non-toxic to human cells and tissues. Striking the right balance between antibacterial potency and biocompatibility is a complex task that often involves trade-offs.
The development of broad-spectrum antibacterial coatings that can effectively combat a wide range of pathogens is another ongoing challenge. Different bacterial species exhibit varying susceptibilities to antibacterial agents, making it difficult to create a one-size-fits-all solution.
Environmental concerns and regulatory hurdles pose additional challenges. Many traditional antibacterial agents, such as silver nanoparticles, have raised concerns about their potential environmental impact and long-term safety. Stricter regulations are pushing researchers to develop more sustainable and eco-friendly alternatives.
Cost-effectiveness and scalability of antibacterial coatings present practical challenges for widespread adoption in medical devices. Complex manufacturing processes or expensive materials can limit the commercial viability of otherwise promising technologies.
The integration of antibacterial properties with other desired surface characteristics, such as lubricity or anti-fouling properties, adds another layer of complexity to coating development. Multifunctional coatings that can address multiple surface requirements simultaneously are highly desirable but technically challenging to achieve.
In the context of microcrystalline cellulose (MCC) in antibacterial surface coatings, specific challenges include optimizing the incorporation of MCC into coating matrices, ensuring uniform distribution, and maintaining the structural integrity of the coating under various conditions. Additionally, enhancing the inherent antibacterial properties of MCC or effectively combining it with other antibacterial agents without compromising its biocompatibility and biodegradability presents ongoing research challenges.
Another critical challenge is maintaining long-term efficacy of antibacterial coatings. Many current solutions exhibit diminishing effectiveness over time due to wear, degradation, or depletion of active agents. This is particularly problematic for implantable medical devices that require prolonged antibacterial protection.
Biocompatibility remains a significant concern in antibacterial surface technologies. While a coating may effectively eliminate bacteria, it must also be non-toxic to human cells and tissues. Striking the right balance between antibacterial potency and biocompatibility is a complex task that often involves trade-offs.
The development of broad-spectrum antibacterial coatings that can effectively combat a wide range of pathogens is another ongoing challenge. Different bacterial species exhibit varying susceptibilities to antibacterial agents, making it difficult to create a one-size-fits-all solution.
Environmental concerns and regulatory hurdles pose additional challenges. Many traditional antibacterial agents, such as silver nanoparticles, have raised concerns about their potential environmental impact and long-term safety. Stricter regulations are pushing researchers to develop more sustainable and eco-friendly alternatives.
Cost-effectiveness and scalability of antibacterial coatings present practical challenges for widespread adoption in medical devices. Complex manufacturing processes or expensive materials can limit the commercial viability of otherwise promising technologies.
The integration of antibacterial properties with other desired surface characteristics, such as lubricity or anti-fouling properties, adds another layer of complexity to coating development. Multifunctional coatings that can address multiple surface requirements simultaneously are highly desirable but technically challenging to achieve.
In the context of microcrystalline cellulose (MCC) in antibacterial surface coatings, specific challenges include optimizing the incorporation of MCC into coating matrices, ensuring uniform distribution, and maintaining the structural integrity of the coating under various conditions. Additionally, enhancing the inherent antibacterial properties of MCC or effectively combining it with other antibacterial agents without compromising its biocompatibility and biodegradability presents ongoing research challenges.
Existing MCC-based Antibacterial Coating Solutions
01 Antibacterial properties of microcrystalline cellulose
Microcrystalline cellulose exhibits inherent antibacterial properties, making it useful in various applications. Its unique structure and surface characteristics contribute to its ability to inhibit bacterial growth, potentially through mechanisms such as physical barrier formation or interaction with bacterial cell membranes.- Antibacterial properties of microcrystalline cellulose: Microcrystalline cellulose exhibits inherent antibacterial properties, making it useful in various applications. Its unique structure and surface characteristics contribute to its ability to inhibit bacterial growth, potentially through mechanisms such as physical barrier formation or interaction with bacterial cell membranes.
- Modification of microcrystalline cellulose for enhanced antibacterial activity: Various methods can be employed to modify microcrystalline cellulose, enhancing its antibacterial properties. These modifications may include chemical treatments, surface functionalization, or incorporation of additional antibacterial agents, resulting in a more potent antibacterial material for diverse applications.
