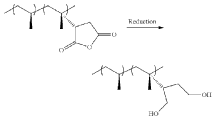
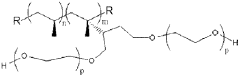
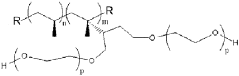
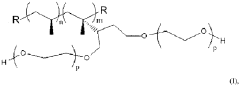
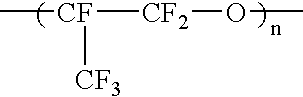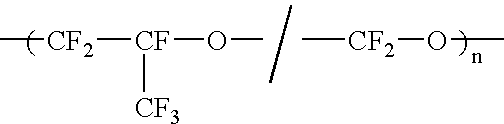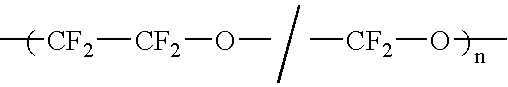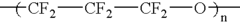Polypropylene’s Contribution to Wearable Medical Device Advancements
JUL 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PP in Wearables: Background and Objectives
Polypropylene (PP) has emerged as a pivotal material in the rapidly evolving field of wearable medical devices. The journey of PP in this domain traces back to its initial applications in disposable medical supplies, gradually expanding into more sophisticated wearable technologies. This evolution has been driven by the increasing demand for lightweight, durable, and biocompatible materials in healthcare applications.
The primary objective of incorporating PP into wearable medical devices is to enhance their performance, comfort, and reliability. PP's unique properties, including its low density, high tensile strength, and excellent chemical resistance, make it an ideal candidate for various components in wearable devices. These characteristics contribute to the development of devices that are not only functional but also comfortable for long-term wear.
In recent years, the wearable medical device market has experienced exponential growth, fueled by advancements in sensor technologies, miniaturization, and the increasing focus on preventive healthcare. PP has played a crucial role in this growth, enabling the creation of flexible, skin-friendly, and moisture-resistant device housings and components. The material's versatility allows for its use in a wide range of applications, from simple fitness trackers to complex medical monitoring systems.
The technological evolution of PP in wearables has been marked by several key milestones. These include the development of specialized PP grades with enhanced properties, such as improved flexibility and biocompatibility, as well as the integration of PP with other materials to create hybrid solutions. Researchers and manufacturers have also focused on optimizing PP's processing techniques to achieve finer details and more complex geometries in wearable device components.
Looking ahead, the objectives for PP in wearable medical devices are multifaceted. There is a strong emphasis on further improving the material's properties to meet the increasingly stringent requirements of medical-grade wearables. This includes enhancing PP's barrier properties to protect sensitive electronic components, improving its antimicrobial characteristics, and developing PP composites that offer better thermal management and electrical conductivity.
Another key objective is to leverage PP's recyclability to address the growing concern of electronic waste in the medical device industry. Researchers are exploring ways to design wearable devices with PP components that can be easily disassembled and recycled, aligning with the principles of circular economy and sustainable healthcare practices.
The primary objective of incorporating PP into wearable medical devices is to enhance their performance, comfort, and reliability. PP's unique properties, including its low density, high tensile strength, and excellent chemical resistance, make it an ideal candidate for various components in wearable devices. These characteristics contribute to the development of devices that are not only functional but also comfortable for long-term wear.
In recent years, the wearable medical device market has experienced exponential growth, fueled by advancements in sensor technologies, miniaturization, and the increasing focus on preventive healthcare. PP has played a crucial role in this growth, enabling the creation of flexible, skin-friendly, and moisture-resistant device housings and components. The material's versatility allows for its use in a wide range of applications, from simple fitness trackers to complex medical monitoring systems.
The technological evolution of PP in wearables has been marked by several key milestones. These include the development of specialized PP grades with enhanced properties, such as improved flexibility and biocompatibility, as well as the integration of PP with other materials to create hybrid solutions. Researchers and manufacturers have also focused on optimizing PP's processing techniques to achieve finer details and more complex geometries in wearable device components.
Looking ahead, the objectives for PP in wearable medical devices are multifaceted. There is a strong emphasis on further improving the material's properties to meet the increasingly stringent requirements of medical-grade wearables. This includes enhancing PP's barrier properties to protect sensitive electronic components, improving its antimicrobial characteristics, and developing PP composites that offer better thermal management and electrical conductivity.
