Use of Microcrystalline Cellulose Nanoparticles in Targeted Gene Therapy
JUL 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
MCC Nanoparticles in Gene Therapy: Background and Objectives
Microcrystalline cellulose (MCC) nanoparticles have emerged as a promising vehicle for targeted gene therapy, representing a significant advancement in the field of nanomedicine. The development of MCC nanoparticles for gene delivery stems from the broader context of nanotechnology applications in healthcare, which has seen rapid growth over the past two decades.
The use of nanoparticles in gene therapy addresses several key challenges that have historically limited the efficacy of genetic treatments. Traditional gene delivery methods often struggle with issues such as low transfection efficiency, rapid degradation of genetic material, and off-target effects. MCC nanoparticles offer potential solutions to these problems due to their unique physicochemical properties and biocompatibility.
Cellulose, as the most abundant natural polymer on Earth, provides an attractive source material for nanoparticle synthesis. The transition from bulk cellulose to nanostructured MCC involves sophisticated processing techniques that have been refined over time. These advancements have enabled the production of nanoparticles with precise size control, high surface area, and modifiable surface chemistry, all of which are crucial for effective gene delivery.
The primary objective of utilizing MCC nanoparticles in targeted gene therapy is to develop a safe, efficient, and versatile delivery system that can protect genetic material during transport and facilitate its uptake by specific cells or tissues. This approach aims to improve the therapeutic index of genetic treatments while minimizing side effects associated with non-specific delivery.
Research in this field is driven by the potential to treat a wide range of genetic disorders, cancers, and other diseases that have proven resistant to conventional therapies. The ability to deliver genes or gene-editing tools with high precision could revolutionize personalized medicine, offering tailored treatments based on an individual's genetic profile.
As the field progresses, researchers are focusing on several key areas to enhance the effectiveness of MCC nanoparticles in gene therapy. These include optimizing particle size and shape for improved cellular uptake, developing surface modifications to target specific cell types, and engineering controlled release mechanisms to ensure sustained therapeutic effects.
The evolution of MCC nanoparticles for gene therapy aligns with broader trends in nanomedicine, including the development of smart materials that can respond to biological stimuli and the integration of multifunctional capabilities within a single nanoparticle system. These advancements are expected to lead to more sophisticated and effective gene delivery platforms in the coming years.
The use of nanoparticles in gene therapy addresses several key challenges that have historically limited the efficacy of genetic treatments. Traditional gene delivery methods often struggle with issues such as low transfection efficiency, rapid degradation of genetic material, and off-target effects. MCC nanoparticles offer potential solutions to these problems due to their unique physicochemical properties and biocompatibility.
Cellulose, as the most abundant natural polymer on Earth, provides an attractive source material for nanoparticle synthesis. The transition from bulk cellulose to nanostructured MCC involves sophisticated processing techniques that have been refined over time. These advancements have enabled the production of nanoparticles with precise size control, high surface area, and modifiable surface chemistry, all of which are crucial for effective gene delivery.
The primary objective of utilizing MCC nanoparticles in targeted gene therapy is to develop a safe, efficient, and versatile delivery system that can protect genetic material during transport and facilitate its uptake by specific cells or tissues. This approach aims to improve the therapeutic index of genetic treatments while minimizing side effects associated with non-specific delivery.
Research in this field is driven by the potential to treat a wide range of genetic disorders, cancers, and other diseases that have proven resistant to conventional therapies. The ability to deliver genes or gene-editing tools with high precision could revolutionize personalized medicine, offering tailored treatments based on an individual's genetic profile.
As the field progresses, researchers are focusing on several key areas to enhance the effectiveness of MCC nanoparticles in gene therapy. These include optimizing particle size and shape for improved cellular uptake, developing surface modifications to target specific cell types, and engineering controlled release mechanisms to ensure sustained therapeutic effects.
The evolution of MCC nanoparticles for gene therapy aligns with broader trends in nanomedicine, including the development of smart materials that can respond to biological stimuli and the integration of multifunctional capabilities within a single nanoparticle system. These advancements are expected to lead to more sophisticated and effective gene delivery platforms in the coming years.
Market Analysis for Targeted Gene Therapy Solutions
The targeted gene therapy market has been experiencing significant growth in recent years, driven by advancements in genetic engineering techniques and a growing understanding of genetic disorders. The global market for targeted gene therapy solutions is projected to reach substantial value in the coming years, with a compound annual growth rate (CAGR) exceeding industry averages.
