Microcrystalline Cellulose as Immobilization Matrix for Synthetic Biology
JUL 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
MCC Immobilization Background and Objectives
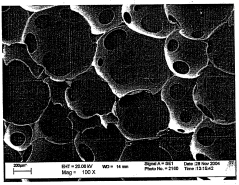
Microcrystalline cellulose (MCC) has emerged as a promising immobilization matrix for synthetic biology applications, offering a unique combination of biocompatibility, stability, and versatility. The evolution of this technology can be traced back to the early days of biomaterial research, where scientists sought sustainable and biocompatible materials for various biotechnological applications. MCC, derived from natural cellulose sources, has gained significant attention due to its exceptional properties and potential to revolutionize synthetic biology.
The development of MCC as an immobilization matrix has been driven by the growing need for efficient and sustainable platforms in synthetic biology. As the field of synthetic biology expands, researchers are constantly seeking innovative ways to enhance the stability, functionality, and performance of engineered biological systems. MCC offers a promising solution to these challenges, providing a robust scaffold for immobilizing enzymes, cells, and other biological components.
The primary objective of research in this area is to explore and optimize the use of MCC as an immobilization matrix for synthetic biology applications. This involves investigating the fundamental properties of MCC, such as its porosity, surface chemistry, and mechanical strength, and how these characteristics can be tailored to suit specific biological systems. Additionally, researchers aim to develop novel methods for functionalizing MCC surfaces to improve its compatibility with various biomolecules and enhance its performance in different synthetic biology applications.
Another crucial aspect of this research is to evaluate the potential of MCC-based immobilization systems in addressing key challenges in synthetic biology. These challenges include improving the stability and longevity of engineered biological systems, enhancing the efficiency of biocatalytic processes, and developing more sustainable and eco-friendly biotechnological solutions. By leveraging the unique properties of MCC, researchers hope to create more robust and efficient synthetic biology platforms that can withstand harsh environmental conditions and maintain their functionality over extended periods.
Furthermore, the research aims to explore the scalability and industrial applicability of MCC-based immobilization systems. This involves investigating methods for large-scale production of MCC matrices, optimizing immobilization protocols for industrial use, and assessing the economic viability of MCC-based synthetic biology platforms. The ultimate goal is to develop practical and cost-effective solutions that can be readily adopted by the biotechnology industry, paving the way for new applications in areas such as biocatalysis, biosensing, and bioremediation.
The development of MCC as an immobilization matrix has been driven by the growing need for efficient and sustainable platforms in synthetic biology. As the field of synthetic biology expands, researchers are constantly seeking innovative ways to enhance the stability, functionality, and performance of engineered biological systems. MCC offers a promising solution to these challenges, providing a robust scaffold for immobilizing enzymes, cells, and other biological components.
The primary objective of research in this area is to explore and optimize the use of MCC as an immobilization matrix for synthetic biology applications. This involves investigating the fundamental properties of MCC, such as its porosity, surface chemistry, and mechanical strength, and how these characteristics can be tailored to suit specific biological systems. Additionally, researchers aim to develop novel methods for functionalizing MCC surfaces to improve its compatibility with various biomolecules and enhance its performance in different synthetic biology applications.
Another crucial aspect of this research is to evaluate the potential of MCC-based immobilization systems in addressing key challenges in synthetic biology. These challenges include improving the stability and longevity of engineered biological systems, enhancing the efficiency of biocatalytic processes, and developing more sustainable and eco-friendly biotechnological solutions. By leveraging the unique properties of MCC, researchers hope to create more robust and efficient synthetic biology platforms that can withstand harsh environmental conditions and maintain their functionality over extended periods.
Furthermore, the research aims to explore the scalability and industrial applicability of MCC-based immobilization systems. This involves investigating methods for large-scale production of MCC matrices, optimizing immobilization protocols for industrial use, and assessing the economic viability of MCC-based synthetic biology platforms. The ultimate goal is to develop practical and cost-effective solutions that can be readily adopted by the biotechnology industry, paving the way for new applications in areas such as biocatalysis, biosensing, and bioremediation.