Another key objective is to leverage PP's recyclability to address the growing concern of electronic waste in the medical device industry. Researchers are exploring ways to design wearable devices with PP components that can be easily disassembled and recycled, aligning with the principles of circular economy and sustainable healthcare practices.
Market Analysis for PP-based Medical Wearables
The market for polypropylene-based medical wearables is experiencing significant growth, driven by the increasing demand for lightweight, durable, and cost-effective medical devices. Polypropylene's unique properties, including its chemical resistance, flexibility, and biocompatibility, make it an ideal material for various wearable medical applications. The global market for medical wearables is projected to expand rapidly, with polypropylene playing a crucial role in this growth.
In the healthcare sector, there is a growing trend towards remote patient monitoring and personalized medicine, which has accelerated the adoption of wearable medical devices. Polypropylene-based wearables are particularly well-suited for applications such as continuous glucose monitors, smart bandages, and wearable ECG monitors. These devices offer patients greater autonomy in managing their health conditions while providing healthcare providers with real-time data for more effective treatment decisions.
The aging population in many developed countries is another significant driver for the PP-based medical wearables market. As the elderly population increases, there is a higher demand for devices that can monitor chronic conditions and provide early warning signs of health issues. Polypropylene's lightweight nature and skin-friendly properties make it an excellent choice for creating comfortable, long-term wear devices for this demographic.
In terms of regional market dynamics, North America currently leads in the adoption of PP-based medical wearables, followed closely by Europe. However, the Asia-Pacific region is expected to show the highest growth rate in the coming years, driven by improving healthcare infrastructure, increasing disposable income, and a large patient population.
The COVID-19 pandemic has further accelerated the market for PP-based medical wearables. The need for remote patient monitoring and reducing in-person hospital visits has led to increased interest in wearable devices that can track vital signs and symptoms. This trend is likely to continue even post-pandemic, as healthcare systems recognize the benefits of these technologies in managing patient care more efficiently.
Despite the positive outlook, there are challenges in the market. Regulatory hurdles, data privacy concerns, and the need for seamless integration with existing healthcare systems are factors that manufacturers of PP-based medical wearables must address. Additionally, competition from other materials and the constant pressure to innovate and improve device performance will shape the market landscape in the coming years.
In the healthcare sector, there is a growing trend towards remote patient monitoring and personalized medicine, which has accelerated the adoption of wearable medical devices. Polypropylene-based wearables are particularly well-suited for applications such as continuous glucose monitors, smart bandages, and wearable ECG monitors. These devices offer patients greater autonomy in managing their health conditions while providing healthcare providers with real-time data for more effective treatment decisions.
The aging population in many developed countries is another significant driver for the PP-based medical wearables market. As the elderly population increases, there is a higher demand for devices that can monitor chronic conditions and provide early warning signs of health issues. Polypropylene's lightweight nature and skin-friendly properties make it an excellent choice for creating comfortable, long-term wear devices for this demographic.
In terms of regional market dynamics, North America currently leads in the adoption of PP-based medical wearables, followed closely by Europe. However, the Asia-Pacific region is expected to show the highest growth rate in the coming years, driven by improving healthcare infrastructure, increasing disposable income, and a large patient population.
The COVID-19 pandemic has further accelerated the market for PP-based medical wearables. The need for remote patient monitoring and reducing in-person hospital visits has led to increased interest in wearable devices that can track vital signs and symptoms. This trend is likely to continue even post-pandemic, as healthcare systems recognize the benefits of these technologies in managing patient care more efficiently.
Despite the positive outlook, there are challenges in the market. Regulatory hurdles, data privacy concerns, and the need for seamless integration with existing healthcare systems are factors that manufacturers of PP-based medical wearables must address. Additionally, competition from other materials and the constant pressure to innovate and improve device performance will shape the market landscape in the coming years.
Current PP Tech in Wearables: Challenges
Polypropylene (PP) has emerged as a key material in the development of wearable medical devices, offering a unique combination of properties that make it suitable for various applications. However, the current use of PP in wearables faces several challenges that need to be addressed to fully harness its potential.
One of the primary challenges is the limited flexibility of PP compared to other polymers used in wearable devices. While PP offers excellent durability and chemical resistance, its inherent stiffness can compromise the comfort and conformability of wearable devices, especially those designed for long-term use. This limitation has led researchers to explore various modification techniques, such as blending PP with elastomers or incorporating plasticizers, to enhance its flexibility without significantly compromising its other desirable properties.
