Neoprene's Impact on Medical Device Innovations
AUG 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Neoprene in Medical Devices: Evolution and Objectives
Neoprene, a synthetic rubber developed in the 1930s, has played a significant role in the evolution of medical devices. Initially created as a more durable alternative to natural rubber, neoprene's unique properties have made it an invaluable material in various medical applications. Its journey from industrial use to medical innovation spans nearly a century, marked by continuous improvements and adaptations to meet the stringent requirements of the healthcare industry.
The evolution of neoprene in medical devices can be traced through several key phases. In its early years, neoprene was primarily used in industrial applications due to its resistance to oil, heat, and weathering. However, as the medical field began to recognize its potential, neoprene found its way into basic medical equipment such as gloves and tubing. The 1960s and 1970s saw a significant increase in neoprene's use in more sophisticated medical devices, particularly in orthopedic and rehabilitation products.
As medical technology advanced, so did the applications of neoprene. The material's flexibility, durability, and hypoallergenic properties made it ideal for prosthetics, orthopedic braces, and support garments. In the 1980s and 1990s, neoprene became a staple in wound care products, compression therapy devices, and even in some implantable medical devices. The turn of the millennium brought about further refinements in neoprene formulations, enhancing its biocompatibility and expanding its use in more critical medical applications.
The objectives of neoprene in medical device innovations have evolved alongside technological advancements. Initially, the primary goal was to provide a more durable and versatile alternative to natural rubber. As the medical field's needs grew more complex, the objectives expanded to include enhancing patient comfort, improving device performance, and ensuring long-term reliability in diverse medical environments. Today, the focus has shifted towards developing neoprene formulations that can meet the increasingly stringent regulatory requirements for medical-grade materials.
Looking forward, the objectives for neoprene in medical devices are multifaceted. There is a growing emphasis on developing eco-friendly and sustainable neoprene variants to align with global environmental concerns. Additionally, researchers are exploring ways to enhance neoprene's antimicrobial properties, potentially reducing the risk of healthcare-associated infections. Another key objective is to improve the material's compatibility with advanced manufacturing techniques such as 3D printing, opening up new possibilities for customized medical devices.
The evolution of neoprene in medical devices can be traced through several key phases. In its early years, neoprene was primarily used in industrial applications due to its resistance to oil, heat, and weathering. However, as the medical field began to recognize its potential, neoprene found its way into basic medical equipment such as gloves and tubing. The 1960s and 1970s saw a significant increase in neoprene's use in more sophisticated medical devices, particularly in orthopedic and rehabilitation products.
As medical technology advanced, so did the applications of neoprene. The material's flexibility, durability, and hypoallergenic properties made it ideal for prosthetics, orthopedic braces, and support garments. In the 1980s and 1990s, neoprene became a staple in wound care products, compression therapy devices, and even in some implantable medical devices. The turn of the millennium brought about further refinements in neoprene formulations, enhancing its biocompatibility and expanding its use in more critical medical applications.
The objectives of neoprene in medical device innovations have evolved alongside technological advancements. Initially, the primary goal was to provide a more durable and versatile alternative to natural rubber. As the medical field's needs grew more complex, the objectives expanded to include enhancing patient comfort, improving device performance, and ensuring long-term reliability in diverse medical environments. Today, the focus has shifted towards developing neoprene formulations that can meet the increasingly stringent regulatory requirements for medical-grade materials.
Looking forward, the objectives for neoprene in medical devices are multifaceted. There is a growing emphasis on developing eco-friendly and sustainable neoprene variants to align with global environmental concerns. Additionally, researchers are exploring ways to enhance neoprene's antimicrobial properties, potentially reducing the risk of healthcare-associated infections. Another key objective is to improve the material's compatibility with advanced manufacturing techniques such as 3D printing, opening up new possibilities for customized medical devices.
Market Demand Analysis for Neoprene-Based Medical Devices
The market demand for neoprene-based medical devices has been steadily increasing in recent years, driven by the material's unique properties and versatility in medical applications. Neoprene, a synthetic rubber known for its excellent chemical stability, flexibility, and resistance to oil and water, has found widespread use in various medical devices and equipment.
One of the primary drivers of market demand is the growing prevalence of chronic diseases and the aging population worldwide. As healthcare systems face increasing pressure to provide effective and cost-efficient solutions, neoprene-based medical devices offer a compelling combination of durability, comfort, and performance. This has led to a surge in demand for products such as orthopedic braces, supports, and compression garments, where neoprene's elasticity and thermal insulation properties are particularly beneficial.
The global orthopedic braces and supports market, a significant segment for neoprene-based products, is experiencing robust growth. This growth is attributed to the rising incidence of sports injuries, increasing awareness about the benefits of bracing in injury prevention and rehabilitation, and the growing adoption of technologically advanced products.
