Role of Microcrystalline Cellulose in Oral Chemotherapeutic Systems
JUL 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
MCC in Chemotherapy: Background and Objectives
Microcrystalline cellulose (MCC) has emerged as a pivotal excipient in the development of oral chemotherapeutic systems, revolutionizing the landscape of cancer treatment. The evolution of MCC in this field traces back to the mid-20th century when researchers first recognized its potential as a pharmaceutical excipient. Since then, its application in oral chemotherapy has grown exponentially, driven by its unique physicochemical properties and biocompatibility.
The primary objective of incorporating MCC in oral chemotherapeutic systems is to enhance drug delivery efficiency while minimizing adverse effects. This aligns with the broader goal of improving patient outcomes and quality of life during cancer treatment. MCC's role extends beyond mere filler; it serves as a multifunctional excipient capable of modifying drug release profiles, improving tablet compressibility, and enhancing overall formulation stability.
In recent years, the focus has shifted towards leveraging MCC's potential in developing controlled-release formulations for chemotherapeutic agents. This trend is propelled by the need for sustained drug levels in the body, which can potentially reduce dosing frequency and mitigate peak-related side effects. Additionally, researchers are exploring MCC's capacity to protect sensitive drug molecules from degradation in the harsh gastrointestinal environment, thereby improving bioavailability.
The technological trajectory of MCC in oral chemotherapy is closely intertwined with advancements in material science and pharmaceutical engineering. Innovations in MCC production techniques, such as co-processing with other excipients, have opened new avenues for tailoring its properties to specific drug delivery requirements. This has led to the development of various grades of MCC, each optimized for different aspects of oral chemotherapeutic formulations.
Looking ahead, the integration of MCC with novel drug delivery technologies presents exciting possibilities. There is growing interest in combining MCC with smart polymers or nanoparticles to create intelligent drug delivery systems capable of responding to physiological cues. Such advancements could pave the way for personalized chemotherapy regimens, where drug release is precisely controlled based on individual patient needs and tumor characteristics.
As we delve deeper into the role of MCC in oral chemotherapeutic systems, it becomes evident that this versatile excipient stands at the intersection of material science, pharmacology, and oncology. The ongoing research and development in this field aim to unlock MCC's full potential, driving innovations that could significantly impact cancer treatment strategies and patient care in the coming years.
The primary objective of incorporating MCC in oral chemotherapeutic systems is to enhance drug delivery efficiency while minimizing adverse effects. This aligns with the broader goal of improving patient outcomes and quality of life during cancer treatment. MCC's role extends beyond mere filler; it serves as a multifunctional excipient capable of modifying drug release profiles, improving tablet compressibility, and enhancing overall formulation stability.
In recent years, the focus has shifted towards leveraging MCC's potential in developing controlled-release formulations for chemotherapeutic agents. This trend is propelled by the need for sustained drug levels in the body, which can potentially reduce dosing frequency and mitigate peak-related side effects. Additionally, researchers are exploring MCC's capacity to protect sensitive drug molecules from degradation in the harsh gastrointestinal environment, thereby improving bioavailability.
The technological trajectory of MCC in oral chemotherapy is closely intertwined with advancements in material science and pharmaceutical engineering. Innovations in MCC production techniques, such as co-processing with other excipients, have opened new avenues for tailoring its properties to specific drug delivery requirements. This has led to the development of various grades of MCC, each optimized for different aspects of oral chemotherapeutic formulations.
Looking ahead, the integration of MCC with novel drug delivery technologies presents exciting possibilities. There is growing interest in combining MCC with smart polymers or nanoparticles to create intelligent drug delivery systems capable of responding to physiological cues. Such advancements could pave the way for personalized chemotherapy regimens, where drug release is precisely controlled based on individual patient needs and tumor characteristics.
As we delve deeper into the role of MCC in oral chemotherapeutic systems, it becomes evident that this versatile excipient stands at the intersection of material science, pharmacology, and oncology. The ongoing research and development in this field aim to unlock MCC's full potential, driving innovations that could significantly impact cancer treatment strategies and patient care in the coming years.
