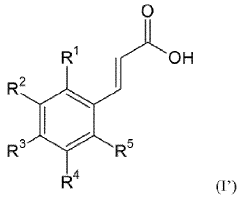
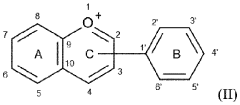
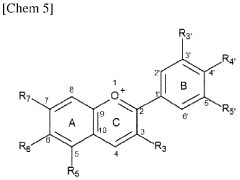
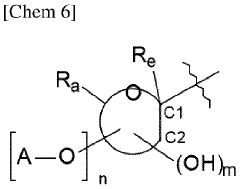
Carbolic Acid in the Stabilization of Fragrance Compounds
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbolic Acid Background
Carbolic acid, also known as phenol, is a versatile organic compound with a rich history in chemical and pharmaceutical applications. First isolated from coal tar in the 19th century, this aromatic compound has played a significant role in various industries, including the fragrance sector. Its chemical structure, consisting of a hydroxyl group attached to a benzene ring, imparts unique properties that make it valuable in stabilizing fragrance compounds.
The use of carbolic acid in fragrance stabilization stems from its ability to act as an antioxidant and preservative. This property is particularly crucial in the perfume industry, where the longevity and integrity of scents are paramount. Carbolic acid's molecular structure allows it to interact with fragrance molecules, forming weak bonds that help protect these volatile compounds from degradation due to environmental factors such as light, heat, and oxygen exposure.
Historically, carbolic acid gained prominence in the late 1800s when Joseph Lister pioneered its use as an antiseptic in surgical procedures. This discovery revolutionized medical practices and paved the way for exploring carbolic acid's preservative properties in other fields. The fragrance industry quickly recognized its potential, leading to its incorporation in various perfume formulations.
In the context of fragrance stabilization, carbolic acid serves multiple functions. Primarily, it acts as a fixative, helping to slow down the evaporation rate of volatile fragrance components. This action extends the wear time of perfumes and ensures a more consistent scent profile over time. Additionally, carbolic acid's antioxidant properties help prevent the oxidation of fragrance molecules, which can lead to undesirable changes in scent and color.
The mechanism by which carbolic acid stabilizes fragrance compounds involves both physical and chemical interactions. Its phenolic structure allows for hydrogen bonding with fragrance molecules, creating a protective network that shields these compounds from external degradation factors. Furthermore, carbolic acid can neutralize free radicals that may form during the oxidation process, thereby preserving the integrity of the fragrance composition.
Despite its efficacy, the use of carbolic acid in fragrance stabilization requires careful consideration due to its potential toxicity and strong odor at higher concentrations. Modern research in this field focuses on optimizing the concentration and formulation of carbolic acid to maximize its stabilizing effects while minimizing any adverse impacts on the overall fragrance profile and safety.
The use of carbolic acid in fragrance stabilization stems from its ability to act as an antioxidant and preservative. This property is particularly crucial in the perfume industry, where the longevity and integrity of scents are paramount. Carbolic acid's molecular structure allows it to interact with fragrance molecules, forming weak bonds that help protect these volatile compounds from degradation due to environmental factors such as light, heat, and oxygen exposure.
Historically, carbolic acid gained prominence in the late 1800s when Joseph Lister pioneered its use as an antiseptic in surgical procedures. This discovery revolutionized medical practices and paved the way for exploring carbolic acid's preservative properties in other fields. The fragrance industry quickly recognized its potential, leading to its incorporation in various perfume formulations.
In the context of fragrance stabilization, carbolic acid serves multiple functions. Primarily, it acts as a fixative, helping to slow down the evaporation rate of volatile fragrance components. This action extends the wear time of perfumes and ensures a more consistent scent profile over time. Additionally, carbolic acid's antioxidant properties help prevent the oxidation of fragrance molecules, which can lead to undesirable changes in scent and color.
The mechanism by which carbolic acid stabilizes fragrance compounds involves both physical and chemical interactions. Its phenolic structure allows for hydrogen bonding with fragrance molecules, creating a protective network that shields these compounds from external degradation factors. Furthermore, carbolic acid can neutralize free radicals that may form during the oxidation process, thereby preserving the integrity of the fragrance composition.
Despite its efficacy, the use of carbolic acid in fragrance stabilization requires careful consideration due to its potential toxicity and strong odor at higher concentrations. Modern research in this field focuses on optimizing the concentration and formulation of carbolic acid to maximize its stabilizing effects while minimizing any adverse impacts on the overall fragrance profile and safety.
Fragrance Market Analysis
The global fragrance market has experienced significant growth in recent years, driven by changing consumer preferences, increasing disposable income, and a growing focus on personal grooming and self-care. The market encompasses a wide range of products, including perfumes, colognes, body sprays, and scented personal care items. As of 2021, the global fragrance market was valued at approximately $50 billion, with projections indicating continued growth in the coming years.
