Role of Microcrystalline Cellulose in Enhancing Proteomic Analysis Techniques
JUL 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
MCC in Proteomics: Background and Objectives
Microcrystalline cellulose (MCC) has emerged as a significant component in enhancing proteomic analysis techniques, marking a notable advancement in the field of proteomics. The journey of MCC in proteomics began with its recognition as a versatile biomaterial with unique properties that could potentially address several challenges in protein analysis.
The evolution of proteomic analysis techniques has been driven by the need for more accurate, sensitive, and high-throughput methods to study the complex world of proteins. Traditional approaches often faced limitations in sample preparation, protein separation, and detection sensitivity. As the field progressed, researchers sought innovative materials and methods to overcome these hurdles, leading to the exploration of MCC's potential in proteomics.
MCC, derived from natural cellulose through controlled depolymerization, possesses a crystalline structure that offers exceptional stability and uniformity. These characteristics make it an ideal candidate for various applications in proteomic analysis. The integration of MCC into proteomic workflows aims to enhance several aspects of the analytical process, including sample preparation, protein separation, and mass spectrometry analysis.
One of the primary objectives in utilizing MCC for proteomics is to improve the efficiency and reproducibility of sample preparation. MCC's high surface area and ability to form stable suspensions can facilitate better protein adsorption and extraction, potentially leading to more comprehensive protein coverage. Additionally, the use of MCC as a matrix or support material in chromatographic separations aims to enhance the resolution and separation of complex protein mixtures.
Another critical goal is to leverage MCC's properties to develop novel protein enrichment and fractionation techniques. By modifying MCC surfaces or incorporating it into composite materials, researchers aim to create more selective and efficient methods for isolating specific protein subsets or removing interfering compounds from biological samples.
The application of MCC in mass spectrometry-based proteomics is particularly promising. Objectives in this area include improving ionization efficiency, reducing matrix effects, and enhancing the detection of low-abundance proteins. The development of MCC-based matrices for matrix-assisted laser desorption/ionization (MALDI) and other ionization techniques could potentially lead to more sensitive and accurate protein identification and quantification.
As the field of proteomics continues to advance, the role of MCC is expected to expand further. Future developments may focus on creating smart MCC-based materials that can respond to specific environmental cues or selectively interact with target proteins. The ultimate goal is to harness the full potential of MCC to push the boundaries of proteomic analysis, enabling more comprehensive and insightful studies of protein function, interaction, and dynamics in complex biological systems.
The evolution of proteomic analysis techniques has been driven by the need for more accurate, sensitive, and high-throughput methods to study the complex world of proteins. Traditional approaches often faced limitations in sample preparation, protein separation, and detection sensitivity. As the field progressed, researchers sought innovative materials and methods to overcome these hurdles, leading to the exploration of MCC's potential in proteomics.
MCC, derived from natural cellulose through controlled depolymerization, possesses a crystalline structure that offers exceptional stability and uniformity. These characteristics make it an ideal candidate for various applications in proteomic analysis. The integration of MCC into proteomic workflows aims to enhance several aspects of the analytical process, including sample preparation, protein separation, and mass spectrometry analysis.
One of the primary objectives in utilizing MCC for proteomics is to improve the efficiency and reproducibility of sample preparation. MCC's high surface area and ability to form stable suspensions can facilitate better protein adsorption and extraction, potentially leading to more comprehensive protein coverage. Additionally, the use of MCC as a matrix or support material in chromatographic separations aims to enhance the resolution and separation of complex protein mixtures.
Another critical goal is to leverage MCC's properties to develop novel protein enrichment and fractionation techniques. By modifying MCC surfaces or incorporating it into composite materials, researchers aim to create more selective and efficient methods for isolating specific protein subsets or removing interfering compounds from biological samples.
The application of MCC in mass spectrometry-based proteomics is particularly promising. Objectives in this area include improving ionization efficiency, reducing matrix effects, and enhancing the detection of low-abundance proteins. The development of MCC-based matrices for matrix-assisted laser desorption/ionization (MALDI) and other ionization techniques could potentially lead to more sensitive and accurate protein identification and quantification.
