Antifreeze Ways to Achieve Goals in Green Chemistry Materials
Antifreeze Tech Evolution
The evolution of antifreeze technology in green chemistry materials has been marked by significant milestones and innovations over the past few decades. Initially, the focus was on developing synthetic antifreeze compounds that could effectively lower the freezing point of water. However, these early solutions often posed environmental risks due to their toxicity and non-biodegradability.
As environmental concerns grew, researchers began exploring more eco-friendly alternatives. The 1990s saw a shift towards the use of propylene glycol-based antifreeze, which offered reduced toxicity compared to traditional ethylene glycol formulations. This marked the beginning of a green revolution in antifreeze technology.
In the early 2000s, scientists started investigating naturally occurring antifreeze proteins (AFPs) found in cold-adapted organisms such as fish, insects, and plants. These proteins demonstrated remarkable ice growth inhibition properties without altering the overall freezing point. This discovery opened up new avenues for biomimetic approaches in antifreeze technology.
The mid-2000s witnessed the development of polymer-based antifreeze additives. These synthetic polymers were designed to mimic the structure and function of natural AFPs, offering improved performance and scalability. Concurrently, research into plant-based antifreeze compounds gained momentum, with extracts from cold-hardy plants showing promising results.
Recent years have seen a surge in nanotechnology applications for antifreeze solutions. Nanoparticles and nanostructured materials have been engineered to enhance the antifreeze properties of existing formulations while minimizing environmental impact. This approach has led to the creation of hybrid systems combining traditional antifreeze compounds with nanomaterials for superior performance.
The latest frontier in antifreeze technology focuses on smart materials that can respond dynamically to temperature changes. These adaptive systems aim to provide optimal protection across a wide range of conditions while minimizing resource consumption. Additionally, there is growing interest in developing antifreeze materials that not only prevent freezing but also offer additional functionalities such as self-healing or energy storage capabilities.
Throughout this evolution, the overarching goal has remained consistent: to achieve effective antifreeze properties while adhering to the principles of green chemistry. This includes reducing toxicity, improving biodegradability, minimizing resource consumption, and enhancing overall sustainability. The journey from synthetic chemicals to bio-inspired solutions and smart materials exemplifies the continuous effort to balance performance with environmental responsibility in the field of antifreeze technology.
Green Chemistry Demand
The demand for green chemistry materials has been steadily increasing in recent years, driven by growing environmental concerns and stricter regulations. This trend is particularly evident in the field of antifreeze solutions, where traditional formulations often contain toxic substances that pose risks to both human health and ecosystems. The market for environmentally friendly antifreeze products is expanding rapidly, with a significant focus on developing solutions that maintain performance while reducing ecological impact.
Industries such as automotive, aerospace, and renewable energy are actively seeking green alternatives to conventional antifreeze materials. These sectors require products that can withstand extreme temperature variations without compromising on safety or efficiency. The push for sustainable practices has led to a surge in research and development activities aimed at creating bio-based and biodegradable antifreeze compounds.
Consumer awareness regarding the environmental implications of chemical products has also contributed to the rising demand for green chemistry materials in antifreeze applications. Homeowners and small-scale users are increasingly opting for eco-friendly options in various household and recreational products, including antifreeze solutions for vehicles and heating systems.
The regulatory landscape plays a crucial role in shaping the demand for green antifreeze materials. Governments worldwide are implementing stricter guidelines on the use and disposal of chemical substances, encouraging manufacturers to invest in sustainable alternatives. This regulatory pressure has created a substantial market opportunity for companies developing innovative, environmentally friendly antifreeze solutions.
In the context of climate change and global warming, there is a growing need for antifreeze materials that can adapt to changing environmental conditions. This has led to increased interest in smart materials and adaptive formulations that can respond to temperature fluctuations more effectively while maintaining their green credentials.
The agriculture sector represents another significant area of demand for green antifreeze materials. As extreme weather events become more frequent, farmers are seeking sustainable solutions to protect crops from frost damage. This has opened up new avenues for research into plant-based antifreeze compounds that can be safely applied to agricultural environments without harming soil quality or biodiversity.
