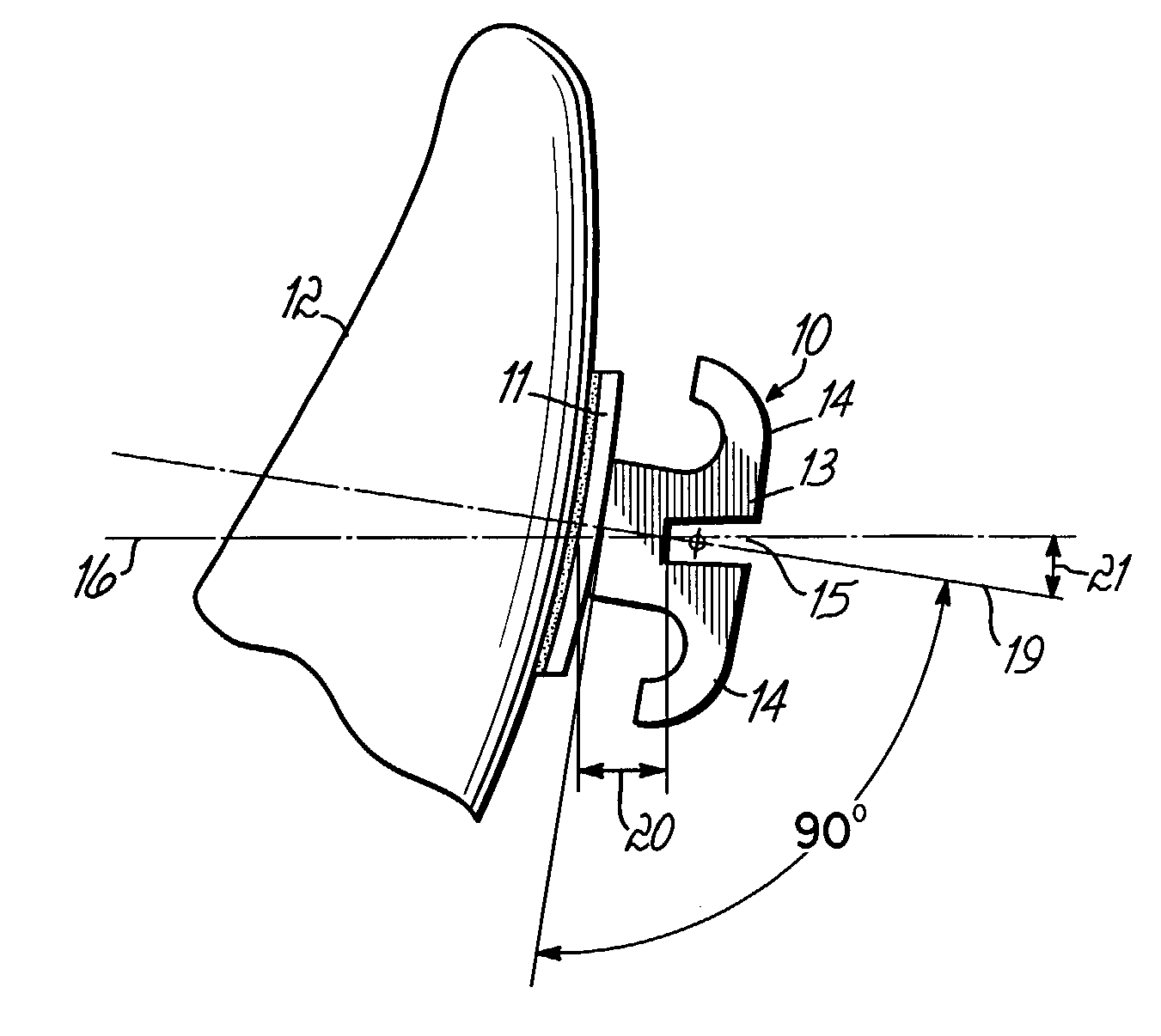
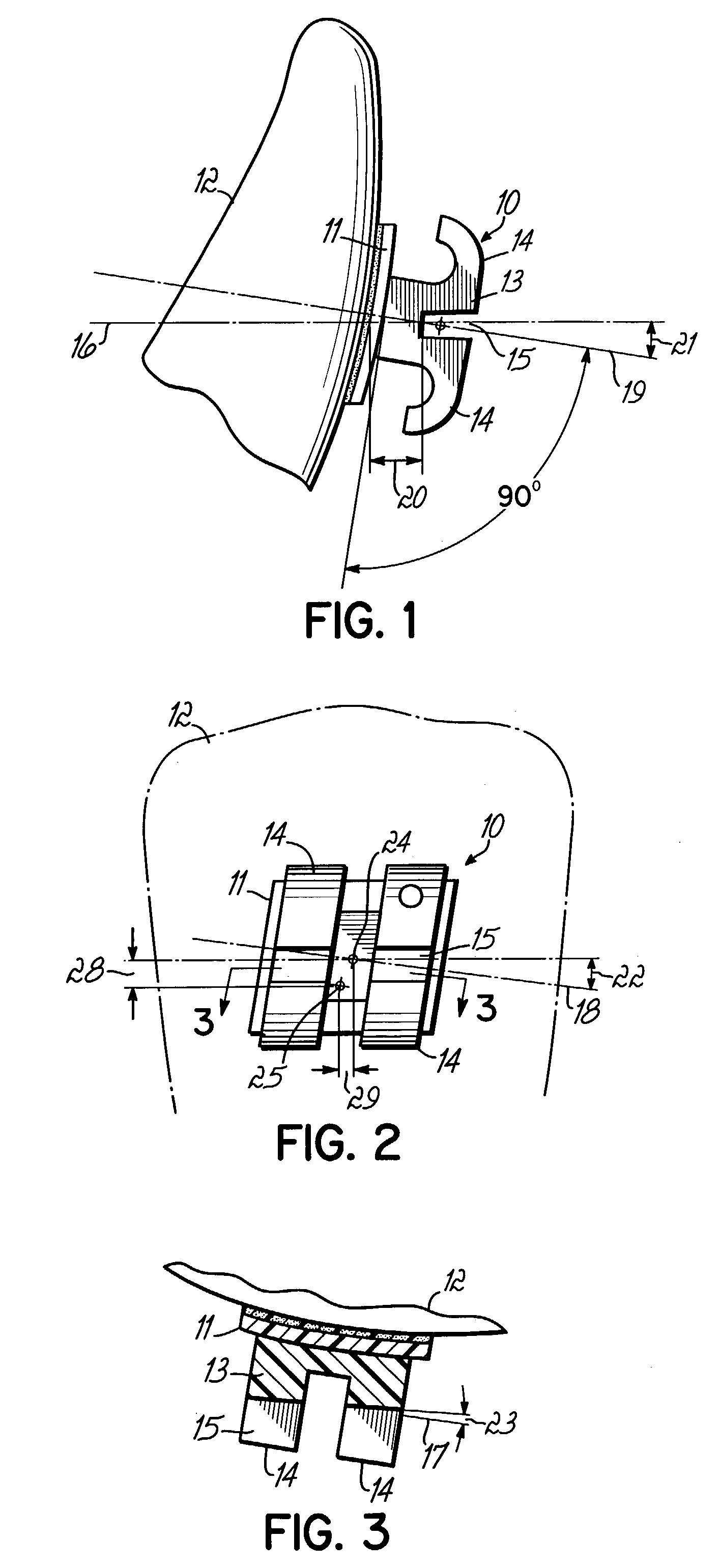
Accura 25 in the Development of Custom Orthotics
JUL 8, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Accura 25 Background and Objectives
Accura 25 is a photopolymer resin developed by 3D Systems, specifically designed for use in stereolithography (SLA) 3D printing processes. This material has gained significant attention in the field of custom orthotics development due to its unique properties and versatility. The evolution of Accura 25 can be traced back to the early 2000s when 3D Systems began exploring advanced materials for rapid prototyping and manufacturing applications.
The primary objective of researching Accura 25 in the context of custom orthotics is to leverage its exceptional characteristics to enhance the production of personalized foot supports. Custom orthotics have traditionally been manufactured using labor-intensive methods, often resulting in inconsistencies and limited customization options. The integration of Accura 25 and 3D printing technology aims to revolutionize this process, offering improved accuracy, repeatability, and patient-specific solutions.