Market Analysis for MCC-based Oral Chemotherapy
The market for microcrystalline cellulose (MCC) in oral chemotherapeutic systems is experiencing significant growth, driven by the increasing prevalence of cancer and the growing demand for more effective and patient-friendly treatment options. The global market for MCC in oral chemotherapy is expected to expand at a robust rate over the next five years, with a particular focus on developed regions such as North America and Europe.
One of the key factors driving market growth is the rising adoption of oral chemotherapy as an alternative to traditional intravenous treatments. Patients and healthcare providers are increasingly recognizing the benefits of oral chemotherapy, including improved quality of life, reduced hospital visits, and greater convenience. This shift in preference is creating a substantial opportunity for MCC-based formulations, which play a crucial role in enhancing drug stability, bioavailability, and controlled release properties.
The market is also benefiting from ongoing advancements in drug delivery technologies and formulation techniques. Pharmaceutical companies are investing heavily in research and development to create innovative MCC-based oral chemotherapy products that offer improved efficacy and reduced side effects. These efforts are expected to result in a pipeline of new products entering the market in the coming years, further driving growth and expanding treatment options for patients.
In terms of market segmentation, the MCC-based oral chemotherapy market can be divided into various categories based on cancer types, drug classes, and formulation types. Solid dosage forms, particularly tablets and capsules, currently dominate the market due to their ease of administration and patient compliance. However, there is growing interest in novel formulations such as orally disintegrating tablets and multi-particulate systems, which offer additional benefits in terms of drug absorption and patient acceptance.
Geographically, North America holds the largest share of the MCC-based oral chemotherapy market, followed by Europe. This dominance can be attributed to the high incidence of cancer in these regions, well-established healthcare infrastructure, and favorable reimbursement policies. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by improving healthcare access, rising disposable incomes, and increasing awareness about advanced cancer treatments.
Despite the positive outlook, the market faces certain challenges. These include stringent regulatory requirements for oral chemotherapy products, concerns about drug-drug interactions, and the need for precise dosing to ensure optimal therapeutic outcomes. Additionally, the high cost of some oral chemotherapy treatments may limit market growth in certain regions, particularly in developing countries with limited healthcare budgets.
One of the key factors driving market growth is the rising adoption of oral chemotherapy as an alternative to traditional intravenous treatments. Patients and healthcare providers are increasingly recognizing the benefits of oral chemotherapy, including improved quality of life, reduced hospital visits, and greater convenience. This shift in preference is creating a substantial opportunity for MCC-based formulations, which play a crucial role in enhancing drug stability, bioavailability, and controlled release properties.
The market is also benefiting from ongoing advancements in drug delivery technologies and formulation techniques. Pharmaceutical companies are investing heavily in research and development to create innovative MCC-based oral chemotherapy products that offer improved efficacy and reduced side effects. These efforts are expected to result in a pipeline of new products entering the market in the coming years, further driving growth and expanding treatment options for patients.
In terms of market segmentation, the MCC-based oral chemotherapy market can be divided into various categories based on cancer types, drug classes, and formulation types. Solid dosage forms, particularly tablets and capsules, currently dominate the market due to their ease of administration and patient compliance. However, there is growing interest in novel formulations such as orally disintegrating tablets and multi-particulate systems, which offer additional benefits in terms of drug absorption and patient acceptance.
Geographically, North America holds the largest share of the MCC-based oral chemotherapy market, followed by Europe. This dominance can be attributed to the high incidence of cancer in these regions, well-established healthcare infrastructure, and favorable reimbursement policies. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by improving healthcare access, rising disposable incomes, and increasing awareness about advanced cancer treatments.
Despite the positive outlook, the market faces certain challenges. These include stringent regulatory requirements for oral chemotherapy products, concerns about drug-drug interactions, and the need for precise dosing to ensure optimal therapeutic outcomes. Additionally, the high cost of some oral chemotherapy treatments may limit market growth in certain regions, particularly in developing countries with limited healthcare budgets.