As the global focus on sustainability intensifies, the demand for green chemistry materials in antifreeze applications is expected to continue its upward trajectory. This trend presents both challenges and opportunities for researchers and manufacturers in the field, driving innovation and fostering the development of next-generation antifreeze solutions that align with the principles of green chemistry.
Antifreeze Challenges
The development of green chemistry materials faces significant challenges in antifreeze applications. One of the primary obstacles is the inherent conflict between traditional antifreeze properties and environmental sustainability. Conventional antifreeze solutions often rely on toxic chemicals, such as ethylene glycol, which pose serious risks to ecosystems and human health when released into the environment.
A major technical hurdle lies in finding eco-friendly alternatives that can match the performance of traditional antifreeze compounds. Green chemistry materials must demonstrate comparable freezing point depression, heat transfer efficiency, and corrosion inhibition properties while maintaining biodegradability and low toxicity. This balance is particularly challenging to achieve, as many naturally derived substances lack the robust antifreeze capabilities of their synthetic counterparts.
Another significant challenge is the scalability and cost-effectiveness of green antifreeze solutions. While some promising bio-based alternatives have been identified in laboratory settings, translating these findings into commercially viable products remains difficult. The production processes for these materials often require complex synthesis routes or expensive raw materials, making them less economically competitive compared to established antifreeze products.
The stability and longevity of green antifreeze materials present additional technical obstacles. Many bio-derived compounds are susceptible to degradation over time, potentially compromising their effectiveness in long-term applications. Researchers must develop innovative stabilization techniques to ensure that these materials maintain their antifreeze properties throughout their intended lifespan without resorting to environmentally harmful additives.
Furthermore, the diverse range of applications for antifreeze materials complicates the development of universal green solutions. Different industries and use cases, such as automotive coolants, industrial heat transfer fluids, and de-icing agents, have varying performance requirements. Creating versatile green chemistry materials that can adapt to these diverse needs while maintaining environmental benefits is a complex challenge that requires multifaceted research approaches.
Regulatory compliance and standardization pose additional hurdles in the advancement of green antifreeze technologies. As environmental regulations become more stringent, new materials must not only demonstrate superior ecological profiles but also meet rigorous safety and performance standards. The lack of established testing protocols and certification processes for green antifreeze materials can impede their adoption and market acceptance.
Current Green Solutions
01 Bio-based antifreeze materials
Green chemistry approaches are being used to develop bio-based antifreeze materials from renewable resources. These environmentally friendly alternatives aim to replace traditional petroleum-based antifreeze products while maintaining or improving antifreeze properties. Research focuses on utilizing plant-derived compounds and biodegradable materials to create effective and sustainable antifreeze solutions.- Bio-based antifreeze materials: Green chemistry approaches are being used to develop bio-based antifreeze materials from renewable resources. These environmentally friendly alternatives aim to replace traditional petroleum-based antifreeze products. Bio-based materials can offer comparable or superior antifreeze properties while reducing environmental impact and toxicity.
- Nanoparticle-enhanced antifreeze solutions: Incorporating nanoparticles into antifreeze solutions can significantly improve their thermal properties and freezing point depression. This green chemistry approach allows for the use of lower concentrations of antifreeze agents, reducing environmental impact while maintaining or enhancing performance.
- Plant-derived antifreeze proteins: Research is focused on identifying and extracting antifreeze proteins from plants that naturally resist freezing. These proteins can be incorporated into green antifreeze formulations, providing effective freeze protection without the need for harmful chemicals. This approach mimics nature's own antifreeze mechanisms.
- Ionic liquid-based antifreeze solutions: Green ionic liquids are being explored as potential antifreeze agents. These non-volatile, thermally stable compounds can offer excellent antifreeze properties while being more environmentally friendly than traditional glycol-based solutions. Their unique properties allow for efficient heat transfer and low freezing points.
- Sustainable production methods for antifreeze materials: Green chemistry principles are being applied to the production processes of antifreeze materials. This includes using renewable energy sources, implementing closed-loop systems to minimize waste, and developing catalysts that enable more efficient and environmentally friendly synthesis of antifreeze compounds.