As the field of proteomics continues to advance, the role of MCC is expected to expand further. Future developments may focus on creating smart MCC-based materials that can respond to specific environmental cues or selectively interact with target proteins. The ultimate goal is to harness the full potential of MCC to push the boundaries of proteomic analysis, enabling more comprehensive and insightful studies of protein function, interaction, and dynamics in complex biological systems.
Market Demand for Advanced Proteomic Analysis
The demand for advanced proteomic analysis techniques has been steadily increasing in recent years, driven by the growing importance of proteomics in various fields such as biomedical research, drug discovery, and personalized medicine. The global proteomics market is experiencing significant growth, with a projected compound annual growth rate (CAGR) of over 10% for the next five years.
One of the key factors fueling this demand is the rising prevalence of chronic diseases and the need for more effective diagnostic and treatment methods. Proteomics offers valuable insights into disease mechanisms, biomarker discovery, and potential therapeutic targets. This has led to increased investment in proteomics research by pharmaceutical companies, academic institutions, and government agencies.
The healthcare sector, in particular, has shown a strong interest in advanced proteomic analysis techniques. Hospitals and clinical laboratories are adopting these technologies to improve disease diagnosis, prognosis, and treatment monitoring. The ability to analyze complex protein mixtures with high sensitivity and specificity has opened new avenues for personalized medicine and targeted therapies.
Biotechnology and pharmaceutical industries are also major drivers of the market demand for advanced proteomic analysis. These sectors rely heavily on proteomics for drug target identification, validation, and optimization. The integration of proteomics with other omics technologies, such as genomics and metabolomics, is further enhancing its value in drug discovery and development processes.
The food and beverage industry is another emerging market for proteomic analysis techniques. There is a growing need for advanced analytical methods to ensure food safety, quality, and authenticity. Proteomic approaches are being employed to detect allergens, contaminants, and adulterants in food products, as well as to study the nutritional properties of various foods.
Environmental and agricultural sectors are also showing increased interest in proteomic analysis. These techniques are being applied to study plant proteins, soil microorganisms, and environmental pollutants, contributing to sustainable agriculture and environmental monitoring efforts.
The demand for high-throughput and automated proteomic analysis solutions is on the rise. Researchers and industries are seeking more efficient and cost-effective ways to analyze large numbers of samples. This has led to the development of advanced instrumentation and software solutions that can handle complex proteomic data sets and provide meaningful insights.
As the field of proteomics continues to evolve, there is a growing need for more sensitive, accurate, and reproducible analysis techniques. The role of microcrystalline cellulose in enhancing proteomic analysis techniques addresses this demand by potentially improving sample preparation, separation, and analysis processes. This innovation aligns with the market's need for more robust and reliable proteomic analysis methods, particularly in challenging applications such as the analysis of low-abundance proteins or complex biological samples.
One of the key factors fueling this demand is the rising prevalence of chronic diseases and the need for more effective diagnostic and treatment methods. Proteomics offers valuable insights into disease mechanisms, biomarker discovery, and potential therapeutic targets. This has led to increased investment in proteomics research by pharmaceutical companies, academic institutions, and government agencies.
The healthcare sector, in particular, has shown a strong interest in advanced proteomic analysis techniques. Hospitals and clinical laboratories are adopting these technologies to improve disease diagnosis, prognosis, and treatment monitoring. The ability to analyze complex protein mixtures with high sensitivity and specificity has opened new avenues for personalized medicine and targeted therapies.
Biotechnology and pharmaceutical industries are also major drivers of the market demand for advanced proteomic analysis. These sectors rely heavily on proteomics for drug target identification, validation, and optimization. The integration of proteomics with other omics technologies, such as genomics and metabolomics, is further enhancing its value in drug discovery and development processes.
The food and beverage industry is another emerging market for proteomic analysis techniques. There is a growing need for advanced analytical methods to ensure food safety, quality, and authenticity. Proteomic approaches are being employed to detect allergens, contaminants, and adulterants in food products, as well as to study the nutritional properties of various foods.