- Composite materials incorporating microcrystalline cellulose for antibacterial effects: Microcrystalline cellulose can be combined with other materials to create composite structures with improved antibacterial properties. These composites may leverage the synergistic effects of multiple components, resulting in enhanced antimicrobial activity and broader application potential in various industries.
- Applications of microcrystalline cellulose in antibacterial products: The antibacterial properties of microcrystalline cellulose make it suitable for use in various products across different industries. These applications may include personal care items, medical devices, packaging materials, and other consumer goods where bacterial control is essential.
- Mechanisms of antibacterial action in microcrystalline cellulose: Research into the mechanisms by which microcrystalline cellulose exhibits antibacterial properties is ongoing. Potential modes of action may include physical interactions with bacterial cell walls, disruption of cellular processes, or the creation of an inhospitable environment for bacterial growth.
02 Modification of microcrystalline cellulose for enhanced antibacterial activity
Various methods can be employed to modify microcrystalline cellulose, enhancing its antibacterial properties. These modifications may include chemical treatments, surface functionalization, or incorporation of additional antibacterial agents, resulting in a more potent antibacterial material for diverse applications.Expand Specific Solutions03 Composite materials incorporating microcrystalline cellulose for antibacterial effects
Microcrystalline cellulose can be combined with other materials to create composite structures with enhanced antibacterial properties. These composites may leverage the synergistic effects of multiple components, resulting in materials with improved performance in various antibacterial applications.Expand Specific Solutions04 Applications of microcrystalline cellulose in antibacterial products
The antibacterial properties of microcrystalline cellulose make it suitable for use in various products across different industries. These may include medical devices, packaging materials, personal care products, and other items where bacterial control is crucial for performance or safety.Expand Specific Solutions05 Mechanisms of antibacterial action in microcrystalline cellulose
Research into the specific mechanisms by which microcrystalline cellulose exerts its antibacterial effects is ongoing. Proposed mechanisms may include physical interactions with bacterial cell walls, disruption of bacterial metabolism, or creation of an unfavorable environment for bacterial growth.Expand Specific Solutions
Key Players in Medical Device Coating Industry
The research on microcrystalline cellulose in antibacterial surface coatings for medical devices is in a growth phase, with increasing market potential due to rising demand for advanced medical technologies. The global market for antimicrobial coatings in medical devices is expanding, driven by the need for infection prevention. While the technology is advancing, it is not yet fully mature, with ongoing research and development efforts. Companies like Fraunhofer-Gesellschaft, Bio-Gate AG, and IBM are actively contributing to this field, leveraging their expertise in materials science and nanotechnology to develop innovative solutions. The involvement of academic institutions such as Cornell University and the University of Birmingham also indicates a strong focus on fundamental research and potential for future breakthroughs in this area.
Fraunhofer-Gesellschaft eV
Technical Solution: Fraunhofer-Gesellschaft has developed an innovative approach to incorporating microcrystalline cellulose (MCC) into antibacterial surface coatings for medical devices. Their method involves creating a nanocomposite material that combines MCC with silver nanoparticles. The MCC acts as a stabilizing matrix for the silver nanoparticles, enhancing their antibacterial efficacy while providing a biocompatible and sustainable base material. This nanocomposite is then integrated into a polymer coating that can be applied to various medical devices. The coating demonstrates excellent adhesion to different substrate materials and maintains its antibacterial properties for extended periods[1][3]. Fraunhofer's research has shown that this MCC-based coating can reduce bacterial colonization by up to 99.9% compared to untreated surfaces[2].
Strengths: Utilizes sustainable and biocompatible MCC, enhances efficacy of silver nanoparticles, long-lasting antibacterial effect. Weaknesses: Potential for silver nanoparticle accumulation in the environment, higher production costs compared to conventional coatings.
Bio-Gate AG
Technical Solution: Bio-Gate AG has pioneered a unique approach to incorporating microcrystalline cellulose (MCC) in antibacterial surface coatings for medical devices. Their proprietary technology, known as MicroSilver BG™, combines MCC with highly pure silver micro-particles. The MCC serves as a carrier for the silver particles, allowing for a controlled release of silver ions over time. This combination is then integrated into various coating matrices suitable for different medical device materials. The coating process involves a specialized spray application technique that ensures uniform distribution of the MCC-silver complex on the device surface. Bio-Gate's research has demonstrated that this coating can maintain its antibacterial efficacy for over 100 days in laboratory conditions[1]. Clinical studies have shown a reduction in device-associated infections by up to 50% when using medical devices treated with this coating[2][3].