Another significant challenge is the relatively poor adhesion properties of PP. This characteristic makes it difficult to bond PP components with other materials commonly used in wearable medical devices, such as sensors, electrodes, or other polymers. The poor adhesion can lead to delamination or separation of layers in multi-component devices, potentially compromising their functionality and reliability. To overcome this, surface modification techniques like plasma treatment or chemical etching are being investigated to improve the adhesion properties of PP.
The hydrophobic nature of PP presents both advantages and challenges in wearable medical applications. While its water-repellent properties can be beneficial for moisture management and device protection, they can also hinder the integration of certain biomedical sensors that require direct contact with bodily fluids. Researchers are exploring ways to selectively modify the surface properties of PP to create hydrophilic regions where necessary, while maintaining its overall hydrophobic characteristics.
Thermal management is another area of concern in PP-based wearable devices. Although PP has good thermal insulation properties, this can lead to heat accumulation in devices worn close to the body for extended periods. This not only affects user comfort but can also impact the performance of heat-sensitive components. Developing PP composites with enhanced thermal conductivity or incorporating heat-dissipating elements into PP-based structures are active areas of research to address this challenge.
Lastly, the recyclability and environmental impact of PP in wearable medical devices pose challenges. While PP is theoretically recyclable, the complex nature of wearable devices, often incorporating multiple materials and electronic components, makes recycling difficult in practice. Developing design strategies that facilitate easy disassembly and material separation, as well as exploring biodegradable PP variants, are crucial steps towards improving the sustainability of PP-based wearable medical devices.
One of the primary challenges is the limited flexibility of PP compared to other polymers used in wearable devices. While PP offers excellent durability and chemical resistance, its inherent stiffness can compromise the comfort and conformability of wearable devices, especially those designed for long-term use. This limitation has led researchers to explore various modification techniques, such as blending PP with elastomers or incorporating plasticizers, to enhance its flexibility without significantly compromising its other desirable properties.
Another significant challenge is the relatively poor adhesion properties of PP. This characteristic makes it difficult to bond PP components with other materials commonly used in wearable medical devices, such as sensors, electrodes, or other polymers. The poor adhesion can lead to delamination or separation of layers in multi-component devices, potentially compromising their functionality and reliability. To overcome this, surface modification techniques like plasma treatment or chemical etching are being investigated to improve the adhesion properties of PP.
The hydrophobic nature of PP presents both advantages and challenges in wearable medical applications. While its water-repellent properties can be beneficial for moisture management and device protection, they can also hinder the integration of certain biomedical sensors that require direct contact with bodily fluids. Researchers are exploring ways to selectively modify the surface properties of PP to create hydrophilic regions where necessary, while maintaining its overall hydrophobic characteristics.
Thermal management is another area of concern in PP-based wearable devices. Although PP has good thermal insulation properties, this can lead to heat accumulation in devices worn close to the body for extended periods. This not only affects user comfort but can also impact the performance of heat-sensitive components. Developing PP composites with enhanced thermal conductivity or incorporating heat-dissipating elements into PP-based structures are active areas of research to address this challenge.
Lastly, the recyclability and environmental impact of PP in wearable medical devices pose challenges. While PP is theoretically recyclable, the complex nature of wearable devices, often incorporating multiple materials and electronic components, makes recycling difficult in practice. Developing design strategies that facilitate easy disassembly and material separation, as well as exploring biodegradable PP variants, are crucial steps towards improving the sustainability of PP-based wearable medical devices.
Existing PP Solutions for Wearable Devices
01 Polypropylene synthesis and production methods
Various methods for synthesizing and producing polypropylene, including catalytic processes and polymerization techniques. These methods aim to improve the efficiency and quality of polypropylene production, resulting in materials with enhanced properties for diverse applications.- Polypropylene composition and manufacturing: Various methods and compositions for manufacturing polypropylene with improved properties are described. These include techniques for controlling molecular weight distribution, enhancing crystallinity, and incorporating additives to achieve desired characteristics such as increased strength, flexibility, or thermal stability.
- Polypropylene blends and copolymers: Development of polypropylene blends and copolymers with other materials to enhance specific properties. This includes creating composite materials, impact-resistant blends, and copolymers with improved processing characteristics or mechanical properties.