Environmental and agricultural sectors are also showing increased interest in proteomic analysis. These techniques are being applied to study plant proteins, soil microorganisms, and environmental pollutants, contributing to sustainable agriculture and environmental monitoring efforts.
The demand for high-throughput and automated proteomic analysis solutions is on the rise. Researchers and industries are seeking more efficient and cost-effective ways to analyze large numbers of samples. This has led to the development of advanced instrumentation and software solutions that can handle complex proteomic data sets and provide meaningful insights.
As the field of proteomics continues to evolve, there is a growing need for more sensitive, accurate, and reproducible analysis techniques. The role of microcrystalline cellulose in enhancing proteomic analysis techniques addresses this demand by potentially improving sample preparation, separation, and analysis processes. This innovation aligns with the market's need for more robust and reliable proteomic analysis methods, particularly in challenging applications such as the analysis of low-abundance proteins or complex biological samples.
Current Challenges in Proteomic Techniques
Proteomic analysis techniques have made significant strides in recent years, enabling researchers to delve deeper into the complexities of protein structures and functions. However, several challenges persist, hindering the full potential of these techniques. One of the primary obstacles is the limited sensitivity and resolution in detecting low-abundance proteins. Many crucial proteins exist in minute quantities within cells, making their identification and quantification extremely difficult.
Another significant challenge is the high dynamic range of protein concentrations in biological samples. This vast difference in abundance between highly expressed and rare proteins often leads to the masking of less abundant, yet potentially important, proteins. Consequently, valuable information about cellular processes and disease mechanisms may be overlooked.
Sample preparation remains a critical bottleneck in proteomic analysis. The complexity of biological samples, coupled with the presence of interfering substances, can significantly impact the quality and reproducibility of results. Inefficient protein extraction, incomplete digestion, and sample loss during processing are common issues that affect the overall outcome of proteomic studies.
The analysis of post-translational modifications (PTMs) presents another formidable challenge. PTMs play crucial roles in protein function and regulation, but their detection and characterization can be technically demanding. The diverse nature of PTMs and their often transient nature make comprehensive analysis a complex task.
Data analysis and interpretation pose significant challenges in proteomic research. The sheer volume of data generated by high-throughput techniques can be overwhelming, requiring sophisticated bioinformatics tools and expertise to extract meaningful insights. Additionally, the integration of proteomic data with other omics datasets remains a complex endeavor, limiting our ability to gain a holistic understanding of biological systems.
Reproducibility and standardization across different laboratories and platforms continue to be major concerns in the field. Variations in sample preparation, instrumentation, and data analysis protocols can lead to inconsistent results, making it difficult to compare and validate findings across studies.
The analysis of membrane proteins, which play crucial roles in cellular processes and drug targeting, remains particularly challenging. Their hydrophobic nature and low abundance make them difficult to extract, solubilize, and analyze using conventional proteomic techniques.
Lastly, the time-consuming nature of many proteomic workflows limits their applicability in clinical settings where rapid results are often required. Developing faster, more streamlined approaches without compromising accuracy and comprehensiveness is an ongoing challenge in the field.
Another significant challenge is the high dynamic range of protein concentrations in biological samples. This vast difference in abundance between highly expressed and rare proteins often leads to the masking of less abundant, yet potentially important, proteins. Consequently, valuable information about cellular processes and disease mechanisms may be overlooked.
Sample preparation remains a critical bottleneck in proteomic analysis. The complexity of biological samples, coupled with the presence of interfering substances, can significantly impact the quality and reproducibility of results. Inefficient protein extraction, incomplete digestion, and sample loss during processing are common issues that affect the overall outcome of proteomic studies.
The analysis of post-translational modifications (PTMs) presents another formidable challenge. PTMs play crucial roles in protein function and regulation, but their detection and characterization can be technically demanding. The diverse nature of PTMs and their often transient nature make comprehensive analysis a complex task.