Strengths: Long-lasting antibacterial effect, controlled release of silver ions, clinically proven efficacy. Weaknesses: Higher initial cost, potential for silver accumulation in long-term use, limited compatibility with certain device materials.
Core Innovations in MCC Surface Modification
Articles provided with microorganism repellent coatings, their preparation and use
PatentInactiveEP0858811A2
Innovation
- Application of a microorganism-repellent coating made of cyanoalkylated hydroxyalkyl cellulose on these surfaces, which reduces bacterial and cell adhesion while maintaining a low coefficient of friction and non-toxicity, achieved through a process involving cyanoethylation and subsequent modification with high-energy rays for enhanced adhesion and insolubility.
Antimicrobial coating for inhibition of bacterial adhesion and biofilm formation
PatentInactiveUS20150010715A1
Innovation
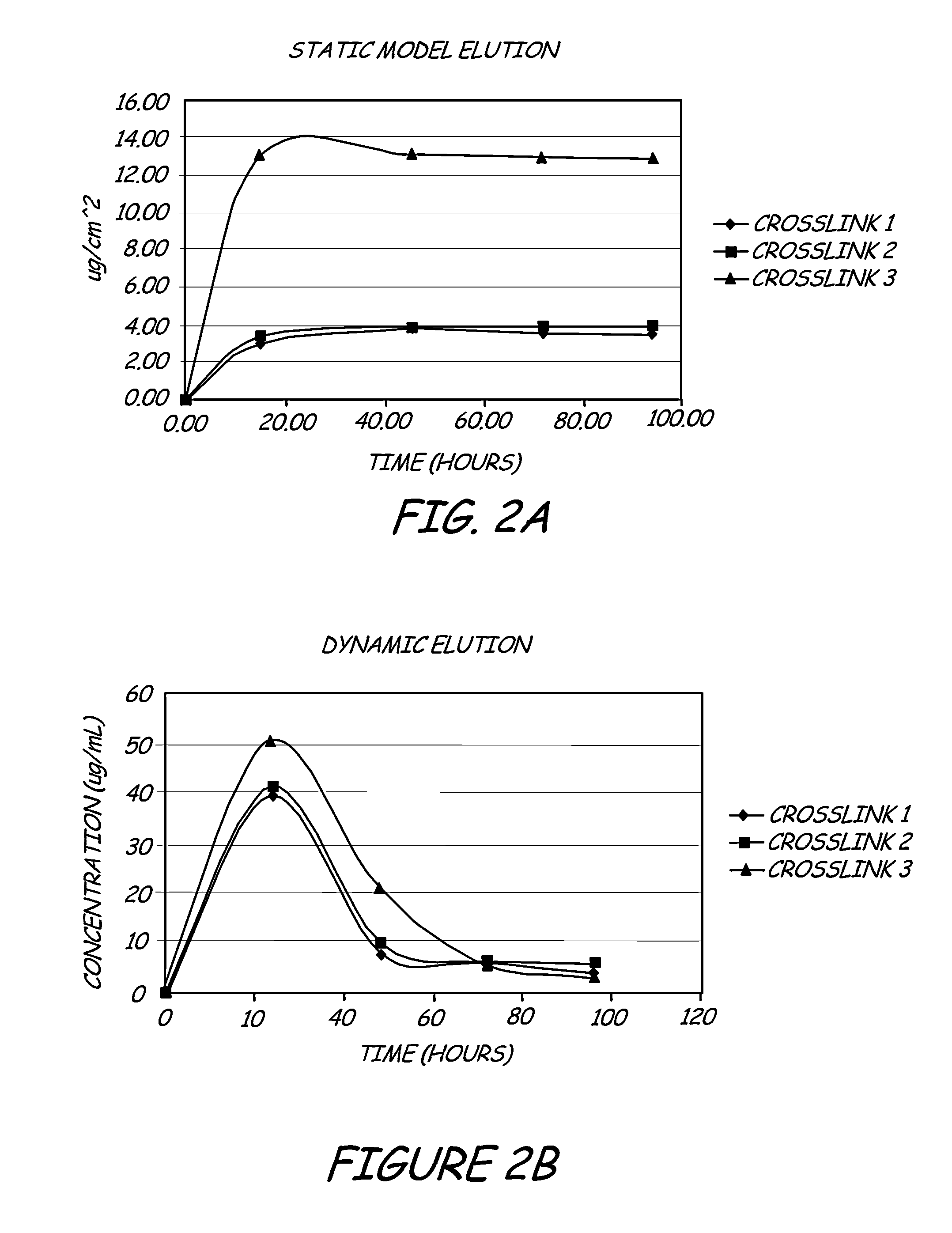
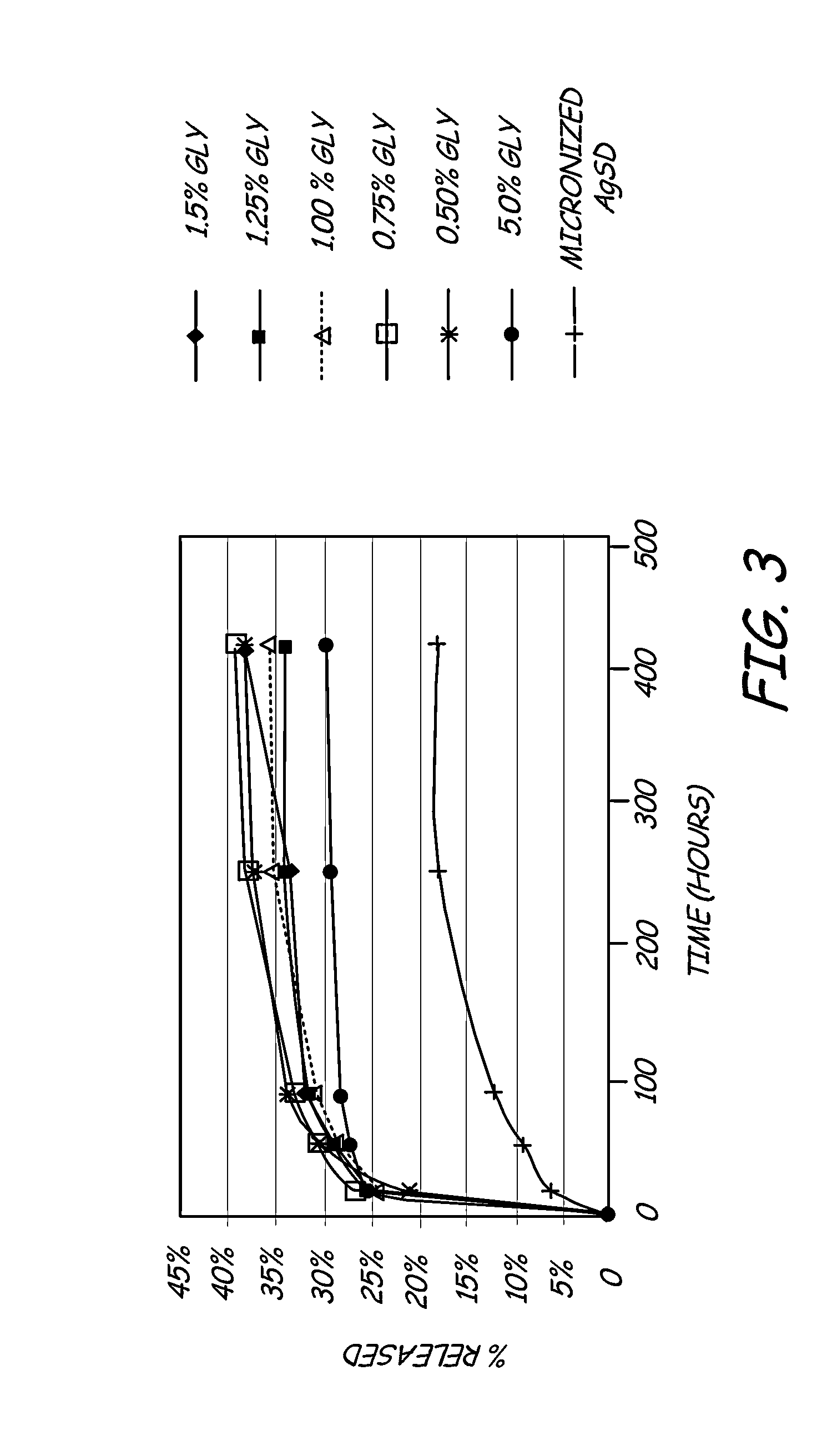
- A hydrophilic polymeric coating with a cross-linked 3D network that maintains a water-insoluble antimicrobial metallic compound, such as silver sulfadiazine, in a solubilized form, allowing high concentrations to be incorporated in thin coatings, inhibiting bacterial adhesion and biofilm formation by releasing bactericidal silver ions.