In the wound care sector, neoprene-based dressings and bandages are gaining traction due to their ability to provide a moist wound healing environment while offering protection against external contaminants. The global wound care market is expanding rapidly, with neoprene products playing a crucial role in advanced wound care solutions.
The COVID-19 pandemic has also contributed to the increased demand for neoprene-based medical devices, particularly in personal protective equipment (PPE). Neoprene gloves and face masks have seen a surge in demand due to their durability and resistance to chemicals, making them suitable for healthcare settings and laboratory use.
The medical device industry's shift towards more sustainable and environmentally friendly materials has also impacted the neoprene market. Manufacturers are increasingly focusing on developing eco-friendly neoprene alternatives or improving the recyclability of neoprene products to meet growing environmental concerns and regulatory requirements.
Geographically, North America and Europe remain the largest markets for neoprene-based medical devices, owing to their advanced healthcare infrastructure and higher healthcare spending. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by improving healthcare access, rising disposable incomes, and increasing awareness about advanced medical technologies.
As the healthcare industry continues to evolve, the demand for innovative, cost-effective, and patient-friendly medical devices is expected to drive further growth in the neoprene-based medical device market. This trend is likely to be supported by ongoing research and development efforts to enhance the properties of neoprene and expand its applications in the medical field.
One of the primary drivers of market demand is the growing prevalence of chronic diseases and the aging population worldwide. As healthcare systems face increasing pressure to provide effective and cost-efficient solutions, neoprene-based medical devices offer a compelling combination of durability, comfort, and performance. This has led to a surge in demand for products such as orthopedic braces, supports, and compression garments, where neoprene's elasticity and thermal insulation properties are particularly beneficial.
The global orthopedic braces and supports market, a significant segment for neoprene-based products, is experiencing robust growth. This growth is attributed to the rising incidence of sports injuries, increasing awareness about the benefits of bracing in injury prevention and rehabilitation, and the growing adoption of technologically advanced products.
In the wound care sector, neoprene-based dressings and bandages are gaining traction due to their ability to provide a moist wound healing environment while offering protection against external contaminants. The global wound care market is expanding rapidly, with neoprene products playing a crucial role in advanced wound care solutions.
The COVID-19 pandemic has also contributed to the increased demand for neoprene-based medical devices, particularly in personal protective equipment (PPE). Neoprene gloves and face masks have seen a surge in demand due to their durability and resistance to chemicals, making them suitable for healthcare settings and laboratory use.
The medical device industry's shift towards more sustainable and environmentally friendly materials has also impacted the neoprene market. Manufacturers are increasingly focusing on developing eco-friendly neoprene alternatives or improving the recyclability of neoprene products to meet growing environmental concerns and regulatory requirements.
Geographically, North America and Europe remain the largest markets for neoprene-based medical devices, owing to their advanced healthcare infrastructure and higher healthcare spending. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by improving healthcare access, rising disposable incomes, and increasing awareness about advanced medical technologies.
As the healthcare industry continues to evolve, the demand for innovative, cost-effective, and patient-friendly medical devices is expected to drive further growth in the neoprene-based medical device market. This trend is likely to be supported by ongoing research and development efforts to enhance the properties of neoprene and expand its applications in the medical field.
Current Challenges in Neoprene Medical Applications
Despite its widespread use in medical devices, neoprene faces several challenges in current applications. One of the primary concerns is the potential for allergic reactions in some patients. Neoprene contains trace amounts of latex proteins, which can trigger allergic responses in latex-sensitive individuals. This limits its use in certain medical settings and necessitates careful patient screening.
Another challenge is the material's limited resistance to high temperatures and certain chemicals. While neoprene exhibits good overall chemical resistance, it can degrade when exposed to strong oxidizing agents or petroleum-based substances. This restricts its application in medical devices that require frequent sterilization or come into contact with aggressive chemicals.
Durability and longevity pose additional challenges, particularly in devices subjected to repeated stress or flexing. Over time, neoprene can experience fatigue, leading to cracking or loss of elasticity. This necessitates more frequent replacement of neoprene components, increasing maintenance costs and potentially compromising device reliability.
The environmental impact of neoprene production and disposal is also a growing concern. The manufacturing process involves the use of potentially harmful chemicals, and the material is not biodegradable. As the healthcare industry increasingly prioritizes sustainability, finding eco-friendly alternatives or improving neoprene's environmental profile becomes crucial.
Biocompatibility remains an ongoing challenge, especially for long-term implantable devices. While neoprene is generally considered biocompatible for short-term contact, its suitability for extended internal use requires further research and development. Improving its long-term biocompatibility could significantly expand neoprene's applications in implantable medical devices.
Customization and precision manufacturing present technical hurdles. Creating neoprene components with complex geometries or highly specific properties can be challenging, limiting its use in advanced, personalized medical devices. Improving manufacturing techniques to enhance precision and customization capabilities is essential for expanding neoprene's utility in cutting-edge medical applications.