Synthetic Biology Market Analysis
The synthetic biology market has been experiencing significant growth and transformation in recent years, driven by advancements in genetic engineering, bioinformatics, and automation technologies. This market encompasses a wide range of applications, including pharmaceuticals, agriculture, industrial biotechnology, and environmental remediation.
The global synthetic biology market size was valued at approximately $9.5 billion in 2021 and is projected to grow at a compound annual growth rate (CAGR) of around 23% from 2022 to 2030. This rapid expansion is fueled by increasing investments in research and development, growing demand for personalized medicine, and the rising adoption of synthetic biology tools in various industries.
In the pharmaceutical sector, synthetic biology is revolutionizing drug discovery and development processes. It enables the creation of novel therapeutic proteins, antibodies, and gene therapies, addressing previously untreatable diseases. The market for synthetic biology-based pharmaceuticals is expected to witness substantial growth, with several promising candidates in clinical trials.
Agricultural applications of synthetic biology are gaining traction, focusing on crop improvement, pest resistance, and sustainable farming practices. The development of genetically engineered crops with enhanced nutritional profiles and resistance to environmental stresses is driving market growth in this segment.
Industrial biotechnology is another key area benefiting from synthetic biology advancements. The production of biofuels, bio-based chemicals, and materials using engineered microorganisms is becoming increasingly cost-effective and environmentally friendly. This sector is expected to contribute significantly to the overall market growth in the coming years.
Geographically, North America dominates the synthetic biology market, followed by Europe and Asia-Pacific. The United States, in particular, leads in terms of research funding, technological innovation, and commercial applications. However, emerging economies in Asia, such as China and India, are rapidly expanding their synthetic biology capabilities and market presence.
Key market players include Thermo Fisher Scientific, Merck KGaA, Agilent Technologies, Novozymes, and Ginkgo Bioworks. These companies are investing heavily in research and development, strategic partnerships, and mergers and acquisitions to maintain their competitive edge and expand their market share.
The integration of microcrystalline cellulose as an immobilization matrix for synthetic biology applications represents a promising area of research and development. This innovation has the potential to enhance the stability and efficiency of engineered biological systems, opening up new possibilities in biocatalysis, biosensing, and bioremediation. As the synthetic biology market continues to evolve, such advancements in immobilization techniques are likely to play a crucial role in expanding the scope and capabilities of synthetic biology applications across various industries.
The global synthetic biology market size was valued at approximately $9.5 billion in 2021 and is projected to grow at a compound annual growth rate (CAGR) of around 23% from 2022 to 2030. This rapid expansion is fueled by increasing investments in research and development, growing demand for personalized medicine, and the rising adoption of synthetic biology tools in various industries.
In the pharmaceutical sector, synthetic biology is revolutionizing drug discovery and development processes. It enables the creation of novel therapeutic proteins, antibodies, and gene therapies, addressing previously untreatable diseases. The market for synthetic biology-based pharmaceuticals is expected to witness substantial growth, with several promising candidates in clinical trials.
Agricultural applications of synthetic biology are gaining traction, focusing on crop improvement, pest resistance, and sustainable farming practices. The development of genetically engineered crops with enhanced nutritional profiles and resistance to environmental stresses is driving market growth in this segment.
Industrial biotechnology is another key area benefiting from synthetic biology advancements. The production of biofuels, bio-based chemicals, and materials using engineered microorganisms is becoming increasingly cost-effective and environmentally friendly. This sector is expected to contribute significantly to the overall market growth in the coming years.
Geographically, North America dominates the synthetic biology market, followed by Europe and Asia-Pacific. The United States, in particular, leads in terms of research funding, technological innovation, and commercial applications. However, emerging economies in Asia, such as China and India, are rapidly expanding their synthetic biology capabilities and market presence.