Technical Challenges in MCC Formulations
Microcrystalline cellulose (MCC) plays a crucial role in oral chemotherapeutic systems, but its formulation presents several technical challenges. One of the primary issues is achieving uniform dispersion of MCC particles within the drug matrix. The hydrophilic nature of MCC can lead to agglomeration, resulting in inconsistent drug release profiles and compromised therapeutic efficacy.
Another significant challenge lies in controlling the moisture content of MCC-based formulations. MCC's hygroscopic properties can lead to water absorption during storage, potentially affecting the stability and shelf life of the chemotherapeutic agents. This necessitates careful consideration of packaging materials and storage conditions to maintain the integrity of the formulation.
The particle size distribution of MCC is another critical factor that impacts the performance of oral chemotherapeutic systems. Achieving a consistent and optimal particle size range is essential for ensuring uniform drug distribution and controlled release kinetics. However, maintaining this consistency across batches can be technically demanding and requires sophisticated processing techniques.
Compatibility between MCC and various chemotherapeutic agents poses another challenge. Some drugs may interact unfavorably with MCC, leading to chemical instability or altered bioavailability. Extensive preformulation studies are necessary to identify and mitigate potential incompatibilities, which can be time-consuming and resource-intensive.
The compressibility and flow properties of MCC-based formulations can also present technical hurdles. While MCC is known for its excellent compression characteristics, the incorporation of chemotherapeutic agents may alter these properties. Achieving the right balance between compressibility and drug loading capacity is crucial for developing effective oral dosage forms.
Furthermore, the impact of MCC on the dissolution profile of chemotherapeutic agents requires careful consideration. The high surface area and porous structure of MCC can significantly influence drug release rates. Tailoring the MCC grade and concentration to achieve the desired dissolution profile while maintaining therapeutic efficacy is a complex optimization process.
Lastly, scale-up and manufacturing challenges associated with MCC-based chemotherapeutic formulations cannot be overlooked. Translating laboratory-scale formulations to commercial production while maintaining consistent quality attributes demands robust process controls and advanced manufacturing technologies.
Another significant challenge lies in controlling the moisture content of MCC-based formulations. MCC's hygroscopic properties can lead to water absorption during storage, potentially affecting the stability and shelf life of the chemotherapeutic agents. This necessitates careful consideration of packaging materials and storage conditions to maintain the integrity of the formulation.
The particle size distribution of MCC is another critical factor that impacts the performance of oral chemotherapeutic systems. Achieving a consistent and optimal particle size range is essential for ensuring uniform drug distribution and controlled release kinetics. However, maintaining this consistency across batches can be technically demanding and requires sophisticated processing techniques.
Compatibility between MCC and various chemotherapeutic agents poses another challenge. Some drugs may interact unfavorably with MCC, leading to chemical instability or altered bioavailability. Extensive preformulation studies are necessary to identify and mitigate potential incompatibilities, which can be time-consuming and resource-intensive.
The compressibility and flow properties of MCC-based formulations can also present technical hurdles. While MCC is known for its excellent compression characteristics, the incorporation of chemotherapeutic agents may alter these properties. Achieving the right balance between compressibility and drug loading capacity is crucial for developing effective oral dosage forms.
Furthermore, the impact of MCC on the dissolution profile of chemotherapeutic agents requires careful consideration. The high surface area and porous structure of MCC can significantly influence drug release rates. Tailoring the MCC grade and concentration to achieve the desired dissolution profile while maintaining therapeutic efficacy is a complex optimization process.
Lastly, scale-up and manufacturing challenges associated with MCC-based chemotherapeutic formulations cannot be overlooked. Translating laboratory-scale formulations to commercial production while maintaining consistent quality attributes demands robust process controls and advanced manufacturing technologies.