Regulatory Framework for Medical Device Coatings
The regulatory framework for medical device coatings, particularly those incorporating microcrystalline cellulose in antibacterial surface coatings, is a complex and evolving landscape. This framework is primarily governed by regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
In the United States, medical device coatings fall under the purview of the FDA's Center for Devices and Radiological Health (CDRH). The regulatory pathway for these coatings depends on their intended use and the risk classification of the medical device they are applied to. Devices with antibacterial coatings may be classified as Class II or Class III devices, requiring either a 510(k) premarket notification or a premarket approval (PMA) application.
The FDA's guidance document "Premarket Notification [510(k)] Submissions for Medical Devices that Include Antimicrobial Agents" provides specific recommendations for manufacturers developing devices with antibacterial coatings. This guidance outlines the necessary data and testing requirements to demonstrate safety and efficacy.
In the European Union, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) govern the approval process for medical devices, including those with antibacterial coatings. The MDR places a strong emphasis on clinical evidence and post-market surveillance, which is particularly relevant for novel coating technologies like those using microcrystalline cellulose.
Both the FDA and EMA require manufacturers to provide comprehensive data on the safety and efficacy of the coating material. This includes biocompatibility testing, leachable and extractable studies, and antimicrobial efficacy testing. For coatings containing microcrystalline cellulose, additional considerations may include the source and purity of the cellulose, as well as its interaction with the base medical device material.
Regulatory bodies also focus on the manufacturing process and quality control measures. Good Manufacturing Practices (GMP) must be followed, and manufacturers must demonstrate consistent production of the coating with specified properties. This includes stability testing to ensure the coating maintains its antibacterial properties over the device's intended shelf life.
Environmental considerations are becoming increasingly important in regulatory frameworks. The biodegradability and environmental impact of microcrystalline cellulose-based coatings may need to be addressed, particularly in light of growing concerns about microplastics and sustainable materials in healthcare.
As research on microcrystalline cellulose in antibacterial surface coatings advances, regulatory frameworks are likely to evolve. Manufacturers and researchers must stay abreast of these changes and engage in early dialogue with regulatory bodies to ensure compliance and streamline the approval process for their innovative coating technologies.
In the United States, medical device coatings fall under the purview of the FDA's Center for Devices and Radiological Health (CDRH). The regulatory pathway for these coatings depends on their intended use and the risk classification of the medical device they are applied to. Devices with antibacterial coatings may be classified as Class II or Class III devices, requiring either a 510(k) premarket notification or a premarket approval (PMA) application.
The FDA's guidance document "Premarket Notification [510(k)] Submissions for Medical Devices that Include Antimicrobial Agents" provides specific recommendations for manufacturers developing devices with antibacterial coatings. This guidance outlines the necessary data and testing requirements to demonstrate safety and efficacy.
In the European Union, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) govern the approval process for medical devices, including those with antibacterial coatings. The MDR places a strong emphasis on clinical evidence and post-market surveillance, which is particularly relevant for novel coating technologies like those using microcrystalline cellulose.
Both the FDA and EMA require manufacturers to provide comprehensive data on the safety and efficacy of the coating material. This includes biocompatibility testing, leachable and extractable studies, and antimicrobial efficacy testing. For coatings containing microcrystalline cellulose, additional considerations may include the source and purity of the cellulose, as well as its interaction with the base medical device material.
Regulatory bodies also focus on the manufacturing process and quality control measures. Good Manufacturing Practices (GMP) must be followed, and manufacturers must demonstrate consistent production of the coating with specified properties. This includes stability testing to ensure the coating maintains its antibacterial properties over the device's intended shelf life.
Environmental considerations are becoming increasingly important in regulatory frameworks. The biodegradability and environmental impact of microcrystalline cellulose-based coatings may need to be addressed, particularly in light of growing concerns about microplastics and sustainable materials in healthcare.