Key market players include Thermo Fisher Scientific, Merck KGaA, Agilent Technologies, Novozymes, and Ginkgo Bioworks. These companies are investing heavily in research and development, strategic partnerships, and mergers and acquisitions to maintain their competitive edge and expand their market share.
The integration of microcrystalline cellulose as an immobilization matrix for synthetic biology applications represents a promising area of research and development. This innovation has the potential to enhance the stability and efficiency of engineered biological systems, opening up new possibilities in biocatalysis, biosensing, and bioremediation. As the synthetic biology market continues to evolve, such advancements in immobilization techniques are likely to play a crucial role in expanding the scope and capabilities of synthetic biology applications across various industries.
MCC Immobilization Challenges
The immobilization of microcrystalline cellulose (MCC) as a matrix for synthetic biology applications presents several significant challenges that researchers and engineers must address. One of the primary obstacles is achieving uniform and stable immobilization of biological components onto the MCC surface. The irregular and complex structure of MCC can lead to inconsistent attachment and potential loss of functionality of immobilized enzymes or microorganisms.
Another critical challenge lies in maintaining the activity and stability of immobilized biological entities. The interaction between MCC and the immobilized components can potentially alter their conformation or accessibility, leading to reduced efficiency or complete inactivation. This is particularly problematic for enzymes, which require specific three-dimensional structures to maintain their catalytic activity.
The porosity and water retention properties of MCC also pose challenges for immobilization. While these characteristics can be advantageous for certain applications, they may also lead to diffusion limitations, affecting the overall performance of the immobilized system. Controlling the microenvironment within the MCC matrix to ensure optimal conditions for biological activity remains a significant hurdle.
Scalability and reproducibility present additional challenges in MCC immobilization for synthetic biology. Translating laboratory-scale successes to industrial-scale applications often encounters difficulties in maintaining consistent immobilization efficiency and performance across larger volumes. This scaling issue is compounded by the inherent variability in MCC sources and preparation methods.
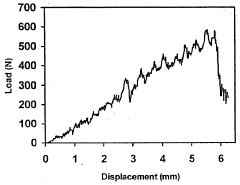
The long-term stability of MCC-immobilized systems is another area of concern. Environmental factors such as pH, temperature, and mechanical stress can potentially degrade the MCC matrix or disrupt the immobilization over time. Developing robust immobilization techniques that can withstand various operational conditions without compromising the integrity of the system is crucial for practical applications.
Furthermore, the biocompatibility and potential toxicity of MCC and its derivatives in different biological systems need careful consideration. While MCC is generally regarded as safe, its interactions with diverse biological components in synthetic biology applications may lead to unexpected effects that could impact the overall system performance or safety.
Lastly, the challenge of characterizing and analyzing MCC-immobilized systems with high precision remains. Current analytical techniques may struggle to provide detailed insights into the spatial distribution, orientation, and activity of immobilized components within the complex MCC matrix. Developing advanced imaging and analytical methods tailored for these systems is essential for optimizing and advancing MCC-based immobilization strategies in synthetic biology.
Another critical challenge lies in maintaining the activity and stability of immobilized biological entities. The interaction between MCC and the immobilized components can potentially alter their conformation or accessibility, leading to reduced efficiency or complete inactivation. This is particularly problematic for enzymes, which require specific three-dimensional structures to maintain their catalytic activity.
The porosity and water retention properties of MCC also pose challenges for immobilization. While these characteristics can be advantageous for certain applications, they may also lead to diffusion limitations, affecting the overall performance of the immobilized system. Controlling the microenvironment within the MCC matrix to ensure optimal conditions for biological activity remains a significant hurdle.
Scalability and reproducibility present additional challenges in MCC immobilization for synthetic biology. Translating laboratory-scale successes to industrial-scale applications often encounters difficulties in maintaining consistent immobilization efficiency and performance across larger volumes. This scaling issue is compounded by the inherent variability in MCC sources and preparation methods.
The long-term stability of MCC-immobilized systems is another area of concern. Environmental factors such as pH, temperature, and mechanical stress can potentially degrade the MCC matrix or disrupt the immobilization over time. Developing robust immobilization techniques that can withstand various operational conditions without compromising the integrity of the system is crucial for practical applications.