Accura 25 exhibits several key properties that make it particularly suitable for orthotic applications. Its high tensile strength and elongation at break provide the necessary durability to withstand the repeated stress of daily use. The material's low viscosity allows for intricate detail reproduction, essential for capturing the unique contours of a patient's foot. Additionally, Accura 25 demonstrates excellent dimensional stability, ensuring that the final orthotic device maintains its shape and functionality over time.
The development trajectory of Accura 25 has been closely aligned with advancements in 3D printing technology. As SLA printers have become more sophisticated, capable of higher resolutions and faster print speeds, the formulation of Accura 25 has been refined to optimize its performance in these advanced systems. This synergistic evolution has opened up new possibilities in the design and manufacture of custom orthotics, allowing for complex geometries and internal structures that were previously impossible to produce.
One of the primary goals in researching Accura 25 for custom orthotics is to establish a standardized workflow that seamlessly integrates 3D scanning, computer-aided design (CAD), and 3D printing. This end-to-end digital process aims to reduce production time, minimize material waste, and improve the overall fit and effectiveness of orthotic devices. By harnessing the capabilities of Accura 25, researchers and manufacturers seek to create orthotics that not only provide superior support and comfort but also offer enhanced customization options to address specific patient needs.
The ongoing research into Accura 25 for custom orthotics also focuses on exploring its potential for creating dynamic and responsive designs. This includes investigating the material's behavior under various loading conditions and its ability to incorporate features such as variable stiffness zones or energy-return mechanisms. As the understanding of Accura 25's properties deepens, the goal is to push the boundaries of orthotic design, potentially leading to innovative solutions that can adapt to changing patient requirements or environmental conditions.
The primary objective of researching Accura 25 in the context of custom orthotics is to leverage its exceptional characteristics to enhance the production of personalized foot supports. Custom orthotics have traditionally been manufactured using labor-intensive methods, often resulting in inconsistencies and limited customization options. The integration of Accura 25 and 3D printing technology aims to revolutionize this process, offering improved accuracy, repeatability, and patient-specific solutions.
Accura 25 exhibits several key properties that make it particularly suitable for orthotic applications. Its high tensile strength and elongation at break provide the necessary durability to withstand the repeated stress of daily use. The material's low viscosity allows for intricate detail reproduction, essential for capturing the unique contours of a patient's foot. Additionally, Accura 25 demonstrates excellent dimensional stability, ensuring that the final orthotic device maintains its shape and functionality over time.
The development trajectory of Accura 25 has been closely aligned with advancements in 3D printing technology. As SLA printers have become more sophisticated, capable of higher resolutions and faster print speeds, the formulation of Accura 25 has been refined to optimize its performance in these advanced systems. This synergistic evolution has opened up new possibilities in the design and manufacture of custom orthotics, allowing for complex geometries and internal structures that were previously impossible to produce.
One of the primary goals in researching Accura 25 for custom orthotics is to establish a standardized workflow that seamlessly integrates 3D scanning, computer-aided design (CAD), and 3D printing. This end-to-end digital process aims to reduce production time, minimize material waste, and improve the overall fit and effectiveness of orthotic devices. By harnessing the capabilities of Accura 25, researchers and manufacturers seek to create orthotics that not only provide superior support and comfort but also offer enhanced customization options to address specific patient needs.
The ongoing research into Accura 25 for custom orthotics also focuses on exploring its potential for creating dynamic and responsive designs. This includes investigating the material's behavior under various loading conditions and its ability to incorporate features such as variable stiffness zones or energy-return mechanisms. As the understanding of Accura 25's properties deepens, the goal is to push the boundaries of orthotic design, potentially leading to innovative solutions that can adapt to changing patient requirements or environmental conditions.
Market Analysis for Custom Orthotics
The custom orthotics market has experienced significant growth in recent years, driven by increasing awareness of foot health, rising prevalence of foot-related disorders, and advancements in manufacturing technologies. The global custom orthotics market size was valued at approximately $3.5 billion in 2020 and is projected to reach $5.2 billion by 2027, growing at a CAGR of 6.2% during the forecast period.
Several factors contribute to the expanding market demand for custom orthotics. The aging population, particularly in developed countries, is a key driver as older individuals are more prone to foot problems and require specialized orthotic solutions. Additionally, the growing incidence of diabetes and obesity has led to an increased need for custom orthotics to address associated foot complications.
