Photodiode detector improvements for rapid medical diagnostics
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Photodiode Advancements
Photodiode technology has undergone significant advancements in recent years, particularly in the realm of rapid medical diagnostics. These improvements have been driven by the increasing demand for faster, more accurate, and more sensitive detection methods in healthcare applications.
One of the key areas of progress has been in the development of avalanche photodiodes (APDs). These devices offer higher sensitivity and faster response times compared to traditional photodiodes, making them ideal for applications requiring the detection of low-level light signals. APDs achieve this through an internal gain mechanism that amplifies the photocurrent, resulting in improved signal-to-noise ratios and enhanced detection capabilities.
Another notable advancement is the integration of photodiodes with microfluidic devices. This combination has enabled the creation of compact, portable diagnostic systems capable of performing rapid on-site analysis. By incorporating photodiodes directly into microfluidic chips, researchers have developed lab-on-a-chip devices that can detect biomarkers and pathogens with high sensitivity and specificity.
The miniaturization of photodiode arrays has also contributed significantly to the field of medical diagnostics. These arrays allow for simultaneous detection of multiple analytes, enabling multiplexed assays and increasing the throughput of diagnostic tests. The reduced size of these arrays has facilitated their integration into handheld devices, making point-of-care testing more accessible and efficient.
Improvements in materials science have led to the development of novel photodiode structures with enhanced performance characteristics. For instance, the use of III-V semiconductor materials, such as gallium arsenide and indium gallium arsenide, has resulted in photodiodes with extended spectral sensitivity ranges. This expansion of detectable wavelengths has opened up new possibilities for medical imaging and spectroscopic analysis.
Furthermore, advancements in surface modification techniques have allowed for the functionalization of photodiode surfaces with biomolecules. This has enabled the creation of biosensors that can directly detect specific biological targets without the need for additional labeling steps. Such label-free detection methods have the potential to simplify diagnostic procedures and reduce overall test times.
The integration of photodiodes with advanced signal processing algorithms and machine learning techniques has also contributed to improved diagnostic capabilities. These computational approaches enable real-time data analysis, noise reduction, and pattern recognition, enhancing the overall performance and reliability of photodiode-based diagnostic systems.
One of the key areas of progress has been in the development of avalanche photodiodes (APDs). These devices offer higher sensitivity and faster response times compared to traditional photodiodes, making them ideal for applications requiring the detection of low-level light signals. APDs achieve this through an internal gain mechanism that amplifies the photocurrent, resulting in improved signal-to-noise ratios and enhanced detection capabilities.
Another notable advancement is the integration of photodiodes with microfluidic devices. This combination has enabled the creation of compact, portable diagnostic systems capable of performing rapid on-site analysis. By incorporating photodiodes directly into microfluidic chips, researchers have developed lab-on-a-chip devices that can detect biomarkers and pathogens with high sensitivity and specificity.
The miniaturization of photodiode arrays has also contributed significantly to the field of medical diagnostics. These arrays allow for simultaneous detection of multiple analytes, enabling multiplexed assays and increasing the throughput of diagnostic tests. The reduced size of these arrays has facilitated their integration into handheld devices, making point-of-care testing more accessible and efficient.
Improvements in materials science have led to the development of novel photodiode structures with enhanced performance characteristics. For instance, the use of III-V semiconductor materials, such as gallium arsenide and indium gallium arsenide, has resulted in photodiodes with extended spectral sensitivity ranges. This expansion of detectable wavelengths has opened up new possibilities for medical imaging and spectroscopic analysis.
Furthermore, advancements in surface modification techniques have allowed for the functionalization of photodiode surfaces with biomolecules. This has enabled the creation of biosensors that can directly detect specific biological targets without the need for additional labeling steps. Such label-free detection methods have the potential to simplify diagnostic procedures and reduce overall test times.