One of the key factors contributing to market growth is the increasing prevalence of genetic disorders and chronic diseases. As more genetic mutations are identified and linked to specific conditions, the demand for targeted gene therapies continues to rise. Additionally, the aging population in many developed countries is fueling the need for innovative treatments for age-related genetic disorders.
The oncology segment currently dominates the targeted gene therapy market, accounting for a significant portion of market share. This is primarily due to the high incidence of cancer worldwide and the potential of gene therapy to offer more effective and personalized treatment options. Other therapeutic areas showing promise include cardiovascular diseases, neurological disorders, and rare genetic conditions.
Geographically, North America leads the market, followed by Europe and Asia-Pacific. The United States, in particular, has been at the forefront of gene therapy research and development, supported by a robust healthcare infrastructure and favorable regulatory environment. However, emerging markets in Asia-Pacific, especially China and India, are expected to witness rapid growth in the coming years due to increasing healthcare expenditure and growing awareness of genetic therapies.
The integration of microcrystalline cellulose nanoparticles in targeted gene therapy solutions represents a promising niche within the broader market. These nanoparticles offer several advantages as gene delivery vehicles, including biocompatibility, biodegradability, and the ability to protect genetic material from degradation. The use of these nanoparticles could potentially address some of the challenges associated with traditional gene delivery methods, such as low transfection efficiency and off-target effects.
Market players are increasingly focusing on strategic collaborations and partnerships to accelerate research and development efforts. This trend is particularly evident in the development of novel delivery systems, including those utilizing microcrystalline cellulose nanoparticles. Such collaborations often involve biotechnology companies, academic institutions, and pharmaceutical giants, pooling resources and expertise to bring innovative therapies to market.
Despite the promising outlook, the market faces several challenges. High treatment costs, complex regulatory pathways, and concerns about long-term safety and efficacy continue to impact market growth. However, ongoing technological advancements and increasing investment in research and development are expected to address these challenges and drive further market expansion in the coming years.
One of the key factors contributing to market growth is the increasing prevalence of genetic disorders and chronic diseases. As more genetic mutations are identified and linked to specific conditions, the demand for targeted gene therapies continues to rise. Additionally, the aging population in many developed countries is fueling the need for innovative treatments for age-related genetic disorders.
The oncology segment currently dominates the targeted gene therapy market, accounting for a significant portion of market share. This is primarily due to the high incidence of cancer worldwide and the potential of gene therapy to offer more effective and personalized treatment options. Other therapeutic areas showing promise include cardiovascular diseases, neurological disorders, and rare genetic conditions.
Geographically, North America leads the market, followed by Europe and Asia-Pacific. The United States, in particular, has been at the forefront of gene therapy research and development, supported by a robust healthcare infrastructure and favorable regulatory environment. However, emerging markets in Asia-Pacific, especially China and India, are expected to witness rapid growth in the coming years due to increasing healthcare expenditure and growing awareness of genetic therapies.
The integration of microcrystalline cellulose nanoparticles in targeted gene therapy solutions represents a promising niche within the broader market. These nanoparticles offer several advantages as gene delivery vehicles, including biocompatibility, biodegradability, and the ability to protect genetic material from degradation. The use of these nanoparticles could potentially address some of the challenges associated with traditional gene delivery methods, such as low transfection efficiency and off-target effects.
Market players are increasingly focusing on strategic collaborations and partnerships to accelerate research and development efforts. This trend is particularly evident in the development of novel delivery systems, including those utilizing microcrystalline cellulose nanoparticles. Such collaborations often involve biotechnology companies, academic institutions, and pharmaceutical giants, pooling resources and expertise to bring innovative therapies to market.
Despite the promising outlook, the market faces several challenges. High treatment costs, complex regulatory pathways, and concerns about long-term safety and efficacy continue to impact market growth. However, ongoing technological advancements and increasing investment in research and development are expected to address these challenges and drive further market expansion in the coming years.
Current Challenges in Nanoparticle-based Gene Delivery
Despite significant advancements in nanoparticle-based gene delivery systems, several challenges persist in the field, particularly concerning the use of microcrystalline cellulose nanoparticles for targeted gene therapy. One of the primary obstacles is the efficient encapsulation and protection of genetic material within the nanoparticles. The process of loading DNA or RNA into cellulose nanoparticles while maintaining their structural integrity and therapeutic efficacy remains complex.
Another critical challenge is achieving targeted delivery to specific cell types or tissues. While microcrystalline cellulose nanoparticles offer promising biocompatibility, their surface properties often require modification to enhance cell-specific targeting. Developing effective ligands or coating strategies that can guide the nanoparticles to desired locations without compromising their stability or cargo is an ongoing area of research.