The sports and athletics segment represents a substantial portion of the custom orthotics market. Professional athletes and fitness enthusiasts are increasingly recognizing the importance of proper foot support in enhancing performance and preventing injuries. This has led to a surge in demand for sport-specific custom orthotics designed to optimize biomechanics and reduce the risk of overuse injuries.
In terms of distribution channels, the market is segmented into hospitals, clinics, online platforms, and specialty stores. While traditional brick-and-mortar outlets still dominate, there is a notable shift towards online sales channels, especially in the wake of the COVID-19 pandemic. E-commerce platforms offering virtual consultations and at-home fitting solutions are gaining traction among consumers seeking convenience and personalized care.
Geographically, North America holds the largest market share, followed by Europe and Asia-Pacific. The United States, in particular, is a key market due to its well-established healthcare infrastructure and high consumer awareness. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness the fastest growth rates in the coming years, driven by improving healthcare access and rising disposable incomes.
The custom orthotics market is characterized by intense competition, with a mix of established players and innovative startups. Key market players are focusing on product innovation, incorporating advanced materials and technologies such as 3D printing and AI-driven design processes to enhance the efficacy and comfort of custom orthotics. The integration of smart technologies, such as embedded sensors for gait analysis and real-time feedback, is emerging as a significant trend in the premium segment of the market.
Looking ahead, the custom orthotics market is poised for continued growth, with several trends shaping its future. The increasing adoption of telemedicine and remote fitting technologies is expected to expand market reach and improve accessibility. Furthermore, the growing emphasis on sustainability is driving research into eco-friendly materials and manufacturing processes for custom orthotics, appealing to environmentally conscious consumers.
Several factors contribute to the expanding market demand for custom orthotics. The aging population, particularly in developed countries, is a key driver as older individuals are more prone to foot problems and require specialized orthotic solutions. Additionally, the growing incidence of diabetes and obesity has led to an increased need for custom orthotics to address associated foot complications.
The sports and athletics segment represents a substantial portion of the custom orthotics market. Professional athletes and fitness enthusiasts are increasingly recognizing the importance of proper foot support in enhancing performance and preventing injuries. This has led to a surge in demand for sport-specific custom orthotics designed to optimize biomechanics and reduce the risk of overuse injuries.
In terms of distribution channels, the market is segmented into hospitals, clinics, online platforms, and specialty stores. While traditional brick-and-mortar outlets still dominate, there is a notable shift towards online sales channels, especially in the wake of the COVID-19 pandemic. E-commerce platforms offering virtual consultations and at-home fitting solutions are gaining traction among consumers seeking convenience and personalized care.
Geographically, North America holds the largest market share, followed by Europe and Asia-Pacific. The United States, in particular, is a key market due to its well-established healthcare infrastructure and high consumer awareness. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness the fastest growth rates in the coming years, driven by improving healthcare access and rising disposable incomes.
The custom orthotics market is characterized by intense competition, with a mix of established players and innovative startups. Key market players are focusing on product innovation, incorporating advanced materials and technologies such as 3D printing and AI-driven design processes to enhance the efficacy and comfort of custom orthotics. The integration of smart technologies, such as embedded sensors for gait analysis and real-time feedback, is emerging as a significant trend in the premium segment of the market.
Looking ahead, the custom orthotics market is poised for continued growth, with several trends shaping its future. The increasing adoption of telemedicine and remote fitting technologies is expected to expand market reach and improve accessibility. Furthermore, the growing emphasis on sustainability is driving research into eco-friendly materials and manufacturing processes for custom orthotics, appealing to environmentally conscious consumers.
Accura 25 Technical Challenges
Accura 25, a photopolymer resin developed by 3D Systems, presents several technical challenges in the context of custom orthotics development. The material's properties, while suitable for many applications, require careful consideration and adaptation for orthotic production.
One of the primary challenges is achieving the optimal balance between flexibility and durability. Custom orthotics need to provide adequate support while also offering a degree of flexibility for comfort. Accura 25's mechanical properties may not inherently meet these requirements, necessitating modifications to the material composition or post-processing techniques to achieve the desired characteristics.