The integration of photodiodes with advanced signal processing algorithms and machine learning techniques has also contributed to improved diagnostic capabilities. These computational approaches enable real-time data analysis, noise reduction, and pattern recognition, enhancing the overall performance and reliability of photodiode-based diagnostic systems.
Medical Diagnostics Market
The medical diagnostics market has experienced significant growth in recent years, driven by increasing demand for rapid and accurate diagnostic tools. This market encompasses a wide range of products and services, including in vitro diagnostics, imaging systems, and point-of-care testing devices. The global medical diagnostics market was valued at approximately $65 billion in 2020 and is projected to reach $90 billion by 2025, with a compound annual growth rate (CAGR) of 6.5%.
One of the key factors driving market growth is the rising prevalence of chronic and infectious diseases worldwide. As populations age and lifestyle-related health issues become more common, there is a growing need for early and accurate diagnosis to improve patient outcomes and reduce healthcare costs. Additionally, the COVID-19 pandemic has further highlighted the importance of rapid and reliable diagnostic tools in managing public health crises.
Technological advancements in medical diagnostics have played a crucial role in market expansion. Innovations in areas such as molecular diagnostics, next-generation sequencing, and artificial intelligence-powered diagnostic tools have significantly improved the speed, accuracy, and accessibility of diagnostic tests. These advancements have not only enhanced the capabilities of traditional laboratory-based diagnostics but have also enabled the development of portable and user-friendly point-of-care devices.
The market for rapid diagnostic tests, in particular, has seen substantial growth. These tests offer quick results, often within minutes, and can be performed at the point of care, reducing the need for specialized laboratory equipment and personnel. The demand for rapid diagnostics has been further accelerated by the need for quick and widespread testing during the COVID-19 pandemic.
Geographically, North America and Europe currently dominate the medical diagnostics market, owing to well-established healthcare infrastructure, high healthcare expenditure, and early adoption of advanced technologies. However, emerging economies in Asia-Pacific and Latin America are expected to witness the fastest growth in the coming years. This growth is attributed to improving healthcare infrastructure, increasing healthcare awareness, and rising disposable incomes in these regions.
Key players in the medical diagnostics market include multinational corporations such as Roche Diagnostics, Abbott Laboratories, Siemens Healthineers, and Thermo Fisher Scientific. These companies are continuously investing in research and development to introduce innovative diagnostic solutions and maintain their competitive edge in the market. Additionally, numerous startups and smaller companies are entering the market with novel technologies, particularly in the areas of molecular diagnostics and point-of-care testing.
One of the key factors driving market growth is the rising prevalence of chronic and infectious diseases worldwide. As populations age and lifestyle-related health issues become more common, there is a growing need for early and accurate diagnosis to improve patient outcomes and reduce healthcare costs. Additionally, the COVID-19 pandemic has further highlighted the importance of rapid and reliable diagnostic tools in managing public health crises.
Technological advancements in medical diagnostics have played a crucial role in market expansion. Innovations in areas such as molecular diagnostics, next-generation sequencing, and artificial intelligence-powered diagnostic tools have significantly improved the speed, accuracy, and accessibility of diagnostic tests. These advancements have not only enhanced the capabilities of traditional laboratory-based diagnostics but have also enabled the development of portable and user-friendly point-of-care devices.
The market for rapid diagnostic tests, in particular, has seen substantial growth. These tests offer quick results, often within minutes, and can be performed at the point of care, reducing the need for specialized laboratory equipment and personnel. The demand for rapid diagnostics has been further accelerated by the need for quick and widespread testing during the COVID-19 pandemic.
Geographically, North America and Europe currently dominate the medical diagnostics market, owing to well-established healthcare infrastructure, high healthcare expenditure, and early adoption of advanced technologies. However, emerging economies in Asia-Pacific and Latin America are expected to witness the fastest growth in the coming years. This growth is attributed to improving healthcare infrastructure, increasing healthcare awareness, and rising disposable incomes in these regions.