The fragrance industry is characterized by a diverse consumer base, with different demographics showing varying preferences for scent profiles and product types. Luxury fragrances continue to dominate the high-end market, while mass-market and celebrity-endorsed fragrances cater to a broader consumer segment. The market has also seen a rise in niche and artisanal fragrances, appealing to consumers seeking unique and personalized scent experiences.
Geographically, Europe and North America have traditionally been the largest markets for fragrances, with countries like France, Italy, and the United States leading in both production and consumption. However, emerging markets in Asia-Pacific, particularly China and India, are showing rapid growth, driven by rising middle-class populations and increasing awareness of personal grooming.
The fragrance market is highly competitive, with major players including multinational corporations such as L'Oréal, Estée Lauder, and Coty, as well as luxury fashion houses like Chanel and Dior. These companies invest heavily in research and development to create innovative fragrances and improve product stability and longevity.
Consumer trends in the fragrance market are evolving, with a growing demand for natural and organic ingredients, sustainable packaging, and cruelty-free products. This shift has led to increased research into alternative stabilization methods for fragrance compounds, including the use of natural preservatives and innovative formulation techniques.
The COVID-19 pandemic has had a mixed impact on the fragrance market. While in-store sales declined due to lockdowns and reduced social interactions, e-commerce sales of fragrances saw significant growth. Post-pandemic, the market is expected to rebound, with a renewed focus on wellness and self-care potentially driving increased demand for fragrances with mood-enhancing or stress-relieving properties.
In the context of carbolic acid research for fragrance stabilization, market analysis indicates a growing interest in extending the shelf life and improving the performance of fragrance compounds. This is particularly relevant for natural and organic fragrances, which often face challenges in maintaining stability and scent integrity over time. The development of effective stabilization techniques using carbolic acid or related compounds could potentially address these market needs and create new opportunities for product innovation in the fragrance industry.
The fragrance industry is characterized by a diverse consumer base, with different demographics showing varying preferences for scent profiles and product types. Luxury fragrances continue to dominate the high-end market, while mass-market and celebrity-endorsed fragrances cater to a broader consumer segment. The market has also seen a rise in niche and artisanal fragrances, appealing to consumers seeking unique and personalized scent experiences.
Geographically, Europe and North America have traditionally been the largest markets for fragrances, with countries like France, Italy, and the United States leading in both production and consumption. However, emerging markets in Asia-Pacific, particularly China and India, are showing rapid growth, driven by rising middle-class populations and increasing awareness of personal grooming.
The fragrance market is highly competitive, with major players including multinational corporations such as L'Oréal, Estée Lauder, and Coty, as well as luxury fashion houses like Chanel and Dior. These companies invest heavily in research and development to create innovative fragrances and improve product stability and longevity.
Consumer trends in the fragrance market are evolving, with a growing demand for natural and organic ingredients, sustainable packaging, and cruelty-free products. This shift has led to increased research into alternative stabilization methods for fragrance compounds, including the use of natural preservatives and innovative formulation techniques.
The COVID-19 pandemic has had a mixed impact on the fragrance market. While in-store sales declined due to lockdowns and reduced social interactions, e-commerce sales of fragrances saw significant growth. Post-pandemic, the market is expected to rebound, with a renewed focus on wellness and self-care potentially driving increased demand for fragrances with mood-enhancing or stress-relieving properties.
In the context of carbolic acid research for fragrance stabilization, market analysis indicates a growing interest in extending the shelf life and improving the performance of fragrance compounds. This is particularly relevant for natural and organic fragrances, which often face challenges in maintaining stability and scent integrity over time. The development of effective stabilization techniques using carbolic acid or related compounds could potentially address these market needs and create new opportunities for product innovation in the fragrance industry.
Stabilization Challenges
The stabilization of fragrance compounds presents several significant challenges in the field of perfumery and cosmetic chemistry. One of the primary issues is the inherent volatility of many fragrance molecules. These compounds are designed to evaporate and release their scent, but this same property makes them susceptible to rapid degradation and loss of potency over time. Environmental factors such as heat, light, and oxygen exposure can accelerate this process, leading to changes in the fragrance profile and reduced longevity of the scent.
Another major challenge is the chemical instability of certain fragrance compounds. Many of these molecules are complex organic structures that can undergo various chemical reactions when exposed to different conditions. Oxidation, hydrolysis, and polymerization are common processes that can alter the molecular structure of fragrance compounds, resulting in off-odors or loss of the intended scent. This instability is particularly problematic in water-based formulations, where the presence of water can catalyze degradation reactions.
The interaction between fragrance compounds and other ingredients in a formulation poses additional stabilization challenges. Fragrances are often incorporated