Optimizing Kaolinite's Role in Carbon Capture Technologies
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Kaolinite Carbon Capture Background and Objectives
Carbon capture and storage (CCS) has emerged as a critical technology in the global effort to mitigate climate change by reducing atmospheric carbon dioxide concentrations. Within this domain, kaolinite, a common clay mineral with the chemical formula Al₂Si₂O₅(OH)₄, has attracted significant attention for its potential role in carbon sequestration processes. The historical development of kaolinite-based carbon capture technologies can be traced back to early geological studies of natural carbon mineralization processes, where researchers observed carbon dioxide naturally binding with various minerals including clay structures.
The evolution of kaolinite applications in carbon capture has accelerated dramatically over the past decade, driven by the urgent need for scalable, cost-effective carbon management solutions. Initially viewed primarily as a structural material or industrial mineral, kaolinite's unique physicochemical properties—including its high surface area, ion exchange capacity, and structural stability—have positioned it as a promising candidate for engineered carbon capture systems.
Recent technological trends indicate a shift toward hybrid approaches that combine kaolinite with other materials to enhance carbon dioxide adsorption capacity and selectivity. These developments align with broader carbon capture technology trajectories that emphasize materials with lower energy penalties and reduced regeneration costs compared to conventional amine-based systems.
The primary technical objectives for optimizing kaolinite's role in carbon capture include: enhancing the CO₂ adsorption capacity through surface modification techniques; improving the kinetics of carbon dioxide capture under various operational conditions; developing cost-effective methods for kaolinite functionalization; and ensuring long-term stability during multiple adsorption-desorption cycles.
Additionally, researchers aim to understand the fundamental mechanisms of CO₂ interaction with kaolinite surfaces at the molecular level, which would enable rational design of next-generation kaolinite-based sorbents. Computational studies using density functional theory and molecular dynamics simulations have begun to elucidate these interactions, providing valuable insights for experimental work.
The technological roadmap for kaolinite in carbon capture applications envisions progression from laboratory-scale demonstrations to pilot implementations and eventually commercial deployment. Current research focuses on overcoming key challenges including relatively low CO₂ uptake compared to some competing materials, sensitivity to moisture, and potential structural degradation during cycling operations.
Ultimately, the goal is to develop kaolinite-based materials that can be integrated into industrial carbon capture systems, offering advantages in terms of abundance, cost, environmental compatibility, and performance stability under real-world conditions.
The evolution of kaolinite applications in carbon capture has accelerated dramatically over the past decade, driven by the urgent need for scalable, cost-effective carbon management solutions. Initially viewed primarily as a structural material or industrial mineral, kaolinite's unique physicochemical properties—including its high surface area, ion exchange capacity, and structural stability—have positioned it as a promising candidate for engineered carbon capture systems.
Recent technological trends indicate a shift toward hybrid approaches that combine kaolinite with other materials to enhance carbon dioxide adsorption capacity and selectivity. These developments align with broader carbon capture technology trajectories that emphasize materials with lower energy penalties and reduced regeneration costs compared to conventional amine-based systems.
The primary technical objectives for optimizing kaolinite's role in carbon capture include: enhancing the CO₂ adsorption capacity through surface modification techniques; improving the kinetics of carbon dioxide capture under various operational conditions; developing cost-effective methods for kaolinite functionalization; and ensuring long-term stability during multiple adsorption-desorption cycles.
Additionally, researchers aim to understand the fundamental mechanisms of CO₂ interaction with kaolinite surfaces at the molecular level, which would enable rational design of next-generation kaolinite-based sorbents. Computational studies using density functional theory and molecular dynamics simulations have begun to elucidate these interactions, providing valuable insights for experimental work.
The technological roadmap for kaolinite in carbon capture applications envisions progression from laboratory-scale demonstrations to pilot implementations and eventually commercial deployment. Current research focuses on overcoming key challenges including relatively low CO₂ uptake compared to some competing materials, sensitivity to moisture, and potential structural degradation during cycling operations.
Ultimately, the goal is to develop kaolinite-based materials that can be integrated into industrial carbon capture systems, offering advantages in terms of abundance, cost, environmental compatibility, and performance stability under real-world conditions.