Key players in the medical diagnostics market include multinational corporations such as Roche Diagnostics, Abbott Laboratories, Siemens Healthineers, and Thermo Fisher Scientific. These companies are continuously investing in research and development to introduce innovative diagnostic solutions and maintain their competitive edge in the market. Additionally, numerous startups and smaller companies are entering the market with novel technologies, particularly in the areas of molecular diagnostics and point-of-care testing.
Current Photodiode Limitations
Photodiodes are crucial components in rapid medical diagnostic devices, offering high sensitivity and fast response times. However, current photodiode technology faces several limitations that hinder its optimal performance in medical applications.
One significant limitation is the dark current, which is the small electric current that flows through photodiodes even when no photons are entering the device. This background noise can mask weak signals, reducing the overall sensitivity of the detector. In medical diagnostics, where detecting low concentrations of biomarkers is often critical, this limitation can lead to false negatives or decreased accuracy in test results.
Another challenge is the spectral response range of photodiodes. While silicon-based photodiodes are widely used due to their cost-effectiveness and ease of integration, they have limited sensitivity in the near-infrared and infrared regions. This restriction can be problematic for medical applications that rely on detecting signals in these wavelength ranges, such as blood oxygen saturation measurements or certain types of tissue imaging.
The quantum efficiency of photodiodes also presents a limitation. Not all incident photons generate electron-hole pairs, leading to a less than ideal conversion of light to electrical current. This inefficiency can result in reduced sensitivity, particularly when dealing with low-intensity light signals common in many medical diagnostic scenarios.
Response time is another critical factor that current photodiodes struggle with in rapid diagnostic applications. While photodiodes are generally faster than many other types of light detectors, there is still room for improvement in applications requiring ultra-fast detection, such as time-resolved fluorescence measurements or high-speed imaging in medical diagnostics.
Temperature sensitivity is a further limitation of current photodiode technology. The performance of photodiodes can vary significantly with temperature changes, affecting their dark current, responsivity, and overall reliability. This sensitivity can be particularly problematic in point-of-care diagnostic devices that may be used in various environmental conditions.
Lastly, the physical size of photodiodes can be a limiting factor in the miniaturization of medical diagnostic devices. While progress has been made in reducing photodiode dimensions, further miniaturization is needed to enable more compact and portable diagnostic tools without compromising performance.
Addressing these limitations is crucial for advancing the capabilities of rapid medical diagnostic devices. Improvements in photodiode technology could lead to more sensitive, accurate, and versatile diagnostic tools, ultimately enhancing patient care and medical research outcomes.
One significant limitation is the dark current, which is the small electric current that flows through photodiodes even when no photons are entering the device. This background noise can mask weak signals, reducing the overall sensitivity of the detector. In medical diagnostics, where detecting low concentrations of biomarkers is often critical, this limitation can lead to false negatives or decreased accuracy in test results.
Another challenge is the spectral response range of photodiodes. While silicon-based photodiodes are widely used due to their cost-effectiveness and ease of integration, they have limited sensitivity in the near-infrared and infrared regions. This restriction can be problematic for medical applications that rely on detecting signals in these wavelength ranges, such as blood oxygen saturation measurements or certain types of tissue imaging.
The quantum efficiency of photodiodes also presents a limitation. Not all incident photons generate electron-hole pairs, leading to a less than ideal conversion of light to electrical current. This inefficiency can result in reduced sensitivity, particularly when dealing with low-intensity light signals common in many medical diagnostic scenarios.
Response time is another critical factor that current photodiodes struggle with in rapid diagnostic applications. While photodiodes are generally faster than many other types of light detectors, there is still room for improvement in applications requiring ultra-fast detection, such as time-resolved fluorescence measurements or high-speed imaging in medical diagnostics.
Temperature sensitivity is a further limitation of current photodiode technology. The performance of photodiodes can vary significantly with temperature changes, affecting their dark current, responsivity, and overall reliability. This sensitivity can be particularly problematic in point-of-care diagnostic devices that may be used in various environmental conditions.