Another significant challenge lies in the material's long-term stability. Orthotics are subjected to repeated stress and varying environmental conditions. Ensuring that Accura 25-based orthotics maintain their shape, support, and functionality over extended periods of use is crucial. This requires extensive testing and potentially the development of protective coatings or treatments to enhance the material's resistance to wear, moisture, and temperature fluctuations.
The biocompatibility of Accura 25 also presents a technical hurdle. While the material is generally considered safe for skin contact, prolonged exposure in orthotic applications may require additional certifications or modifications to ensure patient safety and comfort. This may involve developing specialized surface treatments or incorporating biocompatible additives into the resin formulation.
Precision and accuracy in the 3D printing process using Accura 25 is another critical challenge. Custom orthotics demand high levels of dimensional accuracy to ensure proper fit and function. Achieving consistent results across different print batches and accounting for potential shrinkage or warping during the curing process requires fine-tuning of printing parameters and post-processing techniques.
The post-processing of Accura 25 orthotics also presents technical challenges. Removing support structures and achieving the desired surface finish without compromising the orthotic's structural integrity or dimensional accuracy requires the development of specialized techniques and tools. Additionally, ensuring that any post-processing methods are scalable for commercial production adds another layer of complexity.
Lastly, the integration of Accura 25 into existing orthotic manufacturing workflows poses a significant challenge. Adapting traditional orthotic design and production processes to accommodate the unique properties and requirements of 3D printed Accura 25 components necessitates the development of new software tools, design guidelines, and quality control measures. This integration must be seamless to ensure efficiency and cost-effectiveness in the production of custom orthotics.
One of the primary challenges is achieving the optimal balance between flexibility and durability. Custom orthotics need to provide adequate support while also offering a degree of flexibility for comfort. Accura 25's mechanical properties may not inherently meet these requirements, necessitating modifications to the material composition or post-processing techniques to achieve the desired characteristics.
Another significant challenge lies in the material's long-term stability. Orthotics are subjected to repeated stress and varying environmental conditions. Ensuring that Accura 25-based orthotics maintain their shape, support, and functionality over extended periods of use is crucial. This requires extensive testing and potentially the development of protective coatings or treatments to enhance the material's resistance to wear, moisture, and temperature fluctuations.
The biocompatibility of Accura 25 also presents a technical hurdle. While the material is generally considered safe for skin contact, prolonged exposure in orthotic applications may require additional certifications or modifications to ensure patient safety and comfort. This may involve developing specialized surface treatments or incorporating biocompatible additives into the resin formulation.
Precision and accuracy in the 3D printing process using Accura 25 is another critical challenge. Custom orthotics demand high levels of dimensional accuracy to ensure proper fit and function. Achieving consistent results across different print batches and accounting for potential shrinkage or warping during the curing process requires fine-tuning of printing parameters and post-processing techniques.
The post-processing of Accura 25 orthotics also presents technical challenges. Removing support structures and achieving the desired surface finish without compromising the orthotic's structural integrity or dimensional accuracy requires the development of specialized techniques and tools. Additionally, ensuring that any post-processing methods are scalable for commercial production adds another layer of complexity.
Lastly, the integration of Accura 25 into existing orthotic manufacturing workflows poses a significant challenge. Adapting traditional orthotic design and production processes to accommodate the unique properties and requirements of 3D printed Accura 25 components necessitates the development of new software tools, design guidelines, and quality control measures. This integration must be seamless to ensure efficiency and cost-effectiveness in the production of custom orthotics.
Current Accura 25 Applications in Orthotics
01 Pharmaceutical compositions containing Accura 25
Accura 25 is used in various pharmaceutical compositions for treating different medical conditions. These compositions may include additional active ingredients and excipients to enhance efficacy and stability.- Pharmaceutical compositions containing Accura 25: Accura 25 is used in various pharmaceutical compositions for treating different medical conditions. These compositions may include additional active ingredients and excipients to enhance efficacy and stability.
- Manufacturing processes for Accura 25: Different methods and processes are employed in the production of Accura 25, including synthesis techniques, purification steps, and quality control measures to ensure consistent product quality.
- Analytical methods for Accura 25: Various analytical techniques are used to characterize and quantify Accura 25 in pharmaceutical formulations and biological samples. These methods may include chromatography, spectroscopy, and other advanced analytical tools.