Current MCC Formulation Strategies
01 Production and modification of microcrystalline cellulose
Various methods are employed to produce and modify microcrystalline cellulose, including acid hydrolysis, enzymatic treatment, and mechanical processing. These techniques aim to improve the properties of microcrystalline cellulose for specific applications, such as enhancing its stability, particle size distribution, or functionality.- Production and modification of microcrystalline cellulose: Various methods are employed to produce and modify microcrystalline cellulose, including acid hydrolysis, enzymatic treatments, and chemical modifications. These processes aim to enhance the properties of microcrystalline cellulose for specific applications, such as improving its stability, dispersibility, or functionality in different formulations.
- Applications in pharmaceutical formulations: Microcrystalline cellulose is widely used in pharmaceutical formulations as an excipient. It serves various functions such as a binder, disintegrant, and filler in tablet and capsule formulations. Its unique properties contribute to improved drug release, stability, and overall performance of pharmaceutical products.
- Use in food and cosmetic industries: Microcrystalline cellulose finds applications in food and cosmetic products as a stabilizer, thickener, and texturizing agent. It is used to improve the consistency, mouthfeel, and shelf-life of various food products. In cosmetics, it serves as a bulking agent and helps in controlling the viscosity of formulations.
- Composite materials and reinforcement applications: Microcrystalline cellulose is utilized in the development of composite materials, where it acts as a reinforcing agent. It enhances the mechanical properties, thermal stability, and biodegradability of various materials, including plastics, paper, and construction materials. This application leverages the high strength-to-weight ratio of microcrystalline cellulose.
- Purification and characterization techniques: Various methods are employed for the purification and characterization of microcrystalline cellulose. These include spectroscopic techniques, chromatography, and microscopy. The purification processes aim to remove impurities and achieve desired particle sizes, while characterization techniques help in determining the physical and chemical properties of the material for quality control and research purposes.
02 Applications in pharmaceutical formulations
Microcrystalline cellulose is widely used in pharmaceutical formulations as an excipient. It serves various functions, including as a binder, disintegrant, and filler in tablet and capsule formulations. Its properties contribute to improved drug release, stability, and overall performance of pharmaceutical products.Expand Specific Solutions03 Use in food and cosmetic industries
Microcrystalline cellulose finds applications in food and cosmetic products as a stabilizer, thickener, and texturizing agent. It is used to improve the consistency, mouthfeel, and shelf-life of various food products, as well as in cosmetic formulations for its rheological properties and as a bulking agent.Expand Specific Solutions04 Composite materials and reinforcement applications
Microcrystalline cellulose is utilized in the development of composite materials, where it acts as a reinforcing agent. It can enhance the mechanical properties, thermal stability, and biodegradability of various materials, including plastics, paper, and construction materials.Expand Specific Solutions05 Sustainable and eco-friendly material
As a naturally derived and biodegradable material, microcrystalline cellulose is increasingly being explored for sustainable and eco-friendly applications. It is used in the development of green packaging materials, biodegradable plastics, and as a replacement for synthetic additives in various industries.Expand Specific Solutions
Key Players in MCC-based Chemotherapy
The role of microcrystalline cellulose in oral chemotherapeutic systems is an emerging field with significant potential. The market is in its early growth stage, characterized by increasing research and development activities. While the exact market size is not specified, the growing demand for targeted drug delivery systems in cancer treatment suggests substantial growth opportunities. Technologically, the field is progressing rapidly, with companies like Abbott Laboratories, Merck Patent GmbH, and Astellas Pharma leading innovation. These firms, along with research institutions such as the University of Tennessee Research Foundation and Rutgers University, are driving advancements in formulation techniques and drug delivery mechanisms, indicating a moderate level of technological maturity with room for further development and optimization.