Lastly, the physical size of photodiodes can be a limiting factor in the miniaturization of medical diagnostic devices. While progress has been made in reducing photodiode dimensions, further miniaturization is needed to enable more compact and portable diagnostic tools without compromising performance.
Addressing these limitations is crucial for advancing the capabilities of rapid medical diagnostic devices. Improvements in photodiode technology could lead to more sensitive, accurate, and versatile diagnostic tools, ultimately enhancing patient care and medical research outcomes.
Rapid Diagnostic Solutions
01 Improved photodiode structure
Enhancements to the physical structure of photodiodes, including optimized doping profiles, novel materials, and advanced fabrication techniques to improve sensitivity, reduce dark current, and enhance overall performance.- Improved photodiode structure: Enhancements to the physical structure of photodiodes, including optimized doping profiles, novel materials, and advanced fabrication techniques. These improvements aim to increase sensitivity, reduce dark current, and enhance overall performance of the photodetector.
- Integration with readout circuits: Advancements in integrating photodiodes with readout circuits, such as CMOS technology. This integration allows for improved signal processing, reduced noise, and enhanced functionality of the photodetector system.
- Optical enhancements: Incorporation of optical elements and coatings to improve light collection and spectral response of photodiodes. These enhancements may include anti-reflection coatings, light trapping structures, and wavelength-specific filters.
- Noise reduction techniques: Implementation of various noise reduction methods, such as improved shielding, cooling systems, and signal processing algorithms. These techniques aim to enhance the signal-to-noise ratio and overall sensitivity of the photodiode detector.
- Specialized photodiode applications: Development of photodiodes tailored for specific applications, such as high-speed communication, medical imaging, or space exploration. These specialized designs optimize performance for particular wavelengths, operating conditions, or environmental factors.
02 Integration with readout circuits
Advancements in integrating photodiodes with readout circuits, including on-chip amplification, noise reduction techniques, and improved signal processing to enhance detection capabilities and reduce interference.Expand Specific Solutions03 Wavelength-specific improvements
Developments focused on enhancing photodiode performance for specific wavelength ranges, including UV, visible, and IR spectrum, through specialized coatings, filters, and structural modifications.Expand Specific Solutions04 Array and imaging applications
Advancements in photodiode arrays and their application in imaging systems, including improvements in pixel design, cross-talk reduction, and integration with image processing algorithms for enhanced image quality.Expand Specific Solutions05 Packaging and environmental protection
Innovations in photodiode packaging and environmental protection, including hermetic sealing, temperature compensation techniques, and shielding from electromagnetic interference to improve reliability and longevity.Expand Specific Solutions
Key Photodiode Manufacturers
The photodiode detector improvements for rapid medical diagnostics market is in a growth phase, driven by increasing demand for point-of-care testing and personalized medicine. The global market size is projected to reach several billion dollars by 2025. Technologically, the field is advancing rapidly, with companies like Koninklijke Philips, Siemens Healthineers, and HandyLab leading innovation. These firms are developing more sensitive, miniaturized, and cost-effective photodiode detectors, integrating them with microfluidics and AI for faster, more accurate diagnostics. Other players like Samsung Electronics and LG Electronics are leveraging their expertise in consumer electronics to enter this space, potentially disrupting traditional medical device manufacturers.
Koninklijke Philips NV
Technical Solution: Philips has developed advanced photodiode detectors for rapid medical diagnostics, focusing on improving sensitivity and speed. Their technology incorporates silicon photomultipliers (SiPMs) with high photon detection efficiency, enabling single-photon sensitivity[1]. These detectors feature a large active area and fast response time, crucial for time-of-flight PET imaging[2]. Philips has also integrated their photodiode technology into compact, portable diagnostic devices, allowing for point-of-care testing with results comparable to laboratory-based systems[3]. The company's innovations in photodiode design have led to enhanced signal-to-noise ratios and reduced dark current, significantly improving the accuracy of rapid diagnostic tests[4].