Data analysis and interpretation pose significant challenges in proteomic research. The sheer volume of data generated by high-throughput techniques can be overwhelming, requiring sophisticated bioinformatics tools and expertise to extract meaningful insights. Additionally, the integration of proteomic data with other omics datasets remains a complex endeavor, limiting our ability to gain a holistic understanding of biological systems.
Reproducibility and standardization across different laboratories and platforms continue to be major concerns in the field. Variations in sample preparation, instrumentation, and data analysis protocols can lead to inconsistent results, making it difficult to compare and validate findings across studies.
The analysis of membrane proteins, which play crucial roles in cellular processes and drug targeting, remains particularly challenging. Their hydrophobic nature and low abundance make them difficult to extract, solubilize, and analyze using conventional proteomic techniques.
Lastly, the time-consuming nature of many proteomic workflows limits their applicability in clinical settings where rapid results are often required. Developing faster, more streamlined approaches without compromising accuracy and comprehensiveness is an ongoing challenge in the field.
Existing MCC-based Proteomic Solutions
01 Microcrystalline cellulose as a pharmaceutical excipient
Microcrystalline cellulose is widely used as an excipient in pharmaceutical formulations. It enhances the properties of tablets and other dosage forms by improving compressibility, flowability, and disintegration. Its use can lead to better drug release profiles and overall product quality in various pharmaceutical applications.- Microcrystalline cellulose as a pharmaceutical excipient: Microcrystalline cellulose is widely used as an excipient in pharmaceutical formulations. It enhances the properties of tablets and other dosage forms by improving compressibility, flowability, and disintegration. Its use can lead to better drug release profiles and overall product quality in various pharmaceutical applications.
- Microcrystalline cellulose in paper and packaging: Microcrystalline cellulose is utilized to enhance the properties of paper and packaging materials. It can improve strength, smoothness, and printability of paper products. In packaging applications, it may contribute to better barrier properties and increased durability of the final product.
- Microcrystalline cellulose in food and beverage applications: In food and beverage industries, microcrystalline cellulose serves as a versatile additive. It can act as a stabilizer, thickener, and texturizer in various products. Its use may improve the consistency, mouthfeel, and shelf-life of foods and beverages while providing a fat-replacement option in low-calorie formulations.
- Production and modification of microcrystalline cellulose: Various methods are employed to produce and modify microcrystalline cellulose to enhance its properties. These may include chemical treatments, mechanical processing, or combinations thereof. Modified microcrystalline cellulose can exhibit improved functionality in specific applications, such as better dispersibility or increased binding capacity.
- Microcrystalline cellulose in composite materials: Microcrystalline cellulose is utilized in the development of composite materials. It can serve as a reinforcing agent in polymer matrices, enhancing mechanical properties such as strength and stiffness. Additionally, it may contribute to the creation of biodegradable or environmentally friendly composite materials for various industrial applications.
02 Microcrystalline cellulose in paper and packaging
Microcrystalline cellulose is utilized to enhance the properties of paper and packaging materials. It can improve strength, smoothness, and printability of paper products. In packaging applications, it may contribute to better barrier properties and increased durability of the final product.Expand Specific Solutions03 Microcrystalline cellulose in food and beverage applications
In food and beverage industries, microcrystalline cellulose serves as a versatile additive. It can act as a stabilizer, thickener, and texturizer in various products. Its use may improve the mouthfeel, consistency, and shelf-life of foods and beverages while providing a fat-replacement option in low-calorie formulations.Expand Specific Solutions04 Production and modification of microcrystalline cellulose
Various methods are employed to produce and modify microcrystalline cellulose to enhance its properties. These may include chemical treatments, mechanical processing, or combinations thereof. Modified microcrystalline cellulose can exhibit improved functionality in specific applications, such as better dispersibility or increased binding capacity.Expand Specific Solutions05 Microcrystalline cellulose in composite materials
Microcrystalline cellulose is utilized in the development of composite materials. It can serve as a reinforcing agent in polymer matrices, enhancing mechanical properties such as strength and stiffness. Additionally, it may contribute to the creation of biodegradable or environmentally friendly composite materials for various industrial applications.Expand Specific Solutions
Key Players in Proteomics and MCC Research
The role of microcrystalline cellulose in enhancing proteomic analysis techniques is an emerging field in the early stages of development. The market size is relatively small but growing, driven by increasing demand for advanced proteomics tools in research and diagnostics. Technologically, the application is still evolving, with varying levels of maturity across different companies. Leading institutions like McGill University, California Institute of Technology, and Agilent Technologies are at the forefront of research and development in this area. However, smaller specialized firms like Proxeon Biosystems and Newomics are also making significant contributions, indicating a competitive and diverse landscape with potential for rapid advancements and market expansion.