Market Analysis for Carbon Capture Solutions
The global carbon capture and storage (CCS) market is experiencing significant growth, driven by increasing environmental concerns and regulatory pressures to reduce carbon emissions. As of 2023, the market was valued at approximately $7.5 billion, with projections indicating a compound annual growth rate (CAGR) of 19.2% through 2030, potentially reaching $35.9 billion by the end of the decade. This remarkable growth trajectory underscores the urgent demand for innovative carbon capture solutions.
The industrial sector represents the largest market segment for carbon capture technologies, accounting for roughly 45% of the total market share. Power generation follows closely at 30%, with the remaining market distributed across various applications including transportation and building sectors. Geographically, North America currently leads the market with approximately 40% share, followed by Europe at 30% and Asia-Pacific at 25%, with the latter showing the fastest growth rate.
Kaolinite-based carbon capture solutions are positioned within the emerging materials segment of this market, which currently represents about 15% of total carbon capture technologies but is expected to grow at an above-average rate of 22% annually. The unique properties of kaolinite, including its abundance, low cost, and modifiable surface characteristics, make it particularly attractive for commercial-scale applications.
Market drivers for kaolinite-based carbon capture include increasingly stringent carbon emission regulations across major economies, growing corporate commitments to carbon neutrality, and the expanding carbon credit trading systems. The European Union's Carbon Border Adjustment Mechanism and similar policies in other regions are creating financial incentives for industries to adopt effective carbon capture technologies.
Customer segments showing the highest interest in kaolinite-based solutions include cement manufacturing, steel production, and power generation—industries facing both high carbon taxes and technical challenges in reducing emissions through process modifications alone. These sectors collectively represent a potential addressable market of $12 billion for specialized carbon capture materials.
Competitive analysis reveals that while traditional amine-based carbon capture solutions dominate with approximately 65% market share, novel material-based approaches including metal-organic frameworks (MOFs), zeolites, and clay minerals like kaolinite are gaining traction due to their cost advantages and performance improvements. Kaolinite solutions specifically offer a 30-40% cost reduction compared to conventional technologies, positioning them favorably for price-sensitive market segments.
Market barriers include the need for large-scale demonstration projects, integration challenges with existing industrial infrastructure, and competition from other emerging carbon capture technologies. However, the relatively low raw material costs and scalable production methods for kaolinite-based solutions provide significant competitive advantages that could accelerate market penetration over the next five years.
The industrial sector represents the largest market segment for carbon capture technologies, accounting for roughly 45% of the total market share. Power generation follows closely at 30%, with the remaining market distributed across various applications including transportation and building sectors. Geographically, North America currently leads the market with approximately 40% share, followed by Europe at 30% and Asia-Pacific at 25%, with the latter showing the fastest growth rate.
Kaolinite-based carbon capture solutions are positioned within the emerging materials segment of this market, which currently represents about 15% of total carbon capture technologies but is expected to grow at an above-average rate of 22% annually. The unique properties of kaolinite, including its abundance, low cost, and modifiable surface characteristics, make it particularly attractive for commercial-scale applications.
Market drivers for kaolinite-based carbon capture include increasingly stringent carbon emission regulations across major economies, growing corporate commitments to carbon neutrality, and the expanding carbon credit trading systems. The European Union's Carbon Border Adjustment Mechanism and similar policies in other regions are creating financial incentives for industries to adopt effective carbon capture technologies.
Customer segments showing the highest interest in kaolinite-based solutions include cement manufacturing, steel production, and power generation—industries facing both high carbon taxes and technical challenges in reducing emissions through process modifications alone. These sectors collectively represent a potential addressable market of $12 billion for specialized carbon capture materials.
Competitive analysis reveals that while traditional amine-based carbon capture solutions dominate with approximately 65% market share, novel material-based approaches including metal-organic frameworks (MOFs), zeolites, and clay minerals like kaolinite are gaining traction due to their cost advantages and performance improvements. Kaolinite solutions specifically offer a 30-40% cost reduction compared to conventional technologies, positioning them favorably for price-sensitive market segments.
Market barriers include the need for large-scale demonstration projects, integration challenges with existing industrial infrastructure, and competition from other emerging carbon capture technologies. However, the relatively low raw material costs and scalable production methods for kaolinite-based solutions provide significant competitive advantages that could accelerate market penetration over the next five years.
Current Status and Challenges in Kaolinite-Based Carbon Capture
The global landscape of kaolinite-based carbon capture technologies reveals significant regional disparities in development and implementation. Leading research institutions in North America, particularly in the United States and Canada, have established comprehensive research programs focused on enhancing kaolinite's adsorption capacity through surface modification techniques. Meanwhile, European research centers, especially in Germany and the UK, have concentrated on integrating kaolinite into hybrid carbon capture systems that combine multiple materials for improved efficiency.