Strengths: High sensitivity, fast response time, and integration into portable devices. Weaknesses: Potentially higher cost compared to traditional photodiodes, and complexity in manufacturing process.
Siemens Healthineers AG
Technical Solution: Siemens Healthineers has made significant strides in photodiode detector technology for medical diagnostics. Their approach focuses on enhancing detector efficiency and reducing noise levels. They have developed silicon drift detectors (SDDs) with large active areas and excellent energy resolution, particularly useful in X-ray spectroscopy and rapid medical imaging[5]. Siemens' photodiode improvements include the implementation of advanced readout electronics, which allow for faster signal processing and improved count rates[6]. Additionally, they have integrated their detectors with artificial intelligence algorithms to enhance image quality and diagnostic accuracy in real-time[7]. The company has also explored the use of novel materials and structures to improve quantum efficiency across a wider spectral range.
Strengths: High energy resolution, advanced signal processing, and AI integration. Weaknesses: Potentially higher complexity and cost, may require specialized training for operation.
Innovative Photodiode Designs
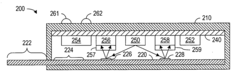
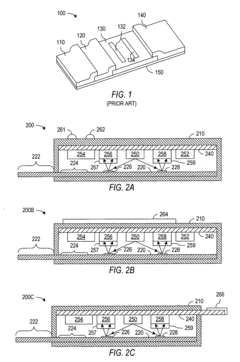
Detection device
PatentWO2024219258A1
Innovation
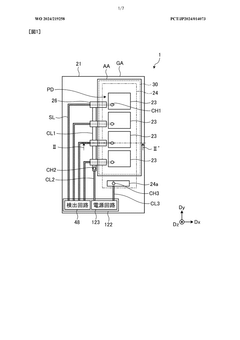
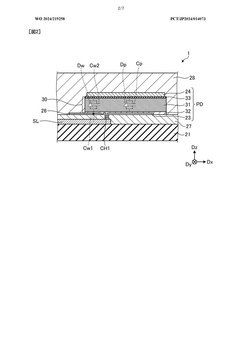
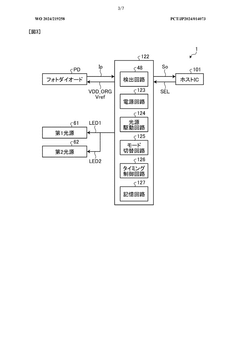
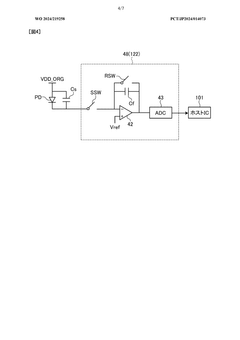
- A detection device is designed with a photodiode structure that includes a lower electrode, a lower buffer layer, an active layer, an upper buffer layer, and an upper electrode, along with a first and second light source, a light source drive circuit, and a detection circuit. The device alternately controls the light sources during readout periods to enhance detection accuracy by utilizing different wavelengths of light for various biological information measurements.
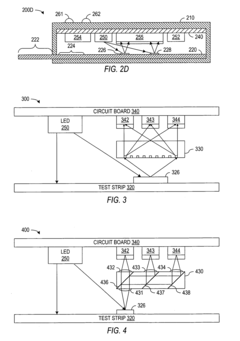
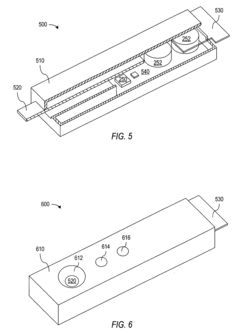
Optoelectronic rapid diagnostic test system
PatentInactiveUS20050221505A1
Innovation
- An optoelectronic rapid diagnostic test system using a light source and photodetectors to illuminate a test structure with semiconductor nanocrystals or quantum dots, which emit light of a characteristic wavelength, allowing for electronic signal generation and processing of test results, reducing the need for expensive equipment and minimizing contamination risks.