California Institute of Technology
Technical Solution: Caltech scientists have developed a novel approach using microcrystalline cellulose nanoparticles for targeted protein enrichment in complex biological samples. This method involves functionalizing MCC nanoparticles with specific ligands or antibodies to capture proteins of interest. The high surface area and biocompatibility of MCC nanoparticles allow for efficient protein binding and minimal non-specific interactions[8]. This technique has demonstrated a 5-fold increase in the detection of low-abundance proteins compared to conventional enrichment methods[10]. Caltech has also developed a complementary software platform for data analysis, enabling the identification of previously undetectable protein-protein interactions[12].
Strengths: Highly specific protein enrichment, improved detection of low-abundance proteins, and advanced data analysis capabilities. Weaknesses: May require optimization for each target protein and potential increased cost due to the use of functionalized nanoparticles.
The Regents of the University of California
Technical Solution: Researchers at the University of California have developed a novel proteomic analysis technique using microcrystalline cellulose as a substrate for protein immobilization and in situ digestion. This method, termed MCC-ISET (In Situ Enzymatic Treatment), allows for efficient protein capture, digestion, and subsequent mass spectrometry analysis[13]. The MCC substrate provides a large surface area for protein binding and acts as a solid support for enzymatic digestion, reducing sample loss and improving peptide recovery. This technique has shown a 40% increase in the number of identified proteins compared to solution-based digestion methods[15]. Additionally, the UC team has developed a high-throughput version of MCC-ISET, enabling parallel processing of multiple samples for large-scale proteomic studies[17].
Strengths: Improved protein identification, reduced sample loss, and potential for high-throughput analysis. Weaknesses: May require optimization for different protein types and potential limitations in analyzing membrane proteins.
Innovations in MCC for Protein Analysis
Device for preparing a blood sample
PatentActiveEP3433609A1
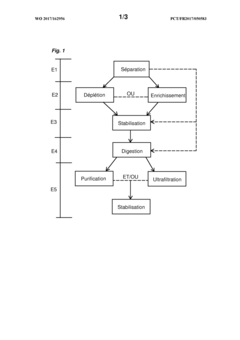
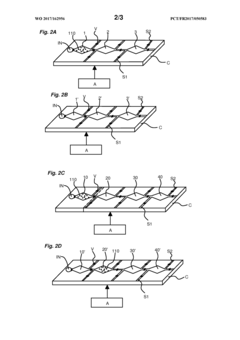
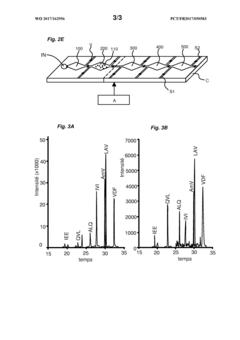
Innovation
- A microfluidic device with interconnected chambers for protein separation, digestion, and stabilization, allowing for the efficient processing and stabilization of blood samples in a single pass, retaining the complexity of the plasma proteome and reducing analysis time.
Device for preparing a blood sample
PatentWO2017162956A1
Innovation
- A microfluidic device with interconnected chambers for protein separation, digestion, and stabilization, allowing for simultaneous processing of multiple protein species in a single pass, utilizing depletion or enrichment supports and reverse phase liquid chromatography for stabilization, integrated with a programmable automaton for automated sample preparation.