In Asia, China has emerged as a dominant force in kaolinite research, leveraging its abundant natural reserves to develop cost-effective carbon capture solutions. Japanese research focuses primarily on precision engineering of kaolinite at the nanoscale to maximize surface area and adsorption sites. Australia, with its significant mining operations, has pioneered industrial-scale applications of kaolinite in carbon capture.
The current technical challenges facing kaolinite-based carbon capture systems are multifaceted. Foremost is the relatively low CO2 adsorption capacity of natural kaolinite compared to other materials such as zeolites or metal-organic frameworks. This limitation necessitates either large quantities of material or significant modification to achieve commercially viable capture rates. Surface area constraints represent another major hurdle, as unmodified kaolinite typically exhibits surface areas of only 10-20 m²/g, substantially lower than competing materials.
Stability issues under industrial conditions pose additional challenges. Kaolinite's performance degrades in high-humidity environments and at elevated temperatures common in flue gas streams. The material also demonstrates relatively slow adsorption kinetics, limiting its effectiveness in rapid-cycle capture systems required for industrial implementation.
Regeneration efficiency remains problematic, with current desorption processes requiring significant energy inputs that reduce the net carbon benefit of the capture system. This energy penalty substantially impacts the economic viability of kaolinite-based solutions. Additionally, scalability concerns persist due to inconsistent quality of natural kaolinite deposits and challenges in standardizing modified materials for industrial deployment.
Recent research has identified promising pathways to address these limitations, including acid activation treatments that can increase surface area by up to 300%, intercalation with organic compounds to enhance interlayer spacing and adsorption capacity, and metal impregnation techniques that introduce new active sites for CO2 binding. Thermal and hydrothermal treatments have shown potential for restructuring kaolinite to create more favorable adsorption properties, while composite formation with complementary materials may overcome inherent limitations of pure kaolinite systems.
In Asia, China has emerged as a dominant force in kaolinite research, leveraging its abundant natural reserves to develop cost-effective carbon capture solutions. Japanese research focuses primarily on precision engineering of kaolinite at the nanoscale to maximize surface area and adsorption sites. Australia, with its significant mining operations, has pioneered industrial-scale applications of kaolinite in carbon capture.
The current technical challenges facing kaolinite-based carbon capture systems are multifaceted. Foremost is the relatively low CO2 adsorption capacity of natural kaolinite compared to other materials such as zeolites or metal-organic frameworks. This limitation necessitates either large quantities of material or significant modification to achieve commercially viable capture rates. Surface area constraints represent another major hurdle, as unmodified kaolinite typically exhibits surface areas of only 10-20 m²/g, substantially lower than competing materials.
Stability issues under industrial conditions pose additional challenges. Kaolinite's performance degrades in high-humidity environments and at elevated temperatures common in flue gas streams. The material also demonstrates relatively slow adsorption kinetics, limiting its effectiveness in rapid-cycle capture systems required for industrial implementation.
Regeneration efficiency remains problematic, with current desorption processes requiring significant energy inputs that reduce the net carbon benefit of the capture system. This energy penalty substantially impacts the economic viability of kaolinite-based solutions. Additionally, scalability concerns persist due to inconsistent quality of natural kaolinite deposits and challenges in standardizing modified materials for industrial deployment.
Recent research has identified promising pathways to address these limitations, including acid activation treatments that can increase surface area by up to 300%, intercalation with organic compounds to enhance interlayer spacing and adsorption capacity, and metal impregnation techniques that introduce new active sites for CO2 binding. Thermal and hydrothermal treatments have shown potential for restructuring kaolinite to create more favorable adsorption properties, while composite formation with complementary materials may overcome inherent limitations of pure kaolinite systems.