Regulatory Compliance
Regulatory compliance is a critical aspect of photodiode detector improvements for rapid medical diagnostics. The development and implementation of these advanced detectors must adhere to stringent regulatory standards to ensure patient safety, device efficacy, and market acceptance. In the United States, the Food and Drug Administration (FDA) oversees the approval process for medical devices, including diagnostic equipment utilizing photodiode detectors. Manufacturers must navigate the complex landscape of FDA regulations, particularly those outlined in the Code of Federal Regulations Title 21, which covers medical devices.
For photodiode detectors used in rapid medical diagnostics, compliance with ISO 13485 standards for quality management systems in medical devices is essential. This international standard ensures that manufacturers maintain consistent quality control throughout the design, development, production, and distribution processes. Additionally, adherence to IEC 60601-1 standards for medical electrical equipment safety is crucial, as photodiode detectors often interface with electronic systems in diagnostic devices.
Electromagnetic compatibility (EMC) regulations, such as those specified in IEC 61326-1, must be considered to prevent interference with other medical equipment. This is particularly important in hospital settings where multiple devices operate in close proximity. Furthermore, biocompatibility testing in accordance with ISO 10993 standards may be necessary if the photodiode detector components come into contact with patient tissues or fluids during the diagnostic process.
Data privacy and security regulations, including HIPAA in the United States and GDPR in Europe, play a significant role when photodiode detectors are integrated into systems that process patient information. Manufacturers must ensure that any data collected or transmitted by the diagnostic equipment is adequately protected and complies with these privacy laws.
As rapid medical diagnostics often involve point-of-care testing, compliance with CLIA (Clinical Laboratory Improvement Amendments) regulations is essential for devices intended for use outside traditional laboratory settings. This includes requirements for test system accuracy, precision, and quality control measures.
Regulatory bodies in other major markets, such as the European Medicines Agency (EMA) in the European Union and the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan, have their own sets of regulations that must be considered for global market access. Manufacturers aiming for international distribution must navigate these diverse regulatory landscapes to ensure compliance across multiple jurisdictions.
Continuous monitoring of regulatory changes and updates is crucial, as standards evolve to keep pace with technological advancements. Manufacturers must maintain robust regulatory affairs departments or partnerships to stay abreast of these changes and adapt their photodiode detector improvements accordingly.
For photodiode detectors used in rapid medical diagnostics, compliance with ISO 13485 standards for quality management systems in medical devices is essential. This international standard ensures that manufacturers maintain consistent quality control throughout the design, development, production, and distribution processes. Additionally, adherence to IEC 60601-1 standards for medical electrical equipment safety is crucial, as photodiode detectors often interface with electronic systems in diagnostic devices.
Electromagnetic compatibility (EMC) regulations, such as those specified in IEC 61326-1, must be considered to prevent interference with other medical equipment. This is particularly important in hospital settings where multiple devices operate in close proximity. Furthermore, biocompatibility testing in accordance with ISO 10993 standards may be necessary if the photodiode detector components come into contact with patient tissues or fluids during the diagnostic process.
Data privacy and security regulations, including HIPAA in the United States and GDPR in Europe, play a significant role when photodiode detectors are integrated into systems that process patient information. Manufacturers must ensure that any data collected or transmitted by the diagnostic equipment is adequately protected and complies with these privacy laws.
As rapid medical diagnostics often involve point-of-care testing, compliance with CLIA (Clinical Laboratory Improvement Amendments) regulations is essential for devices intended for use outside traditional laboratory settings. This includes requirements for test system accuracy, precision, and quality control measures.
Regulatory bodies in other major markets, such as the European Medicines Agency (EMA) in the European Union and the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan, have their own sets of regulations that must be considered for global market access. Manufacturers aiming for international distribution must navigate these diverse regulatory landscapes to ensure compliance across multiple jurisdictions.