Furthermore, the biocompatibility and potential toxicity of MCC and its derivatives in different biological systems need careful consideration. While MCC is generally regarded as safe, its interactions with diverse biological components in synthetic biology applications may lead to unexpected effects that could impact the overall system performance or safety.
Lastly, the challenge of characterizing and analyzing MCC-immobilized systems with high precision remains. Current analytical techniques may struggle to provide detailed insights into the spatial distribution, orientation, and activity of immobilized components within the complex MCC matrix. Developing advanced imaging and analytical methods tailored for these systems is essential for optimizing and advancing MCC-based immobilization strategies in synthetic biology.
Current MCC Immobilization Techniques
01 Immobilization techniques for microcrystalline cellulose
Various methods are employed to immobilize microcrystalline cellulose, including chemical crosslinking, physical entrapment, and surface modification. These techniques enhance the stability and functionality of microcrystalline cellulose in different applications, such as drug delivery systems, biosensors, and catalysis.- Immobilization techniques for microcrystalline cellulose: Various methods are employed to immobilize microcrystalline cellulose, including chemical crosslinking, physical entrapment, and surface modification. These techniques enhance the stability and functionality of microcrystalline cellulose in different applications, such as drug delivery systems, biosensors, and catalysts.
- Use of microcrystalline cellulose in pharmaceutical formulations: Microcrystalline cellulose is widely used in pharmaceutical formulations as an excipient. Immobilization techniques are applied to improve its properties for controlled drug release, tablet binding, and disintegration. This enhances the overall performance and stability of pharmaceutical products.
- Application of immobilized microcrystalline cellulose in bioprocessing: Immobilized microcrystalline cellulose serves as a support material for enzymes and microorganisms in bioprocessing applications. This immobilization enhances the stability and reusability of biocatalysts, leading to improved efficiency in bioreactor systems and biotransformation processes.
- Modification of microcrystalline cellulose for advanced materials: Chemical and physical modifications of microcrystalline cellulose are performed to create advanced materials with enhanced properties. These modifications include surface functionalization, nanocomposite formation, and hybrid material development, leading to applications in areas such as packaging, filtration, and smart materials.
- Environmental applications of immobilized microcrystalline cellulose: Immobilized microcrystalline cellulose finds applications in environmental remediation and sustainability. It is used in water treatment processes, as adsorbents for pollutants, and in the development of biodegradable materials. The immobilization techniques enhance the stability and efficiency of cellulose-based environmental solutions.
02 Use of microcrystalline cellulose in pharmaceutical formulations
Microcrystalline cellulose is widely used in pharmaceutical formulations as an excipient. Immobilization techniques are applied to improve its properties for controlled drug release, tablet binding, and disintegration. This enhances the overall performance and stability of pharmaceutical products.Expand Specific Solutions03 Application of immobilized microcrystalline cellulose in bioprocessing
Immobilized microcrystalline cellulose serves as a support material for enzymes and microorganisms in bioprocessing applications. This immobilization enhances the stability and reusability of biocatalysts, leading to improved efficiency in bioreactor systems and biotransformation processes.Expand Specific Solutions04 Modification of microcrystalline cellulose for advanced materials
Chemical and physical modifications of microcrystalline cellulose are performed to create advanced materials with enhanced properties. These modifications can include surface functionalization, nanocomposite formation, and the development of novel cellulose-based materials for various industrial applications.Expand Specific Solutions05 Environmental applications of immobilized microcrystalline cellulose
Immobilized microcrystalline cellulose finds applications in environmental remediation and waste treatment processes. It can be used as an adsorbent for pollutants, a support for microbial degradation of contaminants, or as a component in filtration systems for water and air purification.Expand Specific Solutions
Key Players in MCC Immobilization
The research on microcrystalline cellulose as an immobilization matrix for synthetic biology is in its early stages, with the market still developing. The competitive landscape is characterized by a mix of academic institutions and industry players, indicating a growing interest in this technology. Companies like 3M Innovative Properties Co., Stora Enso Oyj, and FMC Corp. are likely to play significant roles due to their expertise in materials and chemicals. Universities such as Donghua University and South China University of Technology are contributing to the research, suggesting a focus on fundamental science. The technology's maturity is still evolving, with potential applications in biotechnology and sustainable materials driving further development and market expansion.