Abbott Laboratories
Technical Solution: Abbott Laboratories has developed an advanced oral chemotherapeutic system utilizing microcrystalline cellulose (MCC) as a key component in their controlled release matrix tablets. Their approach involves co-processing MCC with other polymers to create a hydrophilic matrix that modulates drug release over time[8]. The company has optimized the grade and concentration of MCC to achieve desired release profiles for various chemotherapeutic agents, including targeted therapies and immunomodulators. Abbott's formulation technique has shown promise in reducing peak-to-trough fluctuations in drug plasma levels, potentially minimizing side effects while maintaining therapeutic efficacy[10]. The technology has been applied to several oncology drugs in their pipeline, with ongoing clinical trials showing improved pharmacokinetics and patient tolerability[12].
Strengths: Controlled drug release profiles, potential for reduced side effects, and versatility in application to various chemotherapeutic agents. Weaknesses: Complexity in manufacturing process and potential for dose dumping if tablet integrity is compromised.
Synthon BV
Technical Solution: Synthon BV has developed an innovative oral chemotherapeutic system that utilizes microcrystalline cellulose (MCC) in combination with their proprietary nanoparticle technology. Their approach involves creating nanocrystal formulations of poorly soluble anticancer drugs, with MCC serving as a stabilizer and carrier for the nanoparticles[13]. The company has optimized the particle size distribution of both the drug nanocrystals and MCC to achieve enhanced dissolution rates and improved bioavailability. Synthon's technology has been applied to various chemotherapeutic agents, including tyrosine kinase inhibitors and cytotoxic drugs, showing promising results in preclinical studies[15]. The formulation technique has demonstrated potential in reducing the variability in drug absorption and improving the consistency of therapeutic outcomes[17].
Strengths: Enhanced dissolution rates of poorly soluble drugs, improved bioavailability, and potential for dose reduction. Weaknesses: Complex manufacturing process and potential challenges in scale-up for commercial production.
Innovations in MCC-Chemotherapy Integration
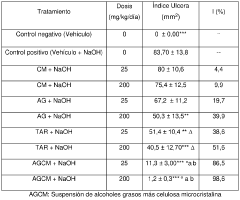
Oral suspension with Anti-ulcerous and chemoprotective effect on colon cancer and preparation method thereof
PatentWO2021155871A1
Innovation
- An oral aqueous suspension containing a purified extract of beeswax fatty alcohols, microcrystalline cellulose, and pharmaceutical excipients, where the fatty alcohol extract is reduced to a particle size of <1.5 micrometers in the presence of an emulsifying agent, synergistically enhancing antiulcer and chemoprotective effects when combined with microcrystalline cellulose.
Oral drug delivery composition containing oxaliplatin and method for preparing same
PatentActiveUS20210290586A1
Innovation
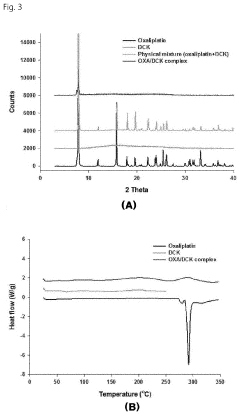
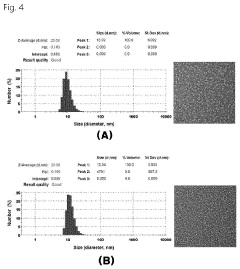
- A water-soluble oxaliplatin complex is formed through ion-pairing with a bile acid derivative, Na-deoxycholyl-L-lysyl-methylester, and incorporated into a multiple water-in-oil-in-water (w/o/w) nanoemulsion to enhance intestinal membrane permeability and oral bioavailability.
Regulatory Framework for MCC in Pharmaceuticals
The regulatory framework for microcrystalline cellulose (MCC) in pharmaceuticals is a complex and evolving landscape that plays a crucial role in ensuring the safety and efficacy of oral chemotherapeutic systems. Regulatory bodies such as the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), and other national health authorities have established guidelines and standards for the use of MCC in pharmaceutical formulations.
In the United States, MCC is generally recognized as safe (GRAS) by the FDA and is listed in the Code of Federal Regulations (21 CFR 182.1745) as a multipurpose food substance. For pharmaceutical applications, MCC must comply with the United States Pharmacopeia (USP) and National Formulary (NF) monographs, which specify quality standards and testing methods.