Current Kaolinite Modification Techniques
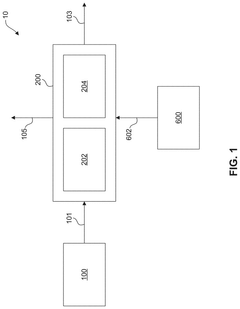
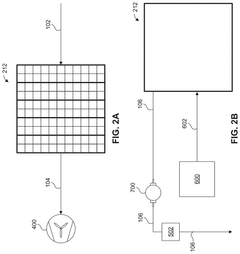
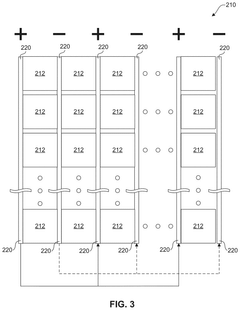
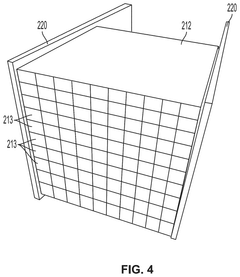
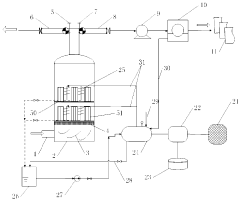
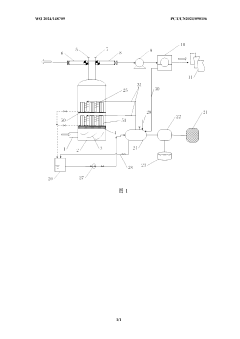
01 Kaolinite-based adsorbents for carbon capture
Kaolinite can be modified to create effective adsorbents for carbon dioxide capture. These modifications enhance the surface area and adsorption capacity of kaolinite, making it more efficient for CO2 capture. Various treatment methods, including thermal activation, acid treatment, and functionalization with amines, can transform kaolinite into a high-performance carbon capture material. The modified kaolinite adsorbents show improved selectivity for CO2 over other gases and can be regenerated for multiple adsorption cycles.- Kaolinite-based adsorbents for carbon capture: Kaolinite can be modified to create effective adsorbents for carbon dioxide capture. These modifications enhance the surface area and adsorption capacity of kaolinite, making it more efficient for CO2 sequestration. Various treatment methods, including thermal activation and chemical modification, can transform raw kaolinite into materials with improved carbon capture properties. These adsorbents offer a cost-effective and environmentally friendly approach to reducing atmospheric CO2 levels.
- Carbon capture systems incorporating kaolinite filters: Carbon capture systems can be designed with kaolinite-based filters to remove CO2 from industrial emissions or ambient air. These systems typically include a filtration unit containing modified kaolinite materials that selectively adsorb carbon dioxide. The integration of kaolinite filters into existing industrial processes provides an efficient method for reducing carbon emissions. These systems can be scaled for various applications, from small-scale air purification to large industrial emission control.
- Functionalized kaolinite composites for enhanced CO2 adsorption: Kaolinite can be functionalized with various compounds to create composite materials with superior carbon capture capabilities. These composites often combine kaolinite with metal oxides, amines, or other functional groups that have high affinity for CO2. The synergistic effect between kaolinite and these additives results in materials with improved selectivity, capacity, and regeneration properties. These functionalized composites represent an advancement in material science for carbon capture applications.
- Regeneration methods for kaolinite-based carbon capture materials: Effective regeneration processes are crucial for the practical application of kaolinite-based carbon capture materials. Various methods have been developed to release captured CO2 and restore the adsorption capacity of kaolinite adsorbents. These include temperature swing adsorption, pressure swing adsorption, and vacuum regeneration techniques. Efficient regeneration processes ensure the economic viability of kaolinite-based carbon capture by enabling multiple adsorption-desorption cycles without significant loss of performance.
- Integration of kaolinite carbon capture with industrial processes: Kaolinite-based carbon capture technologies can be integrated with various industrial processes to achieve carbon neutrality or negative emissions. This integration involves designing systems that capture CO2 directly from industrial exhaust streams using kaolinite-derived materials. Applications include power generation, cement production, and chemical manufacturing. The integration approach offers dual benefits of reducing carbon footprint while potentially utilizing the captured CO2 for other industrial applications, creating a circular economy model.