- Surface treatment and modification of polypropylene: Techniques for modifying the surface properties of polypropylene, including plasma treatment, chemical etching, and coating applications. These methods aim to improve adhesion, printability, or compatibility with other materials in various applications.
- Polypropylene in packaging and films: Applications of polypropylene in packaging materials and film production, focusing on improvements in barrier properties, sealability, and recyclability. This includes multilayer film structures and oriented polypropylene films for various packaging solutions.
- Recycling and sustainability of polypropylene: Methods for recycling polypropylene and developing more sustainable production processes. This includes chemical recycling techniques, incorporation of recycled content, and development of bio-based polypropylene alternatives to reduce environmental impact.
02 Polypropylene composites and blends
Development of polypropylene-based composites and blends with other materials to enhance specific properties such as strength, durability, or thermal resistance. These combinations create new materials with improved characteristics for specialized applications in various industries.Expand Specific Solutions03 Polypropylene modification techniques
Methods for modifying polypropylene to enhance its properties, including chemical treatments, additives, and surface modifications. These techniques aim to improve characteristics such as adhesion, printability, or compatibility with other materials, expanding the range of applications for polypropylene.Expand Specific Solutions04 Polypropylene in packaging and films
Applications of polypropylene in packaging materials and films, focusing on improvements in barrier properties, strength, and processability. This includes the development of specialized polypropylene grades for food packaging, flexible packaging, and other film applications.Expand Specific Solutions05 Recycling and sustainability of polypropylene
Advancements in polypropylene recycling technologies and the development of more sustainable production methods. This includes improved recycling processes, the use of bio-based feedstocks, and the creation of polypropylene grades with enhanced recyclability to address environmental concerns.Expand Specific Solutions
Key Players in PP-based Wearable Development
The market for polypropylene in wearable medical devices is in a growth phase, driven by increasing demand for lightweight, durable, and biocompatible materials. The global market size is expanding rapidly, with major players like Boston Scientific, Mitsui Chemicals, and LG Chem leading innovation. Technological maturity varies across applications, with companies such as Medtronic Vascular and Cardiac Pacemakers advancing in implantable devices, while others like Liquidia Technologies focus on drug delivery systems. The competitive landscape is diverse, with both established medical device manufacturers and chemical companies vying for market share through R&D and strategic partnerships.
Boston Scientific Scimed, Inc.
Technical Solution: Boston Scientific Scimed has developed advanced polypropylene-based materials for wearable medical devices, focusing on improving biocompatibility and durability. Their proprietary PP blend incorporates nanoparticles to enhance flexibility and reduce inflammatory responses in long-term implantable devices [1]. The company has also pioneered a surface modification technique that allows for better integration of polypropylene with body tissues, reducing the risk of rejection and improving overall device performance [3]. Their latest innovation involves a multi-layer PP structure that enables controlled drug release in wearable drug delivery systems, potentially revolutionizing patient care for chronic conditions [5].
Strengths: Excellent biocompatibility, enhanced durability, and innovative drug delivery capabilities. Weaknesses: Higher production costs and potential regulatory hurdles for novel materials.
Mitsui Chemicals, Inc.
Technical Solution: Mitsui Chemicals has developed a high-performance polypropylene grade specifically for wearable medical devices. Their PRIME POLYPRO™ series features enhanced flexibility and impact resistance, making it ideal for devices that need to conform to body contours [2]. The company has also introduced a proprietary additive technology that imparts antimicrobial properties to their PP, addressing concerns about infection in wearable devices [4]. Mitsui's latest innovation is a breathable PP film that allows for moisture management in skin-contact applications, improving patient comfort in long-term use scenarios [6]. Additionally, they have developed a PP compound with improved barrier properties, essential for protecting sensitive electronic components in wearable devices [8].
Strengths: Versatile PP grades with tailored properties for medical applications, strong focus on patient comfort and safety. Weaknesses: May face competition from alternative materials in certain niche applications.
Innovative PP Formulations for Medical Wearables
Enhancing bond strength of medical devices
PatentWO2018057873A1
Innovation
- Incorporating a polypropylene-poly(ethylene oxide) amphiphilic graft copolymer (PP-g-PEO) into the base polymeric formulation of medical devices, specifically in the range of 0.01 to 5.0% by weight, enhances the bonding strength between the tubing and connectors by acting as a chemical bridge between immiscible polymers during the solvent bonding process.