02 Nanoparticle-enhanced antifreeze formulations
Incorporating nanoparticles into antifreeze formulations is an emerging area of green chemistry research. These nanoparticles can enhance the thermal conductivity and heat transfer properties of the antifreeze solution, potentially improving its overall performance. This approach allows for the use of lower concentrations of traditional antifreeze chemicals, reducing environmental impact while maintaining or improving antifreeze properties.Expand Specific Solutions03 Ionic liquid-based antifreeze solutions
Ionic liquids are being explored as green alternatives to conventional antifreeze materials. These compounds have unique properties such as low volatility, high thermal stability, and excellent solvation capabilities. Ionic liquid-based antifreeze solutions can potentially offer improved performance and reduced environmental impact compared to traditional glycol-based products.Expand Specific Solutions04 Natural polymer-derived antifreeze agents
Research is being conducted on the development of antifreeze agents derived from natural polymers such as cellulose, chitosan, and other polysaccharides. These biodegradable and renewable materials can be chemically modified to enhance their antifreeze properties, providing environmentally friendly alternatives to synthetic antifreeze compounds.Expand Specific Solutions05 Green corrosion inhibitors for antifreeze formulations
Green chemistry principles are being applied to develop environmentally friendly corrosion inhibitors for use in antifreeze formulations. These inhibitors aim to protect metal components in cooling systems while minimizing environmental impact. Research focuses on plant extracts, amino acids, and other naturally derived compounds as potential green corrosion inhibitors in antifreeze applications.Expand Specific Solutions
Key Industry Players
The research on antifreeze ways to achieve goals in green chemistry materials is in a developing stage, with growing market potential due to increasing environmental concerns. The technology's maturity varies across different applications, with some solutions already commercialized and others still in the research phase. Companies like Advanced Industrial Science & Technology, Kaneka Corp., and Otsuka Pharmaceutical are leading the field with their innovative approaches. The competitive landscape is diverse, including both established chemical companies and emerging biotech firms, reflecting the interdisciplinary nature of this research area. As sustainability becomes a key focus in the chemical industry, this field is expected to see significant growth and investment in the coming years.
Advanced Industrial Science & Technology
King Abdullah University of Science & Technology
Innovative Antifreeze
- Adoption of continuous flow processing technology, integrating with green chemistry principles and enabling technologies like microwave irradiation and microreactor technology, to enhance synthesis, analysis, purification, and formulation processes, utilizing micro- or meso-scaled flow reactors for improved efficiency and sustainability.
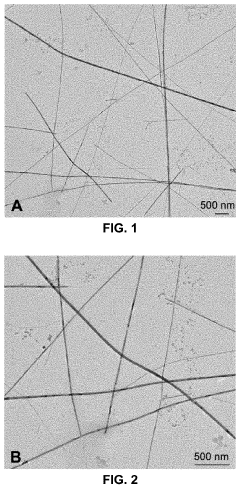
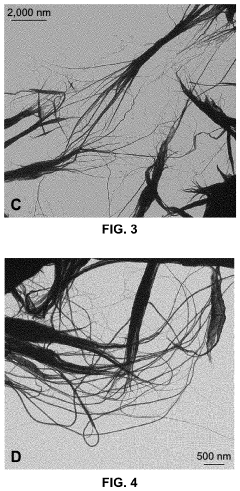
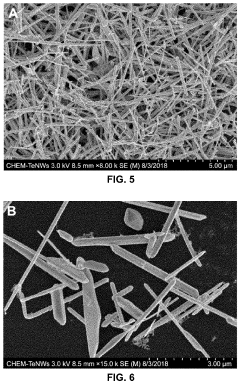
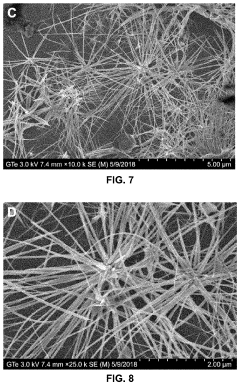
- Green chemistry approaches using natural materials like bacteria, fungi, plants, and organic compounds to synthesize tellurium nanowires through hydrothermal methods, which are safer, more environmentally friendly, and biocompatible, and exhibit novel cytocompatibility and anticancer properties.