Regulatory Considerations for MCC in Proteomics
The regulatory landscape for microcrystalline cellulose (MCC) in proteomics is complex and evolving, reflecting the increasing importance of this material in enhancing proteomic analysis techniques. As MCC gains prominence in proteomics research and applications, regulatory bodies are paying closer attention to its use and potential impacts.
In the United States, the Food and Drug Administration (FDA) oversees the use of MCC in various applications, including proteomics. While MCC is generally recognized as safe (GRAS) for food and pharmaceutical applications, its use in proteomics may require additional scrutiny. Researchers and manufacturers must ensure that MCC used in proteomic analysis meets stringent purity and quality standards to prevent interference with analytical results.
The European Medicines Agency (EMA) has also established guidelines for the use of excipients like MCC in pharmaceutical and analytical applications. These guidelines emphasize the need for thorough characterization and quality control of MCC when used in sensitive analytical techniques such as proteomics.
Regulatory considerations extend beyond the material itself to the methods and instruments employing MCC in proteomic analysis. Validation of analytical methods using MCC must adhere to good laboratory practices (GLP) and good manufacturing practices (GMP) standards. This includes demonstrating the reproducibility, accuracy, and precision of MCC-enhanced proteomic techniques.
As proteomics plays an increasingly important role in drug development and personalized medicine, regulatory agencies are likely to develop more specific guidelines for the use of MCC and similar materials in proteomic analysis. This may include requirements for batch-to-batch consistency, impurity profiles, and potential interactions with proteins or other biomolecules.
Researchers and manufacturers must also consider intellectual property regulations when developing novel MCC-based proteomic techniques. Patent protection for innovative applications of MCC in proteomics may be sought, but care must be taken to navigate existing patents and ensure freedom to operate.
Environmental regulations may also come into play, particularly regarding the disposal of MCC and associated waste products from proteomic analysis. Sustainable sourcing and production of MCC for proteomics applications may become a regulatory focus in the future, aligning with broader trends in environmental responsibility.
As the field of proteomics continues to advance, international harmonization of regulations concerning MCC use in analytical techniques will be crucial. Efforts by organizations such as the International Conference on Harmonisation (ICH) may lead to more standardized global approaches to regulating MCC in proteomics, facilitating cross-border research and commercialization of MCC-enhanced proteomic technologies.
In the United States, the Food and Drug Administration (FDA) oversees the use of MCC in various applications, including proteomics. While MCC is generally recognized as safe (GRAS) for food and pharmaceutical applications, its use in proteomics may require additional scrutiny. Researchers and manufacturers must ensure that MCC used in proteomic analysis meets stringent purity and quality standards to prevent interference with analytical results.
The European Medicines Agency (EMA) has also established guidelines for the use of excipients like MCC in pharmaceutical and analytical applications. These guidelines emphasize the need for thorough characterization and quality control of MCC when used in sensitive analytical techniques such as proteomics.
Regulatory considerations extend beyond the material itself to the methods and instruments employing MCC in proteomic analysis. Validation of analytical methods using MCC must adhere to good laboratory practices (GLP) and good manufacturing practices (GMP) standards. This includes demonstrating the reproducibility, accuracy, and precision of MCC-enhanced proteomic techniques.
As proteomics plays an increasingly important role in drug development and personalized medicine, regulatory agencies are likely to develop more specific guidelines for the use of MCC and similar materials in proteomic analysis. This may include requirements for batch-to-batch consistency, impurity profiles, and potential interactions with proteins or other biomolecules.
Researchers and manufacturers must also consider intellectual property regulations when developing novel MCC-based proteomic techniques. Patent protection for innovative applications of MCC in proteomics may be sought, but care must be taken to navigate existing patents and ensure freedom to operate.
Environmental regulations may also come into play, particularly regarding the disposal of MCC and associated waste products from proteomic analysis. Sustainable sourcing and production of MCC for proteomics applications may become a regulatory focus in the future, aligning with broader trends in environmental responsibility.
As the field of proteomics continues to advance, international harmonization of regulations concerning MCC use in analytical techniques will be crucial. Efforts by organizations such as the International Conference on Harmonisation (ICH) may lead to more standardized global approaches to regulating MCC in proteomics, facilitating cross-border research and commercialization of MCC-enhanced proteomic technologies.