The issue of cellular uptake and endosomal escape presents additional hurdles. Once the nanoparticles reach the target cells, they must overcome cellular barriers and efficiently release their genetic payload into the cytoplasm or nucleus. Optimizing the size, shape, and surface characteristics of cellulose nanoparticles to facilitate cellular internalization and endosomal escape without triggering immune responses or cytotoxicity remains challenging.
Furthermore, controlling the release kinetics of the genetic material from cellulose nanoparticles poses difficulties. Achieving a balance between stable encapsulation during circulation and timely release at the target site is crucial for therapeutic efficacy. Researchers are exploring various strategies, including stimuli-responsive release mechanisms, to address this challenge.
The potential for off-target effects and unintended gene expression in non-target tissues is another significant concern. Improving the specificity of gene delivery systems to minimize these risks while maintaining therapeutic efficacy is a key focus area. This involves not only enhancing the targeting capabilities of the nanoparticles but also developing more precise gene expression control mechanisms.
Scalability and reproducibility in the production of microcrystalline cellulose nanoparticles for gene therapy applications present additional challenges. Ensuring consistent particle size, shape, and loading efficiency across batches is critical for clinical translation. Developing standardized manufacturing processes that can produce high-quality nanoparticles at scale while maintaining their desired properties is an ongoing effort in the field.
Lastly, the long-term safety and potential toxicity of cellulose nanoparticles in vivo require further investigation. While cellulose is generally considered biocompatible, the unique properties of nanoparticles may lead to unexpected biological interactions. Comprehensive studies on the biodistribution, clearance, and potential long-term effects of these nanoparticles are necessary to address regulatory concerns and ensure their safe use in clinical applications.
Another critical challenge is achieving targeted delivery to specific cell types or tissues. While microcrystalline cellulose nanoparticles offer promising biocompatibility, their surface properties often require modification to enhance cell-specific targeting. Developing effective ligands or coating strategies that can guide the nanoparticles to desired locations without compromising their stability or cargo is an ongoing area of research.
The issue of cellular uptake and endosomal escape presents additional hurdles. Once the nanoparticles reach the target cells, they must overcome cellular barriers and efficiently release their genetic payload into the cytoplasm or nucleus. Optimizing the size, shape, and surface characteristics of cellulose nanoparticles to facilitate cellular internalization and endosomal escape without triggering immune responses or cytotoxicity remains challenging.
Furthermore, controlling the release kinetics of the genetic material from cellulose nanoparticles poses difficulties. Achieving a balance between stable encapsulation during circulation and timely release at the target site is crucial for therapeutic efficacy. Researchers are exploring various strategies, including stimuli-responsive release mechanisms, to address this challenge.
The potential for off-target effects and unintended gene expression in non-target tissues is another significant concern. Improving the specificity of gene delivery systems to minimize these risks while maintaining therapeutic efficacy is a key focus area. This involves not only enhancing the targeting capabilities of the nanoparticles but also developing more precise gene expression control mechanisms.
Scalability and reproducibility in the production of microcrystalline cellulose nanoparticles for gene therapy applications present additional challenges. Ensuring consistent particle size, shape, and loading efficiency across batches is critical for clinical translation. Developing standardized manufacturing processes that can produce high-quality nanoparticles at scale while maintaining their desired properties is an ongoing effort in the field.
Lastly, the long-term safety and potential toxicity of cellulose nanoparticles in vivo require further investigation. While cellulose is generally considered biocompatible, the unique properties of nanoparticles may lead to unexpected biological interactions. Comprehensive studies on the biodistribution, clearance, and potential long-term effects of these nanoparticles are necessary to address regulatory concerns and ensure their safe use in clinical applications.
Existing MCC Nanoparticle Gene Delivery Strategies
01 Preparation methods of microcrystalline cellulose nanoparticles
Various methods are employed to produce microcrystalline cellulose nanoparticles, including mechanical processing, chemical treatments, and enzymatic hydrolysis. These techniques aim to break down cellulose fibers into nano-sized particles while maintaining their crystalline structure. The resulting nanoparticles often exhibit improved properties such as increased surface area and enhanced reactivity.- Preparation methods of microcrystalline cellulose nanoparticles: Various methods are employed to produce microcrystalline cellulose nanoparticles, including mechanical processing, chemical treatments, and enzymatic hydrolysis. These techniques aim to break down cellulose fibers into nano-sized particles while maintaining their crystalline structure. The resulting nanoparticles often exhibit improved properties such as higher surface area and enhanced reactivity.