02 Kaolinite-carbon composite materials for CO2 sequestration
Composite materials combining kaolinite with carbon-based materials such as graphene, carbon nanotubes, or activated carbon show enhanced carbon dioxide capture capabilities. These composites leverage the structural properties of kaolinite and the high surface area of carbon materials to create synergistic effects for CO2 adsorption. The preparation methods typically involve mixing, co-precipitation, or in-situ growth techniques. These composite materials demonstrate improved thermal stability, mechanical strength, and adsorption capacity compared to pure kaolinite.Expand Specific Solutions03 Kaolinite modification with alkaline compounds for carbon capture
Treating kaolinite with alkaline compounds such as sodium hydroxide, potassium hydroxide, or calcium oxide can significantly enhance its carbon dioxide capture performance. The alkaline treatment alters the surface chemistry and pore structure of kaolinite, creating more active sites for CO2 adsorption. This modification approach is relatively simple and cost-effective compared to other methods. The resulting materials show improved CO2 adsorption capacity under various temperature and pressure conditions relevant to industrial carbon capture applications.Expand Specific Solutions04 Kaolinite-based systems for direct air capture of carbon dioxide
Kaolinite can be incorporated into direct air capture (DAC) systems designed to remove carbon dioxide directly from ambient air. These systems typically involve kaolinite that has been modified with specific functional groups or combined with other materials to enhance CO2 selectivity at low concentrations. The kaolinite-based DAC materials can be integrated into various system configurations, including fixed beds, fluidized beds, or membrane contactors. These systems offer potential for negative emissions technology by capturing CO2 from the atmosphere for subsequent storage or utilization.Expand Specific Solutions05 Industrial processes using kaolinite for carbon capture and storage
Industrial-scale processes have been developed to utilize kaolinite for carbon capture and storage applications. These processes involve the integration of kaolinite-based materials into existing industrial systems such as power plants, cement factories, or steel mills. The processes typically include steps for adsorbent preparation, adsorption, regeneration, and CO2 compression for storage. Various reactor designs and process configurations have been optimized to maximize carbon capture efficiency while minimizing energy penalties. These industrial applications demonstrate the practical feasibility of using kaolinite-based materials for reducing carbon emissions from large point sources.Expand Specific Solutions
Leading Organizations in Carbon Capture Research
The carbon capture technology market utilizing kaolinite is in an early growth phase, with increasing interest due to global decarbonization efforts. The market size is expanding rapidly, projected to reach significant scale as carbon pricing mechanisms mature. Technologically, the field shows varying maturity levels across players. Research institutions like Korea Institute of Energy Research, China University of Geosciences, and Arizona State University are advancing fundamental science, while companies demonstrate different commercialization stages. Noya PBC and Cambridge Carbon Capture are developing proprietary direct air capture systems, while established chemical companies like BASF and DuPont bring industrial scaling expertise. China-Africa Kaolin and Anpeak Specialty Minerals contribute raw material expertise, creating a diverse competitive landscape poised for significant development as carbon capture becomes increasingly critical to climate strategies.
Noya PBC
Technical Solution: Noya has developed a direct air capture (DAC) system that incorporates modified kaolinite as a key component in their carbon capture media. Their approach uses kaolinite that has been thermally and chemically treated to create a hierarchical porous structure with enhanced CO2 adsorption properties. The technology employs a cyclic process where ambient air passes through kaolinite-based sorbent beds that selectively capture CO2. Once saturated, the sorbent undergoes a regeneration phase using low-temperature heat (approximately 80-100°C), which releases concentrated CO2 for subsequent utilization or storage. Noya's innovation includes a proprietary surface modification technique that grafts amine-functional groups onto kaolinite's surface, significantly increasing CO2 binding capacity while maintaining the clay's structural integrity and low cost advantage. Their modular system design allows for distributed deployment near renewable energy sources or waste heat streams, optimizing energy efficiency and operational costs[3].
Strengths: Modular and scalable design allows flexible deployment; utilizes low-grade waste heat for regeneration; maintains relatively low material costs by using modified natural clay. Weaknesses: Requires chemical modification processes that add complexity; sorbent degradation over multiple cycles may necessitate periodic replacement; competition from other DAC technologies with higher capture rates.
Cambridge Carbon Capture Ltd.
Technical Solution: Cambridge Carbon Capture has developed a proprietary mineralization technology that utilizes modified kaolinite to enhance carbon capture efficiency. Their process involves chemical activation of kaolinite through a controlled acid treatment that increases its surface area and reactivity with CO2. The technology creates engineered kaolinite structures with optimized pore sizes and functional groups that can adsorb CO2 more effectively than natural clay. Their system operates at near-ambient conditions, reducing energy requirements compared to traditional carbon capture methods. The process converts captured CO2 into stable carbonate minerals, providing permanent sequestration. Cambridge Carbon Capture's approach integrates with industrial processes, capturing emissions directly from flue gases while producing valuable byproducts including silica and metal carbonates that offset operational costs[1].