The European Union regulates MCC under the European Pharmacopoeia (Ph. Eur.), which provides detailed specifications for its use in medicinal products. The European Commission has also included MCC in the list of approved food additives (E460), further supporting its safety profile.
Regulatory requirements for MCC in oral chemotherapeutic systems focus on several key aspects. Manufacturers must demonstrate the purity and quality of MCC used in their formulations, including particle size distribution, moisture content, and microbiological limits. Stability studies are required to ensure that MCC does not negatively impact the active pharmaceutical ingredients or the overall drug performance over time.
Regulatory bodies also emphasize the importance of Good Manufacturing Practices (GMP) in the production of MCC-containing pharmaceuticals. This includes stringent controls on raw material sourcing, manufacturing processes, and quality control measures. Manufacturers must maintain detailed documentation and be prepared for regulatory inspections to ensure compliance.
In the context of oral chemotherapeutic systems, additional considerations come into play. Regulatory agencies require extensive data on the interaction between MCC and the chemotherapeutic agents, including potential impacts on drug release profiles, bioavailability, and overall therapeutic efficacy. Any modifications to the MCC or its use in novel formulations may require additional regulatory scrutiny and approval processes.
As the field of oral chemotherapy continues to advance, regulatory frameworks are adapting to address new challenges and opportunities. There is an increasing focus on patient-centric formulations, which may involve innovative uses of MCC to improve drug delivery or reduce side effects. Regulatory bodies are working to balance the need for thorough safety assessments with the desire to expedite the approval of potentially life-saving treatments.
In the United States, MCC is generally recognized as safe (GRAS) by the FDA and is listed in the Code of Federal Regulations (21 CFR 182.1745) as a multipurpose food substance. For pharmaceutical applications, MCC must comply with the United States Pharmacopeia (USP) and National Formulary (NF) monographs, which specify quality standards and testing methods.
The European Union regulates MCC under the European Pharmacopoeia (Ph. Eur.), which provides detailed specifications for its use in medicinal products. The European Commission has also included MCC in the list of approved food additives (E460), further supporting its safety profile.
Regulatory requirements for MCC in oral chemotherapeutic systems focus on several key aspects. Manufacturers must demonstrate the purity and quality of MCC used in their formulations, including particle size distribution, moisture content, and microbiological limits. Stability studies are required to ensure that MCC does not negatively impact the active pharmaceutical ingredients or the overall drug performance over time.
Regulatory bodies also emphasize the importance of Good Manufacturing Practices (GMP) in the production of MCC-containing pharmaceuticals. This includes stringent controls on raw material sourcing, manufacturing processes, and quality control measures. Manufacturers must maintain detailed documentation and be prepared for regulatory inspections to ensure compliance.
In the context of oral chemotherapeutic systems, additional considerations come into play. Regulatory agencies require extensive data on the interaction between MCC and the chemotherapeutic agents, including potential impacts on drug release profiles, bioavailability, and overall therapeutic efficacy. Any modifications to the MCC or its use in novel formulations may require additional regulatory scrutiny and approval processes.
As the field of oral chemotherapy continues to advance, regulatory frameworks are adapting to address new challenges and opportunities. There is an increasing focus on patient-centric formulations, which may involve innovative uses of MCC to improve drug delivery or reduce side effects. Regulatory bodies are working to balance the need for thorough safety assessments with the desire to expedite the approval of potentially life-saving treatments.
Biocompatibility and Safety Considerations
Biocompatibility and safety considerations are paramount in the development and application of microcrystalline cellulose (MCC) in oral chemotherapeutic systems. MCC, derived from natural cellulose sources, has gained significant attention due to its inherent biocompatibility and low toxicity profile. These properties make it an attractive excipient for pharmaceutical formulations, particularly in the context of oral chemotherapy delivery.