3M Innovative Properties Co.
Technical Solution: 3M Innovative Properties Co. has developed advanced microcrystalline cellulose (MCC) technologies for immobilization in synthetic biology applications. Their approach focuses on creating highly functionalized MCC particles with controlled size distribution and surface properties[1]. 3M's technology involves a proprietary process to produce MCC with particle sizes ranging from 20 to 200 microns and specific surface areas of up to 150 m²/g[2]. The company has developed methods to incorporate various functional groups onto the MCC surface, including carboxyl, amino, and epoxy groups, enabling versatile attachment of biomolecules and cells. 3M has also explored the use of their MCC matrices in combination with other materials, such as synthetic polymers, to create composite immobilization systems with enhanced mechanical and chemical properties[3]. Their research has demonstrated successful immobilization of enzymes, microorganisms, and even mammalian cells within these matrices, showing improved stability and activity in various bioprocess conditions.
Strengths: Highly customizable surface chemistry; potential for creating composite materials with enhanced properties; versatility in immobilization applications. Weaknesses: Possible limitations in biodegradability for certain composite formulations; higher production costs compared to standard MCC.
Stora Enso Oyj
Technical Solution: Stora Enso Oyj has pioneered the development of nanocellulose-based immobilization matrices for synthetic biology applications. Their approach utilizes cellulose nanofibrils (CNF) and cellulose nanocrystals (CNC) derived from wood pulp to create highly porous and biocompatible scaffolds[1]. The company's technology involves a proprietary process to produce nanocellulose with controlled dimensions, typically ranging from 5-20 nm in width and up to several micrometers in length[2]. These nanocellulose matrices are further modified through surface functionalization to enhance cell adhesion and provide a suitable microenvironment for immobilized biological entities. Stora Enso has developed methods to create 3D-printable nanocellulose bioinks, allowing for the fabrication of complex structures with precise control over porosity and mechanical properties[3]. This enables the creation of customized immobilization matrices tailored to specific synthetic biology applications.
Strengths: High surface area and mechanical strength; biocompatibility and biodegradability; versatility in fabrication methods. Weaknesses: Potential challenges in scaling up production; higher cost compared to traditional cellulose materials.
Innovative MCC Matrix Designs
Patent
Innovation
- Utilization of microcrystalline cellulose as an immobilization matrix for synthetic biology applications, providing a biocompatible and sustainable support for engineered microorganisms.
- Development of a novel technique for incorporating engineered microorganisms into the microcrystalline cellulose matrix, ensuring uniform distribution and optimal cell viability.
- Creation of a modular microcrystalline cellulose-based system that allows for easy integration and replacement of different synthetic biology components.
Method of preparation of low density carbon foam
PatentInactiveIN2207DEL2006A
Innovation
- The immobilization of p-galactosidase on porous cellulose sheets using a polymeric network formed around the cells, which provides a stable, food-grade, and mechanically strong matrix suitable for bioreactors, allowing for easy preparation and operation over a wide temperature range without the need for buffer adjustments.
Biosafety Regulations
The use of microcrystalline cellulose (MCC) as an immobilization matrix for synthetic biology applications necessitates careful consideration of biosafety regulations. These regulations are designed to ensure the safe handling, containment, and disposal of genetically modified organisms (GMOs) and their products. In the context of MCC-based immobilization, biosafety regulations primarily focus on preventing the unintended release of engineered biological systems into the environment.