Continuous monitoring of regulatory changes and updates is crucial, as standards evolve to keep pace with technological advancements. Manufacturers must maintain robust regulatory affairs departments or partnerships to stay abreast of these changes and adapt their photodiode detector improvements accordingly.
Cost-Effectiveness Analysis
Cost-effectiveness analysis is a crucial aspect of evaluating photodiode detector improvements for rapid medical diagnostics. This analysis aims to determine whether the benefits of implementing advanced photodiode technologies outweigh the associated costs in the context of medical diagnostics.
One of the primary considerations in this analysis is the initial investment required for upgrading existing diagnostic equipment or developing new devices incorporating improved photodiode detectors. This includes costs related to research and development, manufacturing, and integration of the new technology into existing medical systems.
The potential cost savings resulting from improved photodiode detectors must be carefully evaluated. These savings may arise from increased diagnostic accuracy, reduced need for repeat tests, and faster turnaround times. For instance, more sensitive photodiode detectors could lead to earlier disease detection, potentially reducing long-term healthcare costs through timely interventions.
Operational costs associated with the new technology should also be factored into the analysis. This includes maintenance, calibration, and training costs for medical personnel. While advanced photodiode detectors may require more specialized maintenance, they could potentially reduce overall operational costs through improved reliability and longer lifespan.
The impact on patient outcomes is a critical factor in assessing cost-effectiveness. Improved photodiode detectors that enable more accurate and rapid diagnoses can lead to better patient care, reduced hospital stays, and improved quality of life. These benefits, while sometimes challenging to quantify, must be considered in the overall cost-effectiveness equation.
Scalability and adaptability of the improved photodiode technology across different medical diagnostic applications should be evaluated. Technologies that can be easily integrated into a wide range of diagnostic tools may offer better cost-effectiveness due to economies of scale and versatility in clinical settings.
Comparative analysis with alternative diagnostic technologies is essential. The cost-effectiveness of improved photodiode detectors should be benchmarked against other emerging technologies in medical diagnostics to ensure that investment in this particular area yields the best value for healthcare systems.
Long-term cost projections should be considered, taking into account potential advancements in photodiode technology and changes in healthcare needs. This forward-looking approach helps in making informed decisions about the sustainability and long-term value of investing in photodiode detector improvements.
One of the primary considerations in this analysis is the initial investment required for upgrading existing diagnostic equipment or developing new devices incorporating improved photodiode detectors. This includes costs related to research and development, manufacturing, and integration of the new technology into existing medical systems.
The potential cost savings resulting from improved photodiode detectors must be carefully evaluated. These savings may arise from increased diagnostic accuracy, reduced need for repeat tests, and faster turnaround times. For instance, more sensitive photodiode detectors could lead to earlier disease detection, potentially reducing long-term healthcare costs through timely interventions.
Operational costs associated with the new technology should also be factored into the analysis. This includes maintenance, calibration, and training costs for medical personnel. While advanced photodiode detectors may require more specialized maintenance, they could potentially reduce overall operational costs through improved reliability and longer lifespan.
The impact on patient outcomes is a critical factor in assessing cost-effectiveness. Improved photodiode detectors that enable more accurate and rapid diagnoses can lead to better patient care, reduced hospital stays, and improved quality of life. These benefits, while sometimes challenging to quantify, must be considered in the overall cost-effectiveness equation.
Scalability and adaptability of the improved photodiode technology across different medical diagnostic applications should be evaluated. Technologies that can be easily integrated into a wide range of diagnostic tools may offer better cost-effectiveness due to economies of scale and versatility in clinical settings.
Comparative analysis with alternative diagnostic technologies is essential. The cost-effectiveness of improved photodiode detectors should be benchmarked against other emerging technologies in medical diagnostics to ensure that investment in this particular area yields the best value for healthcare systems.
Long-term cost projections should be considered, taking into account potential advancements in photodiode technology and changes in healthcare needs. This forward-looking approach helps in making informed decisions about the sustainability and long-term value of investing in photodiode detector improvements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!