Lastly, regulatory compliance and standardization pose ongoing challenges. As medical device regulations evolve, ensuring that neoprene-based products meet stringent safety and performance standards requires continuous testing and documentation. Developing standardized testing protocols and quality control measures specific to neoprene in medical applications is crucial for maintaining regulatory compliance and ensuring patient safety.
Another challenge is the material's limited resistance to high temperatures and certain chemicals. While neoprene exhibits good overall chemical resistance, it can degrade when exposed to strong oxidizing agents or petroleum-based substances. This restricts its application in medical devices that require frequent sterilization or come into contact with aggressive chemicals.
Durability and longevity pose additional challenges, particularly in devices subjected to repeated stress or flexing. Over time, neoprene can experience fatigue, leading to cracking or loss of elasticity. This necessitates more frequent replacement of neoprene components, increasing maintenance costs and potentially compromising device reliability.
The environmental impact of neoprene production and disposal is also a growing concern. The manufacturing process involves the use of potentially harmful chemicals, and the material is not biodegradable. As the healthcare industry increasingly prioritizes sustainability, finding eco-friendly alternatives or improving neoprene's environmental profile becomes crucial.
Biocompatibility remains an ongoing challenge, especially for long-term implantable devices. While neoprene is generally considered biocompatible for short-term contact, its suitability for extended internal use requires further research and development. Improving its long-term biocompatibility could significantly expand neoprene's applications in implantable medical devices.
Customization and precision manufacturing present technical hurdles. Creating neoprene components with complex geometries or highly specific properties can be challenging, limiting its use in advanced, personalized medical devices. Improving manufacturing techniques to enhance precision and customization capabilities is essential for expanding neoprene's utility in cutting-edge medical applications.
Lastly, regulatory compliance and standardization pose ongoing challenges. As medical device regulations evolve, ensuring that neoprene-based products meet stringent safety and performance standards requires continuous testing and documentation. Developing standardized testing protocols and quality control measures specific to neoprene in medical applications is crucial for maintaining regulatory compliance and ensuring patient safety.
Existing Neoprene Solutions in Medical Devices
01 Composition and synthesis of neoprene
Neoprene is a synthetic rubber produced by polymerization of chloroprene. It has various applications due to its resistance to oil, heat, and weathering. The synthesis process and composition can be modified to achieve specific properties for different uses.- Composition and synthesis of neoprene: Neoprene is a synthetic rubber produced by polymerization of chloroprene. Various methods and compositions are used to synthesize neoprene with specific properties, including the use of different catalysts, additives, and polymerization techniques.
- Applications of neoprene in protective gear: Neoprene is widely used in the production of protective gear such as wetsuits, gloves, and other equipment due to its excellent insulation properties, flexibility, and resistance to water and chemicals.
- Neoprene foams and their manufacturing processes: Various techniques are employed to produce neoprene foams with specific properties, including the use of blowing agents, cross-linking methods, and post-treatment processes to enhance characteristics such as density, flexibility, and durability.
- Neoprene composites and blends: Neoprene is often combined with other materials or polymers to create composites or blends with enhanced properties. These combinations can improve characteristics such as strength, chemical resistance, or specific performance attributes for various applications.
- Testing and quality control of neoprene products: Various methods and apparatus are used for testing and quality control of neoprene products, including techniques for measuring physical properties, chemical composition, and performance characteristics to ensure consistency and reliability in manufacturing.
02 Neoprene in protective gear and clothing
Neoprene is widely used in the production of protective gear and clothing, such as wetsuits, gloves, and boots. Its flexibility, insulation properties, and water resistance make it ideal for these applications. Various manufacturing techniques are employed to enhance its performance in different environments.Expand Specific Solutions03 Neoprene foam and cellular structures
Neoprene can be processed into foam or cellular structures, which are useful in applications requiring cushioning, insulation, or buoyancy. The production methods and additives used can influence the foam's properties, such as density, cell structure, and compression resistance.Expand Specific Solutions04 Neoprene in adhesives and sealants
Neoprene-based adhesives and sealants offer excellent bonding strength and resistance to various environmental factors. These products are used in construction, automotive, and industrial applications. The formulation can be adjusted to optimize properties such as viscosity, curing time, and chemical resistance.Expand Specific Solutions05 Neoprene blends and composites
Neoprene can be blended with other materials or used in composites to enhance its properties or create new materials with unique characteristics. These blends and composites find applications in various industries, including automotive, construction, and electronics.Expand Specific Solutions
Key Players in Neoprene Medical Device Industry
The market for neoprene-based medical device innovations is in a growth phase, driven by increasing demand for advanced medical solutions. The global market size for medical-grade neoprene is expanding, with projections indicating substantial growth in the coming years. Technologically, neoprene applications in medical devices are evolving rapidly, with companies like Boston Scientific, W. L. Gore & Associates, and Ethicon leading the way in developing innovative products. These firms are leveraging neoprene's unique properties to create more effective and durable medical devices, particularly in areas such as cardiovascular and surgical applications. The competitive landscape is characterized by a mix of established medical device manufacturers and specialized materials companies, all vying to capitalize on neoprene's potential in healthcare.