- Formulations containing Accura 25 for specific applications: Accura 25 is incorporated into specialized formulations for targeted applications, such as controlled release systems, topical preparations, or combination therapies with other active ingredients.
- Accura 25 in novel drug delivery systems: Research and development efforts focus on incorporating Accura 25 into innovative drug delivery systems to improve its bioavailability, efficacy, and patient compliance. These may include nanoparticle-based systems, transdermal patches, or other advanced delivery technologies.
02 Chemical synthesis and production methods for Accura 25
Various methods for synthesizing and producing Accura 25 have been developed, including novel reaction pathways and purification techniques to improve yield and purity.Expand Specific Solutions03 Analytical methods for detecting and quantifying Accura 25
Techniques have been developed for the detection and quantification of Accura 25 in various matrices, including chromatographic and spectroscopic methods for quality control and research purposes.Expand Specific Solutions04 Formulations and delivery systems for Accura 25
Novel formulations and delivery systems have been designed to improve the bioavailability, stability, and efficacy of Accura 25, including controlled-release formulations and targeted delivery mechanisms.Expand Specific Solutions05 Applications of Accura 25 in various industries
Accura 25 has found applications in diverse industries beyond pharmaceuticals, including agriculture, materials science, and chemical manufacturing, demonstrating its versatility as a chemical compound.Expand Specific Solutions
Key Players in Custom Orthotics Manufacturing
The research on Accura 25 in custom orthotics development is situated in a rapidly evolving market with significant growth potential. The industry is in a transitional phase, moving from traditional manufacturing methods to more advanced digital technologies. The global custom orthotics market is expanding, driven by increasing awareness of foot health and rising demand for personalized medical solutions. Technologically, the field is progressing from manual fabrication to 3D printing and AI-assisted design. Key players like Align Technology, 3M, and Ottobock are investing heavily in R&D to develop innovative solutions, while newer entrants such as uLab Systems are disrupting the market with digital platforms. The competition is intensifying as companies strive to integrate cutting-edge technologies into their product offerings.
Align Technology, Inc.
Technical Solution: Align Technology has developed a proprietary digital scanning system and 3D printing technology for custom orthotics, particularly in the field of orthodontics. Their Invisalign system uses advanced computer imaging and modeling to create a series of custom-made, clear aligners[1]. For orthotic development, they employ a similar approach, utilizing 3D scans of patients' feet to design and manufacture custom orthotics. The company's iTero Element scanner captures thousands of images per second, creating a precise 3D model of the foot[2]. This model is then used to design orthotics that perfectly match the patient's foot contours and address specific biomechanical issues. The orthotics are manufactured using advanced 3D printing techniques, ensuring high precision and consistency[3].
Strengths: High precision 3D scanning and printing technology, established presence in orthodontics market, proprietary software for custom design. Weaknesses: Primary focus on dental applications may limit orthotic expertise, potentially higher cost due to advanced technology use.
Otto Bock Healthcare
Technical Solution: Otto Bock Healthcare, now known as Ottobock, has developed innovative approaches to custom orthotics, particularly in the field of prosthetics and orthotics. Their research on Accura 25 material has led to advancements in the development of custom orthotics. Ottobock utilizes a combination of traditional casting methods and modern 3D scanning technologies to capture precise measurements of patients' limbs[4]. They have developed proprietary software that analyzes these measurements and biomechanical data to design custom orthotics. The company's approach includes the use of computer-aided design and manufacturing (CAD/CAM) systems to create highly personalized orthotic devices[5]. Ottobock's orthotics are often made using a combination of traditional materials and advanced composites, including carbon fiber, to achieve optimal strength-to-weight ratios[6].
Strengths: Extensive experience in prosthetics and orthotics, advanced CAD/CAM systems, use of high-performance materials. Weaknesses: May have a steeper learning curve for practitioners, potentially higher costs due to advanced materials and technologies.
Accura 25 Material Properties Analysis
Providing custom orthodontic treatment with appliance components from inventory
PatentInactiveUS7229282B2
Innovation
- A system and method that uses computer-aided analysis to evaluate and select standard or pre-manufactured orthodontic appliance components based on customized treatment plans, comparing component geometries to determine the best fit, allowing for the assembly of appliances that closely approximate custom designs while considering cost, availability, and anatomical factors.