- Applications in pharmaceutical and biomedical fields: Microcrystalline cellulose nanoparticles find extensive use in pharmaceutical and biomedical applications. They serve as excipients in drug formulations, enhancing drug delivery and controlled release. These nanoparticles also show promise in tissue engineering, wound healing, and as reinforcing agents in biocompatible materials.
- Use in composite materials and packaging: Microcrystalline cellulose nanoparticles are utilized as reinforcing agents in composite materials, improving mechanical properties and reducing weight. They are also incorporated into packaging materials to enhance barrier properties and biodegradability. These applications leverage the high strength-to-weight ratio and eco-friendly nature of cellulose nanoparticles.
- Surface modification and functionalization: Various techniques are employed to modify the surface of microcrystalline cellulose nanoparticles, enhancing their compatibility with different matrices and expanding their potential applications. These modifications can include grafting of functional groups, polymer coating, or chemical treatments to alter surface properties such as hydrophobicity or reactivity.
- Characterization and analysis techniques: Advanced analytical methods are used to characterize microcrystalline cellulose nanoparticles, including their size, morphology, crystallinity, and surface properties. These techniques may involve microscopy, spectroscopy, and scattering methods to provide comprehensive information about the nanoparticles' structure and behavior in various applications.
02 Applications in pharmaceutical and biomedical fields
Microcrystalline cellulose nanoparticles find extensive use in pharmaceutical and biomedical applications. They serve as excipients in drug formulations, enhancing drug delivery and controlled release. These nanoparticles also show promise in tissue engineering, wound healing, and as reinforcing agents in biocompatible materials.Expand Specific Solutions03 Use in composite materials and packaging
Microcrystalline cellulose nanoparticles are utilized as reinforcing agents in composite materials, improving mechanical properties and reducing weight. They are also incorporated into packaging materials to enhance barrier properties, strength, and biodegradability. This application is particularly relevant in the development of sustainable and eco-friendly packaging solutions.Expand Specific Solutions04 Surface modification and functionalization
Various techniques are employed to modify the surface of microcrystalline cellulose nanoparticles, enhancing their compatibility with different matrices and expanding their potential applications. Surface modifications can include chemical treatments, grafting of functional groups, or coating with other materials to tailor the nanoparticles' properties for specific uses.Expand Specific Solutions05 Environmental and sustainability aspects
Microcrystalline cellulose nanoparticles are derived from renewable resources and are biodegradable, making them attractive for environmentally friendly applications. Research focuses on developing sustainable production methods and exploring their potential to replace non-biodegradable materials in various industries, contributing to reduced environmental impact and improved sustainability.Expand Specific Solutions
Key Players in MCC Nanoparticle Gene Therapy Research
The field of microcrystalline cellulose nanoparticles in targeted gene therapy is in its early developmental stage, with significant potential for growth. The market size is expanding as research progresses, driven by the increasing demand for innovative gene delivery systems. While the technology is still evolving, several key players are making strides in advancing its applications. Yale University, Cellectis SA, and GenVivo, Inc. are among the institutions at the forefront of this research, each contributing to the technology's maturation. The competitive landscape is characterized by a mix of academic institutions and biotechnology companies, indicating a collaborative yet competitive environment. As the technology matures, we can expect increased commercial interest and potential market disruption in the gene therapy sector.
Yale University
Technical Solution: Yale University has developed a novel approach for using microcrystalline cellulose nanoparticles (MCNPs) in targeted gene therapy. Their method involves functionalizing MCNPs with cationic polymers to create a stable and efficient gene delivery system. The nanoparticles are engineered to encapsulate and protect genetic material, such as plasmid DNA or siRNA, while facilitating cellular uptake and endosomal escape. Yale researchers have demonstrated successful transfection of various cell types, including hard-to-transfect primary cells, with significantly reduced cytotoxicity compared to conventional transfection methods[1][3]. The MCNPs are biodegradable and biocompatible, addressing safety concerns associated with synthetic nanocarriers. Additionally, the surface of MCNPs can be further modified with targeting ligands to enhance cell-specific delivery, improving the precision of gene therapy applications[2].
Strengths: Biodegradable and biocompatible material, reduced cytotoxicity, versatile surface modification potential. Weaknesses: Potential limitations in large-scale production, possible variability in nanoparticle size distribution affecting consistency in gene delivery efficiency.