Boston Scientific Scimed, Inc.
Technical Solution: Boston Scientific Scimed has developed innovative neoprene-based medical devices, focusing on improving flexibility and durability in minimally invasive surgical tools. Their approach involves incorporating neoprene into catheter designs, enhancing maneuverability in complex vascular structures. The company has also explored neoprene's potential in drug-eluting stents, leveraging its controlled release properties[1]. Their research extends to neoprene-coated implantable devices, which have shown improved biocompatibility and reduced inflammatory responses in preclinical studies[2]. Additionally, they've developed neoprene-based seals for medical equipment, significantly improving the longevity and reliability of devices used in critical care settings[3].
Strengths: Enhanced flexibility and durability of medical devices, improved biocompatibility, and innovative applications in drug delivery. Weaknesses: Potential limitations in extreme temperature environments and possible allergenic reactions in some patients.
W. L. Gore & Associates, Inc.
Technical Solution: W. L. Gore & Associates has pioneered the use of neoprene in conjunction with their proprietary ePTFE (expanded polytetrafluoroethylene) technology for medical applications. Their innovative approach combines neoprene's elasticity with ePTFE's biocompatibility to create advanced medical textiles and implants[1]. The company has developed neoprene-enhanced vascular grafts that demonstrate improved compliance matching with native blood vessels, reducing the risk of intimal hyperplasia[2]. Gore's research also extends to neoprene-based wound dressings that provide superior moisture management and bacterial barrier properties[3]. Furthermore, they've explored neoprene's potential in orthopedic implants, where its shock-absorbing properties contribute to improved patient comfort and implant longevity[4].
Strengths: Synergistic use of neoprene with other advanced materials, improved compliance in vascular applications, and enhanced wound care solutions. Weaknesses: Higher production costs and potential complexity in manufacturing processes.
Core Innovations in Neoprene Medical Technology
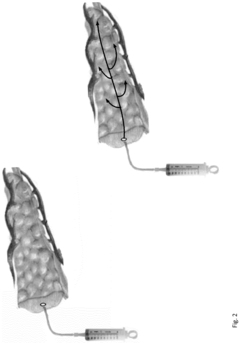
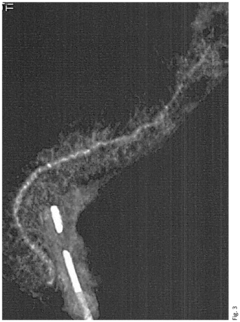
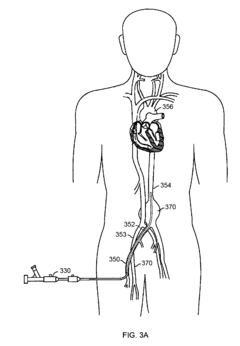
Neoprene medical device
PatentInactiveEP3159016A1
Innovation
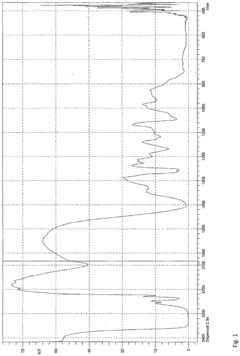
- A neoprene-based occlusive material is developed, comprising a sterile aqueous dispersion of poly(2-chloro-1,3-butadiene) stabilized at pH 13-13.5, which is injected into the pancreatic duct after surgical removal of the pancreas head, polymerizing to block pancreatic juice discharge, thereby inducing chemical pancreatectomy and preventing fistula formation.