Custom Tools for Bonding Orthodontic Appliances and Methods of Bonding Orthodontic Appliances
PatentActiveUS20230026846A1
Innovation
- Custom tools with patient-specific facial and lingual bodies that interlock to provide precise placement and bonding of orthodontic appliances, allowing for accurate measurement and analysis, reducing doctor/patient time, and enabling easier rebonding of brackets.
Regulatory Framework for 3D Printed Orthotics
The regulatory framework for 3D printed orthotics is a complex and evolving landscape that encompasses various aspects of medical device regulation, manufacturing standards, and patient safety. In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing the development and commercialization of 3D printed orthotics. The FDA has established guidelines for additive manufacturing of medical devices, which include orthotics, under the broader category of custom-made devices.
These guidelines address key areas such as design control, material selection, and quality management systems. Manufacturers of 3D printed orthotics must adhere to Good Manufacturing Practices (GMP) and implement robust quality control measures throughout the production process. The FDA also requires manufacturers to maintain detailed documentation of the design, manufacturing, and testing processes to ensure traceability and facilitate regulatory compliance.
In the European Union, 3D printed orthotics fall under the Medical Device Regulation (MDR), which came into effect in May 2021. The MDR introduces more stringent requirements for custom-made devices, including 3D printed orthotics. Manufacturers must comply with essential safety and performance requirements, conduct clinical evaluations, and implement post-market surveillance systems.
International standards organizations, such as the International Organization for Standardization (ISO) and ASTM International, have developed specific standards for additive manufacturing in healthcare applications. These standards provide guidance on material characterization, process validation, and quality assurance for 3D printed medical devices, including orthotics.
Regulatory bodies are also addressing the unique challenges posed by 3D printing technologies, such as ensuring consistency and reproducibility across different printing systems and locations. This has led to the development of guidelines for software validation, process parameter control, and post-processing procedures specific to 3D printed orthotics.
As the technology continues to advance, regulatory frameworks are expected to evolve to keep pace with innovations in materials, design methodologies, and manufacturing processes. This may include the development of new standards for biocompatible materials used in 3D printing, as well as guidelines for integrating digital design tools and artificial intelligence in the production of custom orthotics.
Manufacturers and healthcare providers must stay informed about these regulatory developments to ensure compliance and maintain the highest standards of patient care. Collaboration between industry stakeholders, regulatory bodies, and research institutions will be crucial in shaping a regulatory framework that balances innovation with patient safety in the rapidly evolving field of 3D printed orthotics.
These guidelines address key areas such as design control, material selection, and quality management systems. Manufacturers of 3D printed orthotics must adhere to Good Manufacturing Practices (GMP) and implement robust quality control measures throughout the production process. The FDA also requires manufacturers to maintain detailed documentation of the design, manufacturing, and testing processes to ensure traceability and facilitate regulatory compliance.
In the European Union, 3D printed orthotics fall under the Medical Device Regulation (MDR), which came into effect in May 2021. The MDR introduces more stringent requirements for custom-made devices, including 3D printed orthotics. Manufacturers must comply with essential safety and performance requirements, conduct clinical evaluations, and implement post-market surveillance systems.
International standards organizations, such as the International Organization for Standardization (ISO) and ASTM International, have developed specific standards for additive manufacturing in healthcare applications. These standards provide guidance on material characterization, process validation, and quality assurance for 3D printed medical devices, including orthotics.
Regulatory bodies are also addressing the unique challenges posed by 3D printing technologies, such as ensuring consistency and reproducibility across different printing systems and locations. This has led to the development of guidelines for software validation, process parameter control, and post-processing procedures specific to 3D printed orthotics.
As the technology continues to advance, regulatory frameworks are expected to evolve to keep pace with innovations in materials, design methodologies, and manufacturing processes. This may include the development of new standards for biocompatible materials used in 3D printing, as well as guidelines for integrating digital design tools and artificial intelligence in the production of custom orthotics.
Manufacturers and healthcare providers must stay informed about these regulatory developments to ensure compliance and maintain the highest standards of patient care. Collaboration between industry stakeholders, regulatory bodies, and research institutions will be crucial in shaping a regulatory framework that balances innovation with patient safety in the rapidly evolving field of 3D printed orthotics.