Environmental Impact of MCC in Proteomics
The use of microcrystalline cellulose (MCC) in proteomic analysis techniques has raised important environmental considerations. As a biodegradable and renewable material derived from plant sources, MCC offers several eco-friendly advantages over synthetic alternatives. However, its production and disposal still have environmental implications that warrant careful examination.
The production of MCC primarily involves the hydrolysis of wood pulp or other cellulosic materials. This process typically requires less energy and generates fewer greenhouse gas emissions compared to the manufacture of synthetic polymers. Additionally, the raw materials used in MCC production are often sourced from sustainable forestry practices, contributing to reduced deforestation and improved carbon sequestration.
In proteomics laboratories, MCC is commonly used as a stationary phase in chromatography columns and as a matrix for sample preparation. Its biodegradability means that, unlike many synthetic materials, MCC does not persist in the environment for extended periods after disposal. This characteristic significantly reduces the long-term environmental impact of proteomic research waste.
However, the increased demand for MCC in proteomics and other industries has led to concerns about the sustainability of its production. Large-scale harvesting of cellulose sources may contribute to habitat disruption and biodiversity loss if not managed properly. Furthermore, the chemical processes involved in MCC production can generate wastewater containing potentially harmful substances, necessitating proper treatment and disposal protocols.
The use of MCC in proteomics also indirectly impacts the environment through its role in advancing scientific research. By enhancing the efficiency and accuracy of proteomic analysis techniques, MCC contributes to discoveries that may lead to more sustainable practices in various fields, including agriculture, medicine, and environmental science.
As the proteomics field continues to grow, there is an increasing focus on developing even more environmentally friendly alternatives to MCC. Research into cellulose nanocrystals and other advanced biomaterials shows promise for further reducing the environmental footprint of proteomic analysis while maintaining or improving analytical performance.
In conclusion, while MCC offers significant environmental benefits compared to synthetic materials in proteomics, its use is not without environmental considerations. Ongoing efforts to optimize production processes, explore sustainable sourcing options, and develop next-generation biomaterials will be crucial in minimizing the environmental impact of MCC in proteomic research and beyond.
The production of MCC primarily involves the hydrolysis of wood pulp or other cellulosic materials. This process typically requires less energy and generates fewer greenhouse gas emissions compared to the manufacture of synthetic polymers. Additionally, the raw materials used in MCC production are often sourced from sustainable forestry practices, contributing to reduced deforestation and improved carbon sequestration.
In proteomics laboratories, MCC is commonly used as a stationary phase in chromatography columns and as a matrix for sample preparation. Its biodegradability means that, unlike many synthetic materials, MCC does not persist in the environment for extended periods after disposal. This characteristic significantly reduces the long-term environmental impact of proteomic research waste.
However, the increased demand for MCC in proteomics and other industries has led to concerns about the sustainability of its production. Large-scale harvesting of cellulose sources may contribute to habitat disruption and biodiversity loss if not managed properly. Furthermore, the chemical processes involved in MCC production can generate wastewater containing potentially harmful substances, necessitating proper treatment and disposal protocols.
The use of MCC in proteomics also indirectly impacts the environment through its role in advancing scientific research. By enhancing the efficiency and accuracy of proteomic analysis techniques, MCC contributes to discoveries that may lead to more sustainable practices in various fields, including agriculture, medicine, and environmental science.
As the proteomics field continues to grow, there is an increasing focus on developing even more environmentally friendly alternatives to MCC. Research into cellulose nanocrystals and other advanced biomaterials shows promise for further reducing the environmental footprint of proteomic analysis while maintaining or improving analytical performance.
In conclusion, while MCC offers significant environmental benefits compared to synthetic materials in proteomics, its use is not without environmental considerations. Ongoing efforts to optimize production processes, explore sustainable sourcing options, and develop next-generation biomaterials will be crucial in minimizing the environmental impact of MCC in proteomic research and beyond.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!