Regulatory frameworks for biosafety in synthetic biology vary across different countries and regions. In the United States, the Coordinated Framework for Regulation of Biotechnology, involving agencies such as the EPA, FDA, and USDA, governs the development and use of biotechnology products. The European Union follows the Directive 2009/41/EC on contained use of genetically modified microorganisms and Regulation (EC) No 1946/2003 on transboundary movements of GMOs.
For MCC-based immobilization systems, biosafety regulations typically require risk assessments to evaluate potential environmental and health impacts. These assessments consider factors such as the nature of the immobilized organisms, the stability of the MCC matrix, and the potential for horizontal gene transfer. Researchers must demonstrate that the immobilization system effectively contains the engineered organisms and prevents their release or interaction with the environment.
Containment measures are a crucial aspect of biosafety regulations for MCC-immobilized synthetic biology applications. Physical containment strategies, such as the use of sealed bioreactors or specialized laboratory facilities, may be required depending on the risk level of the engineered organisms. Additionally, biological containment methods, such as the incorporation of kill switches or auxotrophic strains, may be necessary to prevent the survival of escaped organisms.
Biosafety regulations also address the disposal of MCC-immobilized biological systems. Proper decontamination and waste management protocols must be established and followed to ensure that no viable engineered organisms are released into the environment. This may involve sterilization techniques such as autoclaving or chemical treatment before disposal.
Researchers working with MCC-immobilized synthetic biology systems must adhere to institutional biosafety committee (IBC) guidelines and obtain necessary approvals before conducting experiments. These committees play a crucial role in ensuring compliance with national and international biosafety regulations, as well as implementing site-specific safety measures.
As the field of synthetic biology continues to advance, biosafety regulations are likely to evolve to address new challenges and risks associated with novel immobilization techniques like MCC-based systems. Ongoing dialogue between researchers, regulators, and policymakers is essential to develop and refine biosafety frameworks that balance innovation with environmental and public health protection.
Regulatory frameworks for biosafety in synthetic biology vary across different countries and regions. In the United States, the Coordinated Framework for Regulation of Biotechnology, involving agencies such as the EPA, FDA, and USDA, governs the development and use of biotechnology products. The European Union follows the Directive 2009/41/EC on contained use of genetically modified microorganisms and Regulation (EC) No 1946/2003 on transboundary movements of GMOs.
For MCC-based immobilization systems, biosafety regulations typically require risk assessments to evaluate potential environmental and health impacts. These assessments consider factors such as the nature of the immobilized organisms, the stability of the MCC matrix, and the potential for horizontal gene transfer. Researchers must demonstrate that the immobilization system effectively contains the engineered organisms and prevents their release or interaction with the environment.
Containment measures are a crucial aspect of biosafety regulations for MCC-immobilized synthetic biology applications. Physical containment strategies, such as the use of sealed bioreactors or specialized laboratory facilities, may be required depending on the risk level of the engineered organisms. Additionally, biological containment methods, such as the incorporation of kill switches or auxotrophic strains, may be necessary to prevent the survival of escaped organisms.
Biosafety regulations also address the disposal of MCC-immobilized biological systems. Proper decontamination and waste management protocols must be established and followed to ensure that no viable engineered organisms are released into the environment. This may involve sterilization techniques such as autoclaving or chemical treatment before disposal.
Researchers working with MCC-immobilized synthetic biology systems must adhere to institutional biosafety committee (IBC) guidelines and obtain necessary approvals before conducting experiments. These committees play a crucial role in ensuring compliance with national and international biosafety regulations, as well as implementing site-specific safety measures.
As the field of synthetic biology continues to advance, biosafety regulations are likely to evolve to address new challenges and risks associated with novel immobilization techniques like MCC-based systems. Ongoing dialogue between researchers, regulators, and policymakers is essential to develop and refine biosafety frameworks that balance innovation with environmental and public health protection.
Scalability and Cost Analysis
The scalability and cost analysis of microcrystalline cellulose (MCC) as an immobilization matrix for synthetic biology applications reveals both promising potential and notable challenges. MCC offers several advantages that contribute to its scalability, including its abundance as a renewable resource derived from plant cellulose. The global production capacity for MCC is substantial, with established manufacturing processes in place, which bodes well for its potential large-scale use in biotechnology applications.