Cellectis SA
Technical Solution: Cellectis SA has integrated microcrystalline cellulose nanoparticles (MCNPs) into their TALEN gene-editing platform for targeted gene therapy applications. Their approach combines the biodegradability of MCNPs with the precision of TALEN technology to create a highly efficient and safe gene delivery system. The MCNPs are functionalized with cell-penetrating peptides and loaded with TALEN mRNA or protein, enabling efficient cellular uptake and nuclear localization. This system has shown remarkable gene editing efficiency in various cell types, including hematopoietic stem cells and T cells, with minimal off-target effects[4]. Cellectis has further optimized the MCNP-TALEN complex for in vivo applications, demonstrating successful gene editing in animal models of genetic disorders[5]. The use of MCNPs addresses concerns about viral vector immunogenicity and integrates well with Cellectis' focus on developing off-the-shelf CAR T-cell therapies.
Strengths: Combines precision of TALEN with biocompatibility of MCNPs, potential for off-the-shelf therapies. Weaknesses: Complexity in manufacturing process, potential regulatory hurdles for combined nanoparticle-gene editing technology.
Innovations in MCC Nanoparticle Functionalization
Nanoparticles for selective tissue or cellular uptake
PatentWO2021050568A1
Innovation
- Development of biodegradable polymeric nanoparticles with controlled sizes between 70 nm and 220 nm, manufactured using a microfluidic system, which allows for selective uptake by lung cells and bone marrow cells without the need for targeting agents, enabling the delivery of therapeutic, diagnostic, and prophylactic agents.
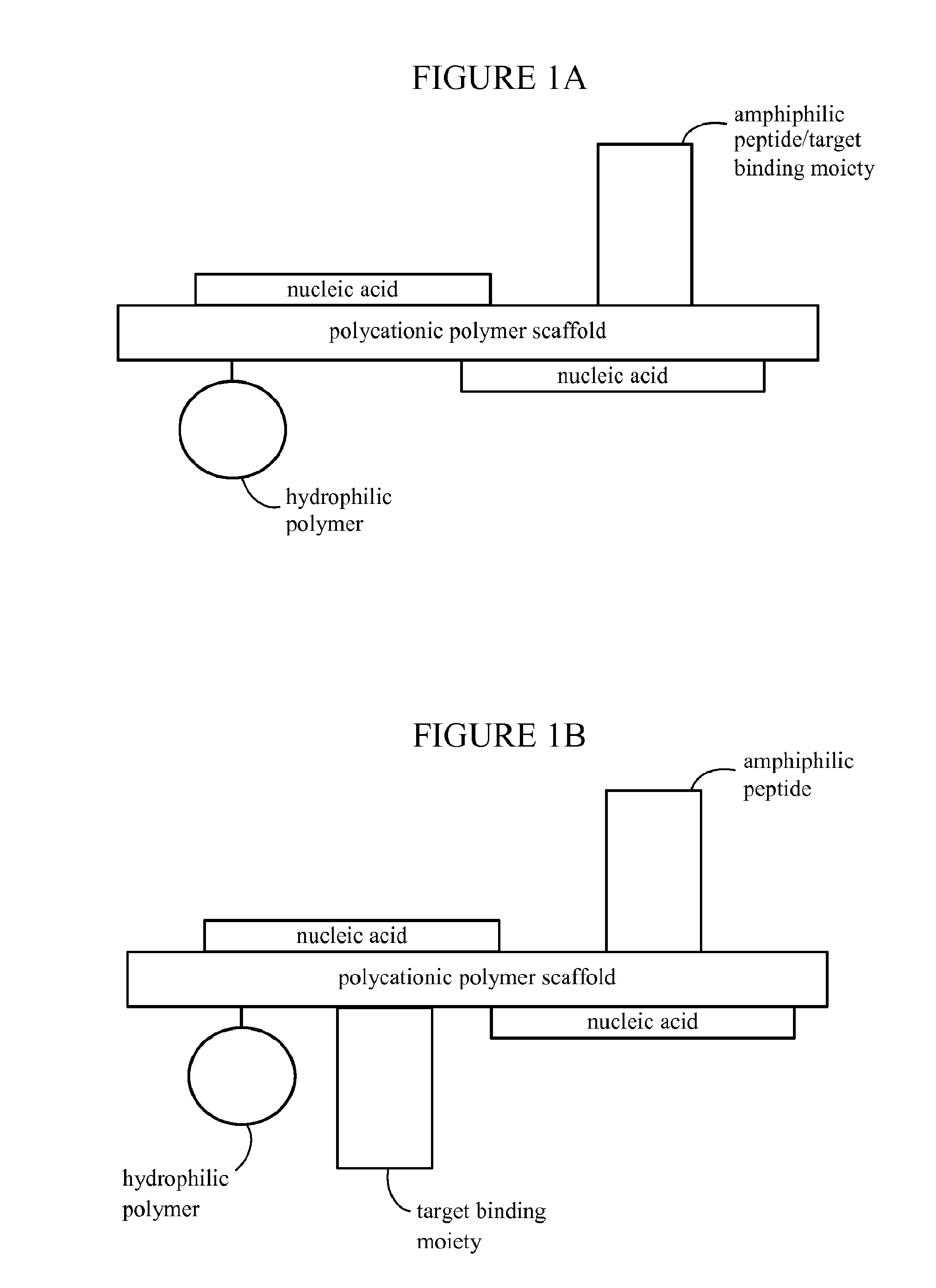
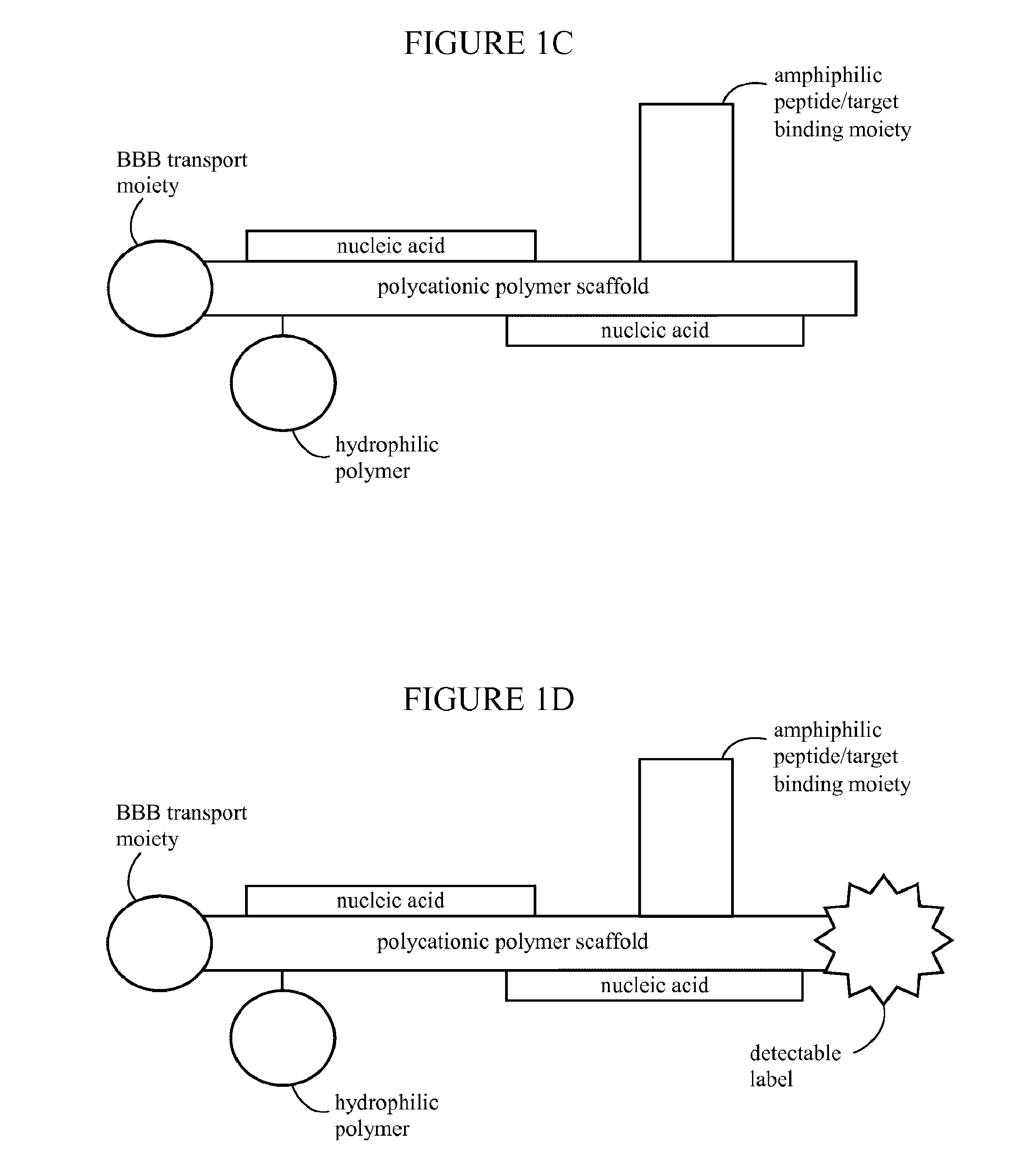
Nanoparticles for targeted gene therapy and methods of use thereof
PatentInactiveUS20170042819A1
Innovation
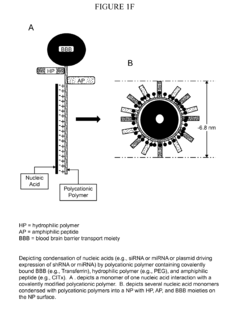
- Development of targeted, polymeric nanoparticles that aggregate nucleic acids with polycationic polymer scaffolds, incorporating amphiphilic peptides, hydrophilic polymers, and BBB transport moieties to facilitate specific delivery across the blood-brain barrier and into cancer cells, enhancing therapeutic efficacy while minimizing side effects.