As research on microcrystalline cellulose in antibacterial surface coatings advances, regulatory frameworks are likely to evolve. Manufacturers and researchers must stay abreast of these changes and engage in early dialogue with regulatory bodies to ensure compliance and streamline the approval process for their innovative coating technologies.
Environmental Impact of MCC-based Coatings
The environmental impact of microcrystalline cellulose (MCC)-based coatings for medical devices is an important consideration in the development and application of this technology. These coatings offer potential benefits in terms of antibacterial properties and biocompatibility, but their environmental implications must be carefully evaluated.
MCC is derived from natural cellulose sources, primarily wood pulp or cotton linters, which are renewable resources. This aspect contributes positively to the sustainability profile of MCC-based coatings. The production process of MCC involves acid hydrolysis and mechanical treatment, which can be optimized to reduce energy consumption and minimize chemical waste.
In terms of biodegradability, MCC-based coatings have an advantage over many synthetic polymer coatings. Cellulose is naturally biodegradable, and MCC retains this property to a large extent. This characteristic reduces the potential for long-term environmental accumulation of coating materials after disposal of medical devices.
However, the antibacterial properties of these coatings often rely on the incorporation of additional substances, such as silver nanoparticles or quaternary ammonium compounds. The environmental fate and potential toxicity of these additives must be carefully considered. For instance, the release of silver nanoparticles into aquatic environments has been associated with potential ecological risks.
The production and application of MCC-based coatings may have lower environmental impacts compared to some traditional coating methods. These coatings often require less toxic solvents and can be applied using water-based systems, reducing volatile organic compound (VOC) emissions during the manufacturing and application processes.
End-of-life considerations for medical devices with MCC-based coatings are generally favorable. The biodegradability of cellulose components can facilitate easier disposal or recycling of devices. However, the presence of non-biodegradable additives or other device components may complicate the overall environmental impact of disposal.
Life cycle assessment (LCA) studies are crucial for comprehensively evaluating the environmental footprint of MCC-based coatings. These assessments should consider raw material extraction, manufacturing processes, use phase, and end-of-life scenarios. Preliminary LCA results suggest that MCC-based coatings may offer environmental advantages over some conventional coating technologies, particularly in terms of resource depletion and global warming potential.
Future research should focus on optimizing the environmental performance of MCC-based coatings. This includes developing more efficient production methods, exploring alternative antibacterial agents with lower environmental impacts, and improving the overall biodegradability of the coating system while maintaining its functional properties for medical device applications.
MCC is derived from natural cellulose sources, primarily wood pulp or cotton linters, which are renewable resources. This aspect contributes positively to the sustainability profile of MCC-based coatings. The production process of MCC involves acid hydrolysis and mechanical treatment, which can be optimized to reduce energy consumption and minimize chemical waste.
In terms of biodegradability, MCC-based coatings have an advantage over many synthetic polymer coatings. Cellulose is naturally biodegradable, and MCC retains this property to a large extent. This characteristic reduces the potential for long-term environmental accumulation of coating materials after disposal of medical devices.
However, the antibacterial properties of these coatings often rely on the incorporation of additional substances, such as silver nanoparticles or quaternary ammonium compounds. The environmental fate and potential toxicity of these additives must be carefully considered. For instance, the release of silver nanoparticles into aquatic environments has been associated with potential ecological risks.
The production and application of MCC-based coatings may have lower environmental impacts compared to some traditional coating methods. These coatings often require less toxic solvents and can be applied using water-based systems, reducing volatile organic compound (VOC) emissions during the manufacturing and application processes.
End-of-life considerations for medical devices with MCC-based coatings are generally favorable. The biodegradability of cellulose components can facilitate easier disposal or recycling of devices. However, the presence of non-biodegradable additives or other device components may complicate the overall environmental impact of disposal.
Life cycle assessment (LCA) studies are crucial for comprehensively evaluating the environmental footprint of MCC-based coatings. These assessments should consider raw material extraction, manufacturing processes, use phase, and end-of-life scenarios. Preliminary LCA results suggest that MCC-based coatings may offer environmental advantages over some conventional coating technologies, particularly in terms of resource depletion and global warming potential.
Future research should focus on optimizing the environmental performance of MCC-based coatings. This includes developing more efficient production methods, exploring alternative antibacterial agents with lower environmental impacts, and improving the overall biodegradability of the coating system while maintaining its functional properties for medical device applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!