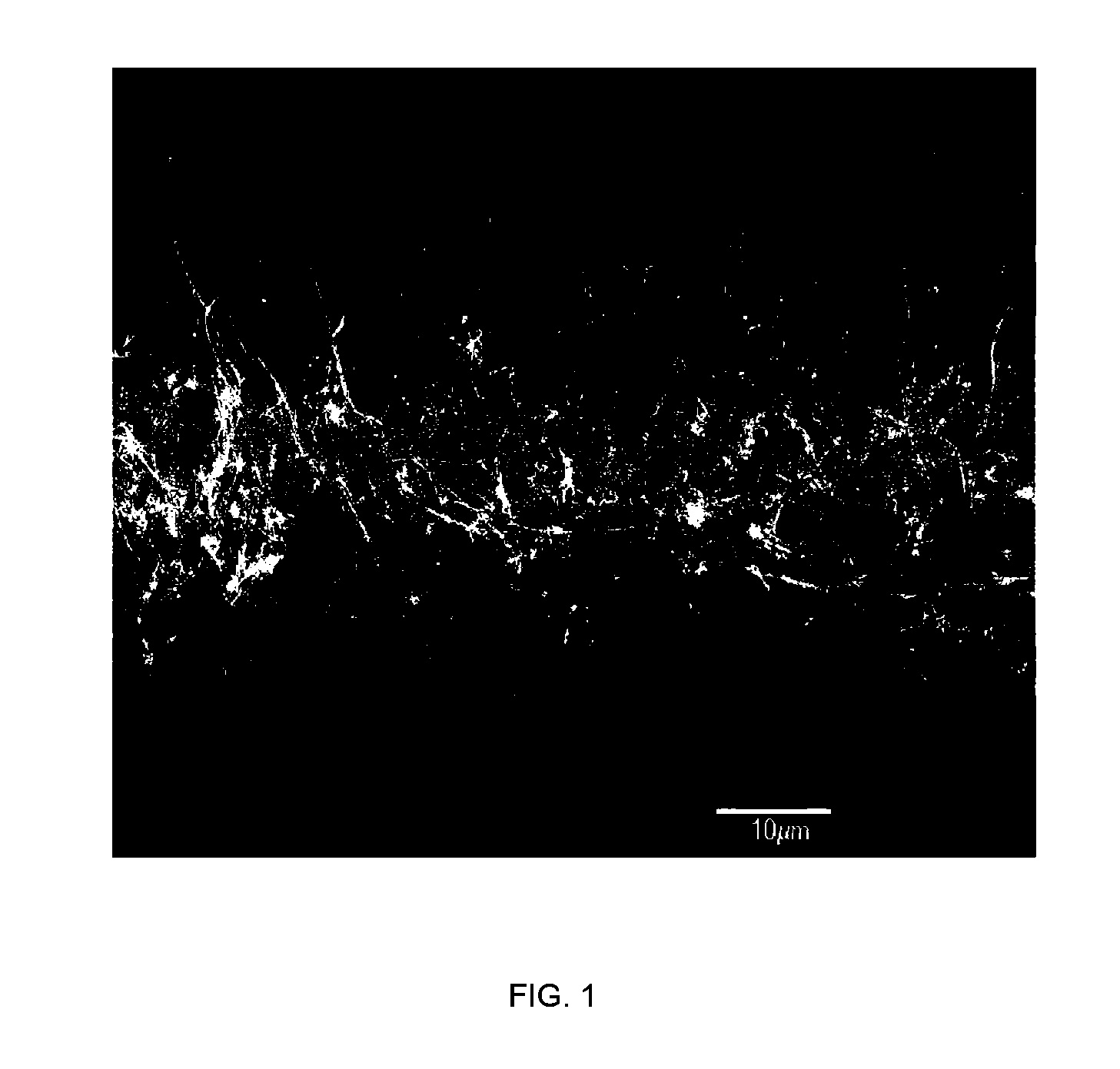
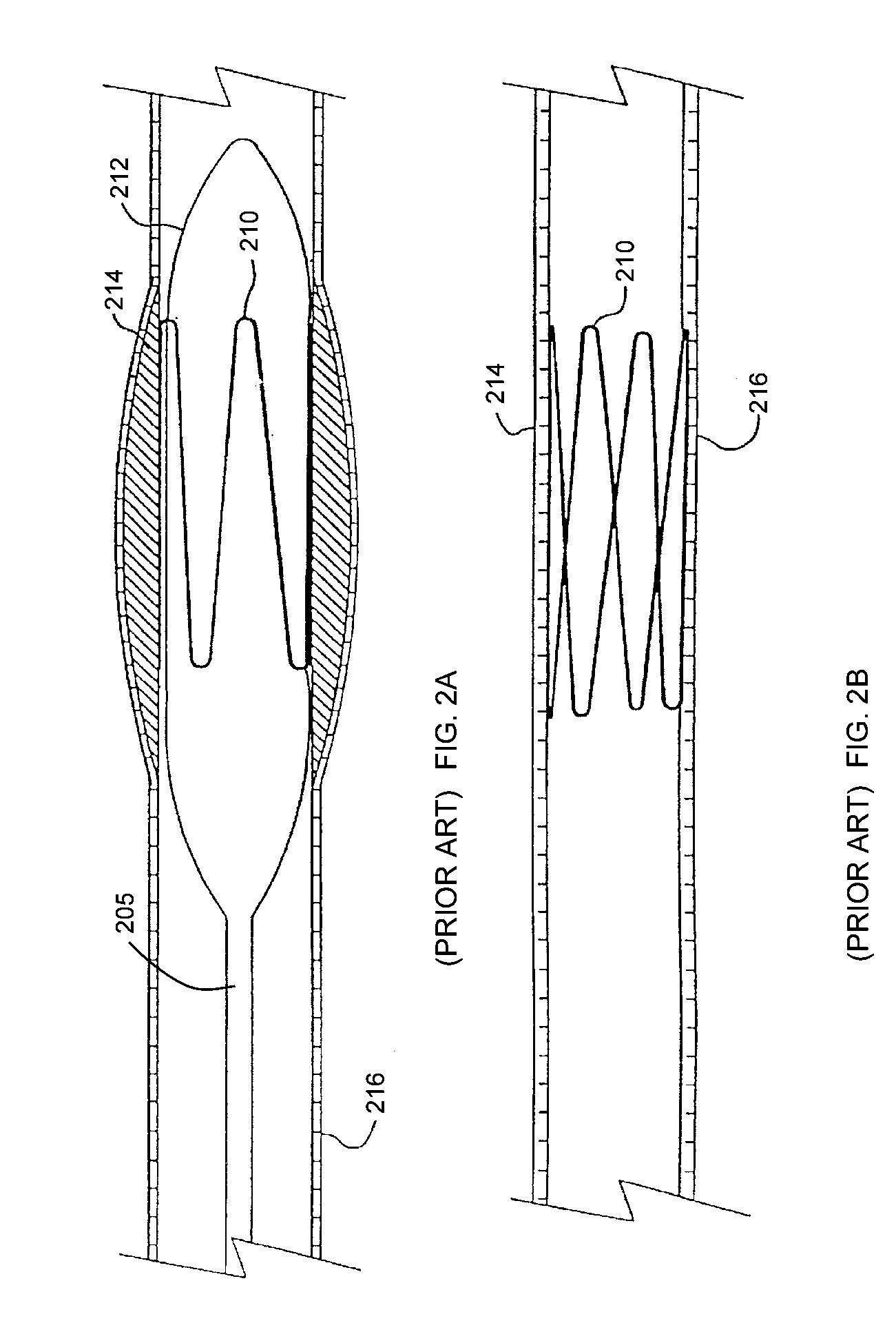
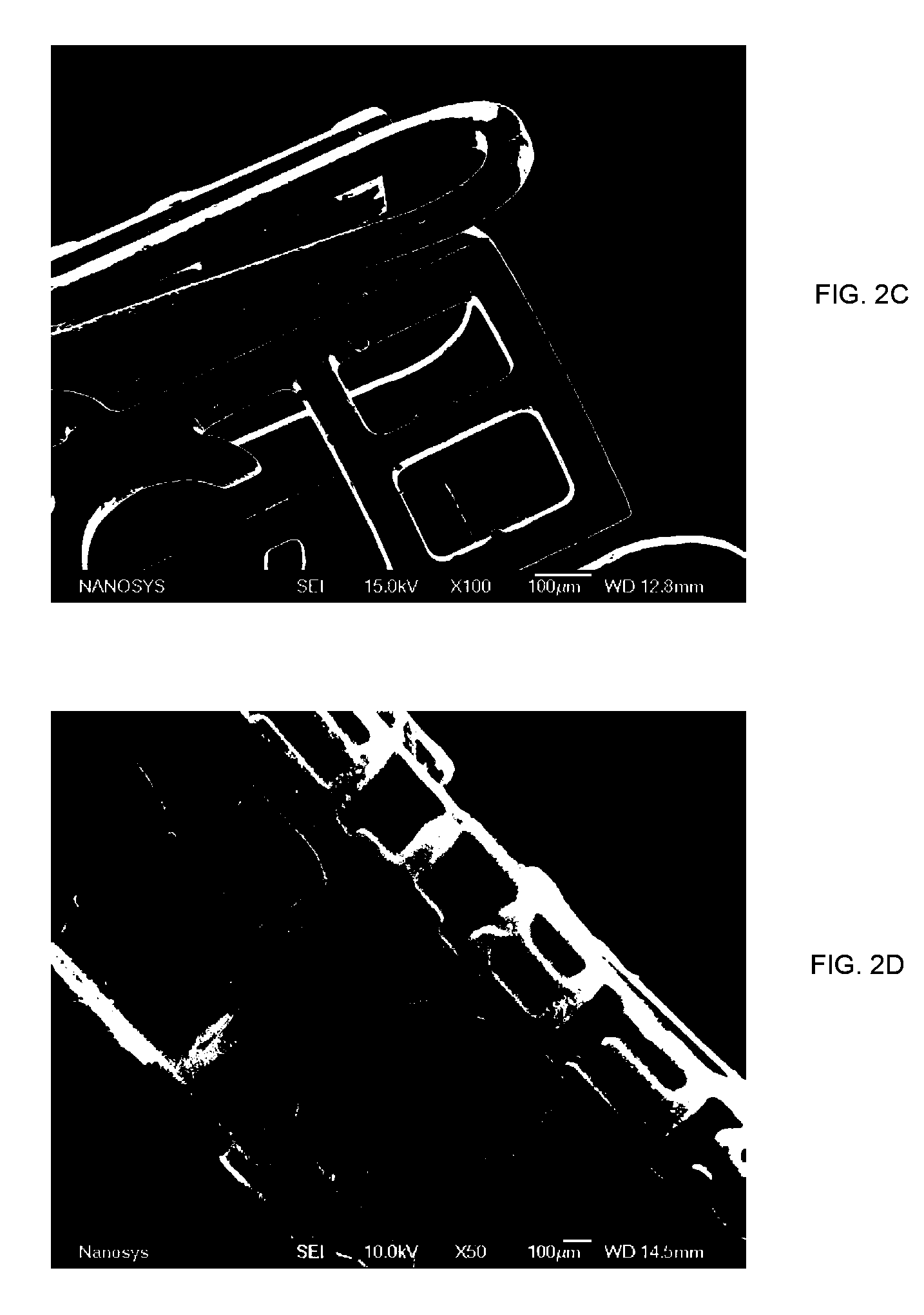
Medical device applications of nanostructured surfaces
PatentInactiveUS7803574B2
Innovation
- Integration of nanostructured surfaces, including nanofibers, nanotubes, and nanoparticles, onto medical devices to enhance biointegration, reduce biofouling, and improve fluid flow, with optional coatings for specific functions like antibacterial properties and drug delivery.