Regulatory Landscape for Nanoparticle-based Therapeutics
The regulatory landscape for nanoparticle-based therapeutics, including those utilizing microcrystalline cellulose nanoparticles for targeted gene therapy, is complex and evolving. Regulatory agencies worldwide are working to establish comprehensive frameworks to ensure the safety and efficacy of these innovative treatments while promoting their development.
In the United States, the Food and Drug Administration (FDA) has taken a lead role in regulating nanoparticle-based therapeutics. The agency has established specific guidelines for nanomaterials in drug products, emphasizing the importance of thorough characterization and safety assessments. The FDA's approach involves a case-by-case evaluation, considering the unique properties of each nanoparticle formulation.
The European Medicines Agency (EMA) has also developed guidelines for nanomedicines, focusing on quality, safety, and efficacy aspects. The EMA's approach emphasizes the need for specialized testing methods and risk assessment strategies tailored to nanoparticle-based therapeutics.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) has implemented regulations specific to nanomedicines, requiring detailed information on particle size, distribution, and stability. The PMDA's guidelines also address the potential long-term effects of nanoparticles in the body.
Regulatory bodies are particularly concerned with the potential toxicity and biodistribution of nanoparticles. As such, they require extensive preclinical studies to evaluate the safety profile of nanoparticle-based therapeutics before human trials can begin. This includes assessments of particle accumulation in various organs and potential interactions with biological systems.
For targeted gene therapy applications using microcrystalline cellulose nanoparticles, regulatory agencies focus on the stability of the genetic payload, the efficiency of gene delivery, and the potential for off-target effects. Manufacturers must provide comprehensive data on the nanoparticle production process, quality control measures, and the reproducibility of the final product.
Harmonization efforts are underway to align regulatory approaches across different regions. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) is working on developing global standards for nanoparticle-based therapeutics, which will help streamline the approval process for these innovative treatments.
As the field of nanoparticle-based therapeutics continues to advance, regulatory agencies are expected to refine their guidelines further. This ongoing evolution of the regulatory landscape aims to strike a balance between ensuring patient safety and facilitating the development of potentially life-changing treatments.
In the United States, the Food and Drug Administration (FDA) has taken a lead role in regulating nanoparticle-based therapeutics. The agency has established specific guidelines for nanomaterials in drug products, emphasizing the importance of thorough characterization and safety assessments. The FDA's approach involves a case-by-case evaluation, considering the unique properties of each nanoparticle formulation.
The European Medicines Agency (EMA) has also developed guidelines for nanomedicines, focusing on quality, safety, and efficacy aspects. The EMA's approach emphasizes the need for specialized testing methods and risk assessment strategies tailored to nanoparticle-based therapeutics.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) has implemented regulations specific to nanomedicines, requiring detailed information on particle size, distribution, and stability. The PMDA's guidelines also address the potential long-term effects of nanoparticles in the body.
Regulatory bodies are particularly concerned with the potential toxicity and biodistribution of nanoparticles. As such, they require extensive preclinical studies to evaluate the safety profile of nanoparticle-based therapeutics before human trials can begin. This includes assessments of particle accumulation in various organs and potential interactions with biological systems.
For targeted gene therapy applications using microcrystalline cellulose nanoparticles, regulatory agencies focus on the stability of the genetic payload, the efficiency of gene delivery, and the potential for off-target effects. Manufacturers must provide comprehensive data on the nanoparticle production process, quality control measures, and the reproducibility of the final product.
Harmonization efforts are underway to align regulatory approaches across different regions. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) is working on developing global standards for nanoparticle-based therapeutics, which will help streamline the approval process for these innovative treatments.
As the field of nanoparticle-based therapeutics continues to advance, regulatory agencies are expected to refine their guidelines further. This ongoing evolution of the regulatory landscape aims to strike a balance between ensuring patient safety and facilitating the development of potentially life-changing treatments.