Cost-Benefit Analysis of Accura 25 in Orthotics Production
The cost-benefit analysis of Accura 25 in orthotics production reveals significant advantages that could revolutionize the custom orthotic manufacturing process. Accura 25, a photopolymer resin developed by 3D Systems, offers exceptional precision and durability, making it an ideal material for producing custom orthotics through additive manufacturing techniques.
From a cost perspective, the initial investment in Accura 25 and associated 3D printing equipment may be substantial. However, the long-term benefits outweigh these upfront costs. The material's high resolution and accuracy reduce the need for post-processing and adjustments, saving both time and labor costs. Additionally, the ability to produce complex geometries in a single print run eliminates the need for multiple manufacturing steps, further reducing production expenses.
The durability of Accura 25 contributes to the longevity of the orthotics, potentially reducing replacement frequency and associated costs for patients. This improved lifespan not only enhances customer satisfaction but also positions manufacturers to offer more competitive pricing or improved profit margins.
In terms of benefits, Accura 25 enables the production of highly customized orthotics with intricate designs that were previously challenging or impossible to manufacture using traditional methods. This level of customization can significantly improve patient outcomes, potentially leading to increased market share and customer loyalty for orthotic providers.
The material's biocompatibility and resistance to moisture absorption make it suitable for long-term wear, addressing common issues with traditional orthotic materials. This characteristic not only improves patient comfort but also reduces the likelihood of material degradation and associated health risks.
From a production efficiency standpoint, Accura 25 allows for rapid prototyping and iteration of designs. This agility in the development process can lead to faster time-to-market for new orthotic products and the ability to quickly respond to individual patient needs. The digital nature of the 3D printing process also facilitates easy storage and reproduction of designs, streamlining inventory management and reducing storage costs.
Environmental considerations also favor the use of Accura 25. The additive manufacturing process results in minimal material waste compared to subtractive methods, aligning with sustainability goals and potentially reducing material costs. Furthermore, the ability to produce orthotics on-demand reduces the need for large inventories, decreasing storage requirements and associated overhead costs.
In conclusion, while the adoption of Accura 25 for custom orthotics production requires initial investment, the long-term cost savings, improved product quality, and enhanced customization capabilities present a compelling case for its implementation. The material's properties align well with the demands of the orthotics industry, offering a competitive edge in a market that increasingly values personalization and performance.
From a cost perspective, the initial investment in Accura 25 and associated 3D printing equipment may be substantial. However, the long-term benefits outweigh these upfront costs. The material's high resolution and accuracy reduce the need for post-processing and adjustments, saving both time and labor costs. Additionally, the ability to produce complex geometries in a single print run eliminates the need for multiple manufacturing steps, further reducing production expenses.
The durability of Accura 25 contributes to the longevity of the orthotics, potentially reducing replacement frequency and associated costs for patients. This improved lifespan not only enhances customer satisfaction but also positions manufacturers to offer more competitive pricing or improved profit margins.
In terms of benefits, Accura 25 enables the production of highly customized orthotics with intricate designs that were previously challenging or impossible to manufacture using traditional methods. This level of customization can significantly improve patient outcomes, potentially leading to increased market share and customer loyalty for orthotic providers.
The material's biocompatibility and resistance to moisture absorption make it suitable for long-term wear, addressing common issues with traditional orthotic materials. This characteristic not only improves patient comfort but also reduces the likelihood of material degradation and associated health risks.
From a production efficiency standpoint, Accura 25 allows for rapid prototyping and iteration of designs. This agility in the development process can lead to faster time-to-market for new orthotic products and the ability to quickly respond to individual patient needs. The digital nature of the 3D printing process also facilitates easy storage and reproduction of designs, streamlining inventory management and reducing storage costs.
Environmental considerations also favor the use of Accura 25. The additive manufacturing process results in minimal material waste compared to subtractive methods, aligning with sustainability goals and potentially reducing material costs. Furthermore, the ability to produce orthotics on-demand reduces the need for large inventories, decreasing storage requirements and associated overhead costs.
In conclusion, while the adoption of Accura 25 for custom orthotics production requires initial investment, the long-term cost savings, improved product quality, and enhanced customization capabilities present a compelling case for its implementation. The material's properties align well with the demands of the orthotics industry, offering a competitive edge in a market that increasingly values personalization and performance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!