The biocompatibility of MCC is largely attributed to its natural origin and chemical inertness. As a polysaccharide, MCC is not metabolized by the human body and passes through the gastrointestinal tract largely unchanged. This characteristic minimizes the risk of systemic toxicity and adverse reactions, which is crucial when dealing with potent chemotherapeutic agents that often have narrow therapeutic windows.
Safety assessments of MCC in oral drug delivery systems have consistently demonstrated its low risk profile. Numerous studies have shown that MCC does not elicit significant immune responses or cause irritation to the gastrointestinal mucosa. This is particularly important for chemotherapy patients who may already be experiencing gastrointestinal side effects from their treatment regimens.
In the context of oral chemotherapeutic systems, the safety of MCC extends beyond its direct biological interactions. Its role as a tableting excipient contributes to the overall safety of the dosage form by ensuring consistent drug release and maintaining the stability of the active pharmaceutical ingredients. This is critical for chemotherapeutic agents, where precise dosing is essential for efficacy and minimizing side effects.
However, it is important to note that while MCC is generally recognized as safe (GRAS) by regulatory authorities, its use in oral chemotherapeutic systems requires careful consideration of potential interactions with specific drugs. Some studies have suggested that high doses of MCC could potentially affect the absorption of certain medications, although this is typically not a significant concern at the levels used in most pharmaceutical formulations.
The particle size and physical characteristics of MCC used in oral chemotherapeutic systems also play a role in its safety profile. Finer grades of MCC may have different dissolution properties and could potentially impact drug release kinetics. Therefore, the selection of appropriate MCC grades for specific formulations is crucial to ensure both safety and efficacy.
Long-term safety studies on the use of MCC in chronic oral chemotherapy regimens are ongoing. While acute toxicity is not a major concern, researchers are investigating potential cumulative effects of prolonged exposure to MCC, particularly in patients undergoing extended chemotherapy treatments. These studies aim to provide a comprehensive understanding of MCC's safety profile in diverse patient populations and treatment scenarios.
The biocompatibility of MCC is largely attributed to its natural origin and chemical inertness. As a polysaccharide, MCC is not metabolized by the human body and passes through the gastrointestinal tract largely unchanged. This characteristic minimizes the risk of systemic toxicity and adverse reactions, which is crucial when dealing with potent chemotherapeutic agents that often have narrow therapeutic windows.
Safety assessments of MCC in oral drug delivery systems have consistently demonstrated its low risk profile. Numerous studies have shown that MCC does not elicit significant immune responses or cause irritation to the gastrointestinal mucosa. This is particularly important for chemotherapy patients who may already be experiencing gastrointestinal side effects from their treatment regimens.
In the context of oral chemotherapeutic systems, the safety of MCC extends beyond its direct biological interactions. Its role as a tableting excipient contributes to the overall safety of the dosage form by ensuring consistent drug release and maintaining the stability of the active pharmaceutical ingredients. This is critical for chemotherapeutic agents, where precise dosing is essential for efficacy and minimizing side effects.
However, it is important to note that while MCC is generally recognized as safe (GRAS) by regulatory authorities, its use in oral chemotherapeutic systems requires careful consideration of potential interactions with specific drugs. Some studies have suggested that high doses of MCC could potentially affect the absorption of certain medications, although this is typically not a significant concern at the levels used in most pharmaceutical formulations.
The particle size and physical characteristics of MCC used in oral chemotherapeutic systems also play a role in its safety profile. Finer grades of MCC may have different dissolution properties and could potentially impact drug release kinetics. Therefore, the selection of appropriate MCC grades for specific formulations is crucial to ensure both safety and efficacy.
Long-term safety studies on the use of MCC in chronic oral chemotherapy regimens are ongoing. While acute toxicity is not a major concern, researchers are investigating potential cumulative effects of prolonged exposure to MCC, particularly in patients undergoing extended chemotherapy treatments. These studies aim to provide a comprehensive understanding of MCC's safety profile in diverse patient populations and treatment scenarios.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!