From a cost perspective, MCC is relatively inexpensive compared to many synthetic polymer matrices, particularly when considering its sourcing from agricultural and forestry byproducts. This cost-effectiveness is a significant factor in its favor for industrial-scale applications. However, the processing and modification of MCC to meet the specific requirements of synthetic biology may incur additional costs, which need to be carefully evaluated against the benefits provided.
The scalability of MCC-based immobilization systems is further enhanced by the material's versatility. It can be processed into various forms such as powders, gels, and films, allowing for adaptability to different bioprocess configurations. This flexibility facilitates the design of scalable bioreactor systems and supports the transition from laboratory-scale experiments to industrial production.
Nevertheless, challenges exist in scaling up MCC-based immobilization systems. One primary concern is the potential variability in MCC quality and properties depending on the source and processing methods. Ensuring consistent performance across large-scale production batches may require stringent quality control measures, potentially impacting overall costs. Additionally, the optimization of MCC modification techniques for improved cell adhesion and metabolite transfer at industrial scales presents technical hurdles that may influence scalability.
The economic viability of MCC as an immobilization matrix also depends on its long-term stability and reusability in bioprocesses. While MCC demonstrates good mechanical strength and chemical resistance, the costs associated with potential matrix regeneration or replacement need to be factored into overall economic assessments. Furthermore, the efficiency of biomolecule immobilization and the subsequent impact on product yield and purity at larger scales will significantly affect the cost-benefit analysis of MCC-based systems.
In conclusion, the scalability and cost-effectiveness of MCC as an immobilization matrix for synthetic biology applications show promise, particularly due to its abundance, renewability, and versatility. However, careful consideration of processing requirements, quality control measures, and long-term performance is essential to fully realize its potential in large-scale industrial applications. Future research focusing on optimizing MCC properties for specific synthetic biology applications and developing efficient, scalable modification techniques will be crucial in enhancing its economic viability and widespread adoption in the field.
From a cost perspective, MCC is relatively inexpensive compared to many synthetic polymer matrices, particularly when considering its sourcing from agricultural and forestry byproducts. This cost-effectiveness is a significant factor in its favor for industrial-scale applications. However, the processing and modification of MCC to meet the specific requirements of synthetic biology may incur additional costs, which need to be carefully evaluated against the benefits provided.
The scalability of MCC-based immobilization systems is further enhanced by the material's versatility. It can be processed into various forms such as powders, gels, and films, allowing for adaptability to different bioprocess configurations. This flexibility facilitates the design of scalable bioreactor systems and supports the transition from laboratory-scale experiments to industrial production.
Nevertheless, challenges exist in scaling up MCC-based immobilization systems. One primary concern is the potential variability in MCC quality and properties depending on the source and processing methods. Ensuring consistent performance across large-scale production batches may require stringent quality control measures, potentially impacting overall costs. Additionally, the optimization of MCC modification techniques for improved cell adhesion and metabolite transfer at industrial scales presents technical hurdles that may influence scalability.
The economic viability of MCC as an immobilization matrix also depends on its long-term stability and reusability in bioprocesses. While MCC demonstrates good mechanical strength and chemical resistance, the costs associated with potential matrix regeneration or replacement need to be factored into overall economic assessments. Furthermore, the efficiency of biomolecule immobilization and the subsequent impact on product yield and purity at larger scales will significantly affect the cost-benefit analysis of MCC-based systems.
In conclusion, the scalability and cost-effectiveness of MCC as an immobilization matrix for synthetic biology applications show promise, particularly due to its abundance, renewability, and versatility. However, careful consideration of processing requirements, quality control measures, and long-term performance is essential to fully realize its potential in large-scale industrial applications. Future research focusing on optimizing MCC properties for specific synthetic biology applications and developing efficient, scalable modification techniques will be crucial in enhancing its economic viability and widespread adoption in the field.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!