Environmental Regulations
Environmental regulations play a crucial role in shaping the development and implementation of green chemistry materials, including antifreeze solutions. These regulations are designed to protect human health and the environment by limiting the use of harmful substances and promoting sustainable practices in the chemical industry.
In recent years, there has been a significant shift towards stricter environmental regulations globally, particularly concerning the use of traditional antifreeze chemicals. Many countries have implemented laws and guidelines that restrict or ban the use of certain antifreeze compounds due to their potential environmental and health impacts. For instance, ethylene glycol, a commonly used antifreeze agent, has been subject to increased scrutiny due to its toxicity and environmental persistence.
The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation has been particularly influential in driving the adoption of greener antifreeze solutions. Under REACH, manufacturers and importers are required to assess and manage the risks associated with the chemicals they produce or use, including antifreeze compounds. This has led to increased research and development efforts in finding environmentally friendly alternatives.
In the United States, the Environmental Protection Agency (EPA) has established guidelines for the proper disposal of antifreeze and encourages the use of less toxic alternatives. The agency's Significant New Alternatives Policy (SNAP) program promotes the transition to safer substitutes for ozone-depleting substances, which has implications for certain antifreeze applications.
Many countries have also implemented regulations targeting specific industries that heavily rely on antifreeze solutions. For example, the automotive sector has seen a push towards more environmentally friendly coolants and antifreeze formulations. This has led to the development of propylene glycol-based antifreeze, which is less toxic than its ethylene glycol counterpart.
The increasing focus on circular economy principles has further influenced environmental regulations related to antifreeze materials. Regulations now often emphasize the importance of recyclability and biodegradability in chemical formulations. This has spurred research into bio-based antifreeze solutions derived from renewable resources, as well as the development of more efficient recycling processes for used antifreeze materials.
As environmental concerns continue to grow, it is expected that regulations will become even more stringent in the coming years. This regulatory landscape is driving innovation in green chemistry, pushing researchers and manufacturers to develop novel antifreeze solutions that not only meet performance requirements but also align with sustainability goals and comply with evolving environmental standards.
Lifecycle Assessment
Lifecycle Assessment (LCA) plays a crucial role in evaluating the environmental impact of antifreeze methods in green chemistry materials. This comprehensive approach examines the entire lifecycle of antifreeze solutions, from raw material extraction to disposal or recycling, providing valuable insights into their sustainability and ecological footprint.
The assessment begins with the production phase, analyzing the energy consumption and emissions associated with manufacturing antifreeze compounds. Green chemistry principles emphasize the use of renewable resources and energy-efficient processes, which can significantly reduce the environmental burden during this stage. For instance, bio-based antifreeze materials derived from agricultural waste or byproducts may offer a more sustainable alternative to traditional petroleum-based options.
Transportation and distribution of antifreeze products contribute to their overall environmental impact. LCA considers factors such as fuel consumption, packaging materials, and logistics efficiency to determine the carbon footprint associated with product delivery. Optimizing transportation routes and utilizing eco-friendly packaging can help minimize these impacts.
The use phase of antifreeze materials is particularly important in the context of green chemistry. LCA evaluates the performance and longevity of antifreeze solutions, considering factors such as thermal efficiency, corrosion resistance, and biodegradability. Innovative antifreeze formulations that offer improved performance while reducing environmental harm are key to achieving sustainability goals in this field.
End-of-life management is a critical aspect of the lifecycle assessment for antifreeze materials. The analysis examines disposal methods, recycling potential, and potential environmental risks associated with improper handling. Green chemistry approaches prioritize the development of antifreeze solutions that are easily recyclable or biodegradable, minimizing their long-term environmental impact.
LCA also considers the potential for unintended consequences and secondary effects of antifreeze materials. This includes assessing the impact on water resources, soil quality, and ecosystem health. By identifying potential risks early in the development process, researchers can refine their formulations to mitigate negative environmental outcomes.
The results of lifecycle assessments inform decision-making processes for both manufacturers and consumers. They provide a basis for comparing different antifreeze solutions and identifying areas for improvement in terms of environmental performance. This data-driven approach supports the continuous evolution of green chemistry materials, driving innovation towards more sustainable and eco-friendly antifreeze technologies.