Regulatory Framework for Neoprene in Medical Devices
The regulatory framework for neoprene in medical devices is a complex and evolving landscape that significantly impacts the development, manufacturing, and marketing of medical products. In the United States, the Food and Drug Administration (FDA) plays a central role in overseeing the use of neoprene in medical devices. The FDA classifies medical devices into three categories based on their risk level, with neoprene-containing devices typically falling under Class I or Class II.
For Class I devices, which pose minimal potential harm, manufacturers must adhere to general controls, including good manufacturing practices and proper labeling. Many neoprene-based products, such as compression sleeves or basic wound dressings, fall into this category. These devices often qualify for 510(k) exemption, streamlining their path to market.
Class II devices, which include more complex neoprene applications like specialized wound dressings or orthopedic supports, require additional special controls. Manufacturers must submit a 510(k) premarket notification, demonstrating that their device is substantially equivalent to a legally marketed predicate device. This process involves providing detailed information on the device's design, materials, and intended use, as well as any biocompatibility testing results.
In the European Union, the regulatory framework is governed by the Medical Device Regulation (MDR), which came into full effect in May 2021. The MDR places a stronger emphasis on clinical evidence and post-market surveillance. Neoprene-containing medical devices must comply with the General Safety and Performance Requirements (GSPRs) outlined in the MDR, which include considerations for chemical, physical, and biological properties of materials used.
Globally, the International Organization for Standardization (ISO) provides standards that are widely recognized and often incorporated into regulatory requirements. ISO 10993, which addresses the biological evaluation of medical devices, is particularly relevant for neoprene applications. This standard outlines testing procedures for biocompatibility, including cytotoxicity, sensitization, and irritation tests.
Manufacturers must also consider environmental regulations when working with neoprene. The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation and similar global initiatives may impact the sourcing and use of certain chemicals in neoprene production for medical devices.
As sustainability concerns grow, regulatory bodies are increasingly focusing on the environmental impact of medical devices. This trend may lead to future regulations addressing the lifecycle management of neoprene-containing devices, including disposal and recycling considerations.
The regulatory landscape for neoprene in medical devices continues to evolve, with a growing emphasis on patient safety, product efficacy, and environmental responsibility. Manufacturers must stay abreast of these changes to ensure compliance and maintain market access for their neoprene-based medical innovations.
For Class I devices, which pose minimal potential harm, manufacturers must adhere to general controls, including good manufacturing practices and proper labeling. Many neoprene-based products, such as compression sleeves or basic wound dressings, fall into this category. These devices often qualify for 510(k) exemption, streamlining their path to market.
Class II devices, which include more complex neoprene applications like specialized wound dressings or orthopedic supports, require additional special controls. Manufacturers must submit a 510(k) premarket notification, demonstrating that their device is substantially equivalent to a legally marketed predicate device. This process involves providing detailed information on the device's design, materials, and intended use, as well as any biocompatibility testing results.
In the European Union, the regulatory framework is governed by the Medical Device Regulation (MDR), which came into full effect in May 2021. The MDR places a stronger emphasis on clinical evidence and post-market surveillance. Neoprene-containing medical devices must comply with the General Safety and Performance Requirements (GSPRs) outlined in the MDR, which include considerations for chemical, physical, and biological properties of materials used.
Globally, the International Organization for Standardization (ISO) provides standards that are widely recognized and often incorporated into regulatory requirements. ISO 10993, which addresses the biological evaluation of medical devices, is particularly relevant for neoprene applications. This standard outlines testing procedures for biocompatibility, including cytotoxicity, sensitization, and irritation tests.
Manufacturers must also consider environmental regulations when working with neoprene. The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation and similar global initiatives may impact the sourcing and use of certain chemicals in neoprene production for medical devices.