Liquid perfluoropolymers and medical applications incorporating same
PatentInactiveUS20050142315A1
Innovation
- The use of liquid curable perfluoropolyether (PFPE) materials for coatings, sealants, and structural parts in medical applications, which do not swell or shrink with solvents, have low surface energy for high lubricity, and are oxygen permeable and bacterial impermeable, allowing for their use in various medical devices and procedures.
Regulatory Compliance for PP Medical Wearables
The regulatory landscape for polypropylene (PP) in wearable medical devices is complex and evolving, requiring manufacturers to navigate a multifaceted compliance framework. In the United States, the Food and Drug Administration (FDA) oversees the regulation of medical devices, including those incorporating PP materials. Manufacturers must adhere to the FDA's Quality System Regulation (QSR) and obtain premarket approval or clearance, depending on the device classification.
For PP-based wearable medical devices, biocompatibility testing is crucial to ensure patient safety. ISO 10993 standards provide guidelines for evaluating the biological safety of medical devices, including those made with PP. Manufacturers must conduct thorough testing to assess potential cytotoxicity, sensitization, and irritation effects of PP components in direct contact with the skin.
In the European Union, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) govern the approval and marketing of medical devices. These regulations emphasize the importance of risk management throughout the product lifecycle. For PP wearables, manufacturers must demonstrate compliance with essential requirements, including safety, performance, and risk mitigation strategies specific to the material properties of polypropylene.
Environmental considerations also play a role in regulatory compliance for PP medical wearables. The European Union's Restriction of Hazardous Substances (RoHS) Directive and Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation impact the use of certain substances in medical devices. Manufacturers must ensure that PP components and any additives comply with these regulations to maintain market access.
Data protection and privacy regulations, such as the General Data Protection Regulation (GDPR) in the EU and the Health Insurance Portability and Accountability Act (HIPAA) in the US, are increasingly relevant for PP-based wearable medical devices that collect and transmit patient data. Manufacturers must implement robust data security measures and ensure compliance with these regulations to protect sensitive health information.
As wearable medical devices become more sophisticated, regulatory bodies are adapting their approaches to keep pace with technological advancements. The FDA's Digital Health Software Precertification (Pre-Cert) Program, for instance, aims to streamline the review process for software-driven medical devices, which may include PP-based wearables with integrated digital components.
Manufacturers of PP medical wearables must also consider international harmonization efforts, such as the Medical Device Single Audit Program (MDSAP), which allows for a single regulatory audit to satisfy the requirements of multiple regulatory jurisdictions. This program can significantly reduce the regulatory burden for companies operating in multiple markets.
For PP-based wearable medical devices, biocompatibility testing is crucial to ensure patient safety. ISO 10993 standards provide guidelines for evaluating the biological safety of medical devices, including those made with PP. Manufacturers must conduct thorough testing to assess potential cytotoxicity, sensitization, and irritation effects of PP components in direct contact with the skin.
In the European Union, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) govern the approval and marketing of medical devices. These regulations emphasize the importance of risk management throughout the product lifecycle. For PP wearables, manufacturers must demonstrate compliance with essential requirements, including safety, performance, and risk mitigation strategies specific to the material properties of polypropylene.
Environmental considerations also play a role in regulatory compliance for PP medical wearables. The European Union's Restriction of Hazardous Substances (RoHS) Directive and Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation impact the use of certain substances in medical devices. Manufacturers must ensure that PP components and any additives comply with these regulations to maintain market access.
Data protection and privacy regulations, such as the General Data Protection Regulation (GDPR) in the EU and the Health Insurance Portability and Accountability Act (HIPAA) in the US, are increasingly relevant for PP-based wearable medical devices that collect and transmit patient data. Manufacturers must implement robust data security measures and ensure compliance with these regulations to protect sensitive health information.
As wearable medical devices become more sophisticated, regulatory bodies are adapting their approaches to keep pace with technological advancements. The FDA's Digital Health Software Precertification (Pre-Cert) Program, for instance, aims to streamline the review process for software-driven medical devices, which may include PP-based wearables with integrated digital components.
Manufacturers of PP medical wearables must also consider international harmonization efforts, such as the Medical Device Single Audit Program (MDSAP), which allows for a single regulatory audit to satisfy the requirements of multiple regulatory jurisdictions. This program can significantly reduce the regulatory burden for companies operating in multiple markets.