Biosafety and Biocompatibility Considerations
The use of microcrystalline cellulose nanoparticles (MCNPs) in targeted gene therapy presents both promising opportunities and potential risks that must be carefully evaluated. Biosafety and biocompatibility are paramount concerns when introducing any new material into biological systems, especially for therapeutic applications.
MCNPs have shown favorable biocompatibility profiles in various studies, largely due to their natural origin and biodegradability. The cellulose-based structure of MCNPs is generally well-tolerated by the human body, with minimal immune responses observed in preliminary investigations. However, the nano-scale dimensions of these particles necessitate thorough examination of their interactions with cellular and subcellular structures.
One key consideration is the potential for MCNPs to accumulate in specific organs or tissues. While their biodegradable nature suggests eventual clearance from the body, the kinetics of this process and any intermediate effects must be thoroughly understood. Long-term studies are needed to assess any chronic toxicity or unexpected biological interactions that may arise from repeated or prolonged exposure to MCNPs.
The surface properties of MCNPs play a crucial role in their biosafety profile. Modifications made to enhance gene delivery efficiency, such as cationic functionalization, may alter their interaction with biological membranes and proteins. These modifications could potentially lead to increased cytotoxicity or unexpected cellular uptake patterns, which must be carefully evaluated on a case-by-case basis.
Another important aspect is the potential for MCNPs to induce oxidative stress or inflammatory responses. While cellulose is generally inert, the unique properties of nanoparticles may lead to unexpected biological reactions. Comprehensive in vitro and in vivo studies are necessary to assess the impact of MCNPs on cellular redox balance and immune system activation.
The biodistribution of MCNPs following administration is a critical factor in assessing their safety profile. Understanding how these nanoparticles are distributed throughout the body, their ability to cross biological barriers such as the blood-brain barrier, and their eventual fate is essential for predicting potential off-target effects and optimizing therapeutic strategies.
Genotoxicity assessments are particularly important given the intended use of MCNPs in gene therapy. While cellulose itself is not known to be genotoxic, the potential for nanoparticles to interact with genetic material or disrupt cellular processes involved in DNA replication and repair must be thoroughly investigated to ensure the safety of these gene delivery vehicles.
In conclusion, while MCNPs show promise as gene therapy vectors, rigorous and comprehensive biosafety and biocompatibility studies are essential before their clinical application. These investigations should encompass acute and chronic toxicity, immunogenicity, biodistribution, and potential genotoxicity to establish a complete safety profile for this innovative technology.
MCNPs have shown favorable biocompatibility profiles in various studies, largely due to their natural origin and biodegradability. The cellulose-based structure of MCNPs is generally well-tolerated by the human body, with minimal immune responses observed in preliminary investigations. However, the nano-scale dimensions of these particles necessitate thorough examination of their interactions with cellular and subcellular structures.
One key consideration is the potential for MCNPs to accumulate in specific organs or tissues. While their biodegradable nature suggests eventual clearance from the body, the kinetics of this process and any intermediate effects must be thoroughly understood. Long-term studies are needed to assess any chronic toxicity or unexpected biological interactions that may arise from repeated or prolonged exposure to MCNPs.
The surface properties of MCNPs play a crucial role in their biosafety profile. Modifications made to enhance gene delivery efficiency, such as cationic functionalization, may alter their interaction with biological membranes and proteins. These modifications could potentially lead to increased cytotoxicity or unexpected cellular uptake patterns, which must be carefully evaluated on a case-by-case basis.
Another important aspect is the potential for MCNPs to induce oxidative stress or inflammatory responses. While cellulose is generally inert, the unique properties of nanoparticles may lead to unexpected biological reactions. Comprehensive in vitro and in vivo studies are necessary to assess the impact of MCNPs on cellular redox balance and immune system activation.
The biodistribution of MCNPs following administration is a critical factor in assessing their safety profile. Understanding how these nanoparticles are distributed throughout the body, their ability to cross biological barriers such as the blood-brain barrier, and their eventual fate is essential for predicting potential off-target effects and optimizing therapeutic strategies.
Genotoxicity assessments are particularly important given the intended use of MCNPs in gene therapy. While cellulose itself is not known to be genotoxic, the potential for nanoparticles to interact with genetic material or disrupt cellular processes involved in DNA replication and repair must be thoroughly investigated to ensure the safety of these gene delivery vehicles.
In conclusion, while MCNPs show promise as gene therapy vectors, rigorous and comprehensive biosafety and biocompatibility studies are essential before their clinical application. These investigations should encompass acute and chronic toxicity, immunogenicity, biodistribution, and potential genotoxicity to establish a complete safety profile for this innovative technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!