Strengths: Low energy requirements compared to conventional methods; produces valuable byproducts that improve economic viability; achieves permanent carbon sequestration through mineralization. Weaknesses: Requires specific chemical modifications that add processing steps; performance may vary with flue gas composition; scaling to industrial levels remains challenging.
Key Patents and Innovations in Clay-Based Carbon Capture
Systems and methods for removing carbon dioxide from a fluid
PatentActiveUS20240424440A1
Innovation
- A modular system that integrates carbon dioxide separation and collection, using a reactor with monoliths impregnated with sorbents like metal carbonates or amines, which adsorb and regenerate carbon dioxide efficiently, allowing for easy integration into existing industrial equipment like cooling towers.
Direct air carbon capture and utilization system and method based on mofs adsorbent
PatentWO2024148709A1
Innovation
- A direct air carbon capture system based on MOFs adsorbents is used, combined with a steam generator and a hydrophobic recovery system, and a solar power generation and energy storage system is used to provide heat and electric energy. MOFs adsorbent modules arranged in a matrix are used for carbon adsorption and desorption, and Efficient regeneration of the adsorbent is achieved through the regeneration cooling water removal system.
Environmental Impact Assessment
The environmental implications of integrating kaolinite into carbon capture technologies extend far beyond the immediate technical benefits. When assessing the full lifecycle impact, kaolinite-based carbon capture systems demonstrate significantly lower environmental footprints compared to conventional amine-based technologies. The mining and processing of kaolinite does create localized environmental disturbances, including habitat disruption and potential water quality issues, but these impacts are generally less severe than those associated with manufacturing synthetic sorbents.
Water consumption represents a critical environmental consideration for kaolinite-based carbon capture. While the material itself has relatively low water requirements during operation, the modification processes to enhance its adsorption properties can be water-intensive. Recent innovations in dry modification techniques have reduced water usage by approximately 40-60% compared to traditional wet chemistry approaches, substantially improving the overall water footprint of these technologies.
Energy requirements for regeneration cycles constitute another significant environmental factor. Kaolinite-based sorbents typically require temperatures between 80-120°C for effective CO2 desorption, representing a 15-30% reduction in energy demand compared to amine-based systems that often operate at 120-150°C. This translates to meaningful reductions in indirect carbon emissions associated with the capture process itself.
Land use considerations reveal that kaolinite mining operations, while disruptive, can be effectively remediated through proper site management and reclamation practices. Studies indicate that well-managed kaolinite extraction sites can return to productive ecological function within 7-10 years post-mining, particularly when native vegetation restoration is prioritized during reclamation.
The waste stream profile of kaolinite-based carbon capture systems presents notable advantages. The primary waste consists of spent sorbent material which, being naturally derived, poses fewer disposal challenges than synthetic alternatives. Furthermore, spent kaolinite can potentially be repurposed for applications in construction materials or soil amendments, creating circular economy opportunities that further enhance environmental performance.
Air quality impacts during operation are minimal, with kaolinite systems generating significantly fewer volatile organic compound (VOC) emissions compared to amine-based technologies. This reduction in harmful air pollutants represents an important co-benefit for communities surrounding carbon capture facilities, particularly in urban or industrial settings where air quality concerns are already pronounced.
Water consumption represents a critical environmental consideration for kaolinite-based carbon capture. While the material itself has relatively low water requirements during operation, the modification processes to enhance its adsorption properties can be water-intensive. Recent innovations in dry modification techniques have reduced water usage by approximately 40-60% compared to traditional wet chemistry approaches, substantially improving the overall water footprint of these technologies.
Energy requirements for regeneration cycles constitute another significant environmental factor. Kaolinite-based sorbents typically require temperatures between 80-120°C for effective CO2 desorption, representing a 15-30% reduction in energy demand compared to amine-based systems that often operate at 120-150°C. This translates to meaningful reductions in indirect carbon emissions associated with the capture process itself.
Land use considerations reveal that kaolinite mining operations, while disruptive, can be effectively remediated through proper site management and reclamation practices. Studies indicate that well-managed kaolinite extraction sites can return to productive ecological function within 7-10 years post-mining, particularly when native vegetation restoration is prioritized during reclamation.