Sustainability in PP-based Wearable Devices
Sustainability in PP-based wearable devices has become a critical focus as the medical industry seeks to balance technological advancements with environmental responsibility. Polypropylene (PP), a versatile thermoplastic polymer, has been instrumental in the development of wearable medical devices due to its excellent properties. However, the increasing demand for these devices has raised concerns about their environmental impact.
One of the primary sustainability challenges in PP-based wearable devices is the end-of-life management. As these devices often contain electronic components and mixed materials, recycling becomes complex. To address this, manufacturers are exploring modular designs that allow for easier disassembly and material separation. This approach not only facilitates recycling but also extends the lifespan of devices by enabling component upgrades.
The production process of PP-based wearable devices is another area where sustainability improvements are being made. Energy-efficient manufacturing techniques, such as low-temperature molding and 3D printing, are being adopted to reduce the carbon footprint of production. Additionally, some companies are investing in renewable energy sources to power their manufacturing facilities, further minimizing environmental impact.
Material innovation plays a crucial role in enhancing the sustainability of PP-based wearable devices. Researchers are developing bio-based polypropylene alternatives derived from renewable resources like corn or sugarcane. These materials offer similar properties to traditional PP while reducing reliance on fossil fuels. Furthermore, efforts are being made to incorporate recycled PP into new devices, creating a circular economy for plastic materials.
Durability and longevity are key factors in the sustainability of wearable medical devices. PP's inherent resistance to chemicals, moisture, and wear makes it an ideal material for creating devices that can withstand frequent use and cleaning. Manufacturers are focusing on enhancing these properties through surface treatments and composite formulations, ensuring that devices remain functional for extended periods, thereby reducing waste.
The medical industry is also exploring the concept of "green electronics" for PP-based wearable devices. This involves the use of biodegradable or easily recyclable electronic components, as well as the development of low-power consumption technologies. These innovations not only reduce the environmental impact of electronic waste but also extend battery life, enhancing the overall sustainability of the devices.
As sustainability becomes increasingly important in healthcare, regulatory bodies are implementing stricter guidelines for the environmental impact of medical devices. This has prompted manufacturers to adopt life cycle assessment (LCA) methodologies to evaluate and improve the sustainability of their PP-based wearable devices throughout their entire life cycle, from raw material extraction to disposal.
One of the primary sustainability challenges in PP-based wearable devices is the end-of-life management. As these devices often contain electronic components and mixed materials, recycling becomes complex. To address this, manufacturers are exploring modular designs that allow for easier disassembly and material separation. This approach not only facilitates recycling but also extends the lifespan of devices by enabling component upgrades.
The production process of PP-based wearable devices is another area where sustainability improvements are being made. Energy-efficient manufacturing techniques, such as low-temperature molding and 3D printing, are being adopted to reduce the carbon footprint of production. Additionally, some companies are investing in renewable energy sources to power their manufacturing facilities, further minimizing environmental impact.
Material innovation plays a crucial role in enhancing the sustainability of PP-based wearable devices. Researchers are developing bio-based polypropylene alternatives derived from renewable resources like corn or sugarcane. These materials offer similar properties to traditional PP while reducing reliance on fossil fuels. Furthermore, efforts are being made to incorporate recycled PP into new devices, creating a circular economy for plastic materials.
Durability and longevity are key factors in the sustainability of wearable medical devices. PP's inherent resistance to chemicals, moisture, and wear makes it an ideal material for creating devices that can withstand frequent use and cleaning. Manufacturers are focusing on enhancing these properties through surface treatments and composite formulations, ensuring that devices remain functional for extended periods, thereby reducing waste.
The medical industry is also exploring the concept of "green electronics" for PP-based wearable devices. This involves the use of biodegradable or easily recyclable electronic components, as well as the development of low-power consumption technologies. These innovations not only reduce the environmental impact of electronic waste but also extend battery life, enhancing the overall sustainability of the devices.
As sustainability becomes increasingly important in healthcare, regulatory bodies are implementing stricter guidelines for the environmental impact of medical devices. This has prompted manufacturers to adopt life cycle assessment (LCA) methodologies to evaluate and improve the sustainability of their PP-based wearable devices throughout their entire life cycle, from raw material extraction to disposal.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!