As sustainability concerns grow, regulatory bodies are increasingly focusing on the environmental impact of medical devices. This trend may lead to future regulations addressing the lifecycle management of neoprene-containing devices, including disposal and recycling considerations.
The regulatory landscape for neoprene in medical devices continues to evolve, with a growing emphasis on patient safety, product efficacy, and environmental responsibility. Manufacturers must stay abreast of these changes to ensure compliance and maintain market access for their neoprene-based medical innovations.
Environmental Impact of Neoprene in Healthcare
The environmental impact of neoprene in healthcare is a growing concern as the medical industry increasingly relies on this synthetic rubber for various applications. Neoprene, also known as polychloroprene, is widely used in medical devices due to its durability, flexibility, and resistance to chemicals and extreme temperatures. However, its production and disposal processes raise significant environmental issues.
The manufacturing of neoprene involves the use of petroleum-based chemicals, which contribute to greenhouse gas emissions and air pollution. The production process releases volatile organic compounds (VOCs) and other harmful substances into the atmosphere, potentially affecting air quality and human health. Additionally, the energy-intensive nature of neoprene production contributes to the overall carbon footprint of medical device manufacturing.
Water pollution is another environmental concern associated with neoprene production. The manufacturing process generates wastewater containing toxic chemicals, which, if not properly treated, can contaminate local water sources and harm aquatic ecosystems. This poses a risk to both wildlife and human communities that rely on these water resources.
The disposal of neoprene-based medical devices presents further environmental challenges. Neoprene is not biodegradable and can persist in landfills for extended periods. When incinerated, it releases harmful chemicals, including hydrogen chloride and dioxins, which are known environmental pollutants and potential carcinogens.
Recycling neoprene is technically possible but often economically unfeasible due to the complex nature of medical devices and the potential contamination with biological materials. This leads to a significant amount of neoprene-containing medical waste being sent to landfills or incineration facilities, exacerbating the environmental impact.
The healthcare industry is increasingly recognizing the need to address these environmental concerns. Some medical device manufacturers are exploring alternative materials with lower environmental impacts, such as bio-based polymers or recycled neoprene. Others are focusing on improving the recyclability of neoprene-containing devices through better design and material separation techniques.
Efforts are also being made to reduce the environmental impact of neoprene production. This includes implementing more efficient manufacturing processes, using renewable energy sources, and developing closed-loop systems to minimize waste and emissions. Some companies are investing in research to develop more environmentally friendly alternatives that maintain the beneficial properties of neoprene for medical applications.
As the healthcare sector continues to prioritize sustainability, the environmental impact of neoprene in medical devices is likely to receive increased scrutiny. This may lead to stricter regulations on neoprene production and disposal, as well as greater incentives for the development of eco-friendly alternatives. The challenge lies in balancing the unique properties that make neoprene valuable in medical applications with the urgent need to reduce its environmental footprint.
The manufacturing of neoprene involves the use of petroleum-based chemicals, which contribute to greenhouse gas emissions and air pollution. The production process releases volatile organic compounds (VOCs) and other harmful substances into the atmosphere, potentially affecting air quality and human health. Additionally, the energy-intensive nature of neoprene production contributes to the overall carbon footprint of medical device manufacturing.
Water pollution is another environmental concern associated with neoprene production. The manufacturing process generates wastewater containing toxic chemicals, which, if not properly treated, can contaminate local water sources and harm aquatic ecosystems. This poses a risk to both wildlife and human communities that rely on these water resources.
The disposal of neoprene-based medical devices presents further environmental challenges. Neoprene is not biodegradable and can persist in landfills for extended periods. When incinerated, it releases harmful chemicals, including hydrogen chloride and dioxins, which are known environmental pollutants and potential carcinogens.
Recycling neoprene is technically possible but often economically unfeasible due to the complex nature of medical devices and the potential contamination with biological materials. This leads to a significant amount of neoprene-containing medical waste being sent to landfills or incineration facilities, exacerbating the environmental impact.
The healthcare industry is increasingly recognizing the need to address these environmental concerns. Some medical device manufacturers are exploring alternative materials with lower environmental impacts, such as bio-based polymers or recycled neoprene. Others are focusing on improving the recyclability of neoprene-containing devices through better design and material separation techniques.
Efforts are also being made to reduce the environmental impact of neoprene production. This includes implementing more efficient manufacturing processes, using renewable energy sources, and developing closed-loop systems to minimize waste and emissions. Some companies are investing in research to develop more environmentally friendly alternatives that maintain the beneficial properties of neoprene for medical applications.
As the healthcare sector continues to prioritize sustainability, the environmental impact of neoprene in medical devices is likely to receive increased scrutiny. This may lead to stricter regulations on neoprene production and disposal, as well as greater incentives for the development of eco-friendly alternatives. The challenge lies in balancing the unique properties that make neoprene valuable in medical applications with the urgent need to reduce its environmental footprint.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!