The waste stream profile of kaolinite-based carbon capture systems presents notable advantages. The primary waste consists of spent sorbent material which, being naturally derived, poses fewer disposal challenges than synthetic alternatives. Furthermore, spent kaolinite can potentially be repurposed for applications in construction materials or soil amendments, creating circular economy opportunities that further enhance environmental performance.
Air quality impacts during operation are minimal, with kaolinite systems generating significantly fewer volatile organic compound (VOC) emissions compared to amine-based technologies. This reduction in harmful air pollutants represents an important co-benefit for communities surrounding carbon capture facilities, particularly in urban or industrial settings where air quality concerns are already pronounced.
Scalability and Economic Viability
The scalability of kaolinite-based carbon capture technologies represents a critical factor in their potential for widespread implementation. Current laboratory-scale applications demonstrate promising CO2 adsorption capacities, but significant challenges emerge when considering industrial-scale deployment. The primary scalability constraint involves the processing and modification of raw kaolinite to enhance its carbon capture properties, which requires substantial energy inputs and specialized equipment. These requirements create bottlenecks in scaling production to meet global carbon reduction targets.
Economic viability analysis reveals a complex cost structure for kaolinite-based carbon capture systems. While the raw material itself is abundant and relatively inexpensive (approximately $30-50 per ton), the total cost of deployment increases substantially when accounting for processing, installation, and operational expenses. Current estimates place the cost of carbon capture using modified kaolinite adsorbents at $60-90 per ton of CO2 captured, which remains higher than the carbon market price in many regions, creating adoption barriers.
Infrastructure requirements present another significant consideration. Retrofitting existing industrial facilities with kaolinite-based carbon capture systems necessitates substantial capital investment and potential operational disruptions. The integration challenges vary significantly across different industries, with power generation and cement production presenting unique technical hurdles that impact overall economic feasibility.
Supply chain considerations for scaled deployment appear favorable, as kaolinite deposits are geographically distributed across multiple continents, reducing geopolitical supply risks. However, the quality of kaolinite varies considerably between deposits, potentially affecting carbon capture performance and necessitating region-specific processing adjustments. This variability introduces additional complexity to standardized industrial implementation.
Recent economic modeling suggests that economies of scale could potentially reduce costs by 30-40% if production volumes reach megaton levels. Several pilot projects have demonstrated promising pathways to cost reduction through process optimization and integration with existing industrial systems. The development of standardized manufacturing processes for modified kaolinite adsorbents represents a critical step toward improving economic viability.
Regulatory frameworks and carbon pricing mechanisms will significantly influence the economic equation. In regions with carbon prices exceeding $75 per ton, kaolinite-based solutions approach economic viability, particularly when considering their potential integration advantages over alternative technologies. As carbon markets mature and pricing mechanisms evolve, the economic case for scaled deployment is expected to strengthen considerably.
Economic viability analysis reveals a complex cost structure for kaolinite-based carbon capture systems. While the raw material itself is abundant and relatively inexpensive (approximately $30-50 per ton), the total cost of deployment increases substantially when accounting for processing, installation, and operational expenses. Current estimates place the cost of carbon capture using modified kaolinite adsorbents at $60-90 per ton of CO2 captured, which remains higher than the carbon market price in many regions, creating adoption barriers.
Infrastructure requirements present another significant consideration. Retrofitting existing industrial facilities with kaolinite-based carbon capture systems necessitates substantial capital investment and potential operational disruptions. The integration challenges vary significantly across different industries, with power generation and cement production presenting unique technical hurdles that impact overall economic feasibility.
Supply chain considerations for scaled deployment appear favorable, as kaolinite deposits are geographically distributed across multiple continents, reducing geopolitical supply risks. However, the quality of kaolinite varies considerably between deposits, potentially affecting carbon capture performance and necessitating region-specific processing adjustments. This variability introduces additional complexity to standardized industrial implementation.
Recent economic modeling suggests that economies of scale could potentially reduce costs by 30-40% if production volumes reach megaton levels. Several pilot projects have demonstrated promising pathways to cost reduction through process optimization and integration with existing industrial systems. The development of standardized manufacturing processes for modified kaolinite adsorbents represents a critical step toward improving economic viability.
Regulatory frameworks and carbon pricing mechanisms will significantly influence the economic equation. In regions with carbon prices exceeding $75 per ton, kaolinite-based solutions approach economic viability, particularly when considering their potential integration advantages over alternative technologies. As carbon markets mature and pricing mechanisms evolve, the economic case for scaled deployment is expected to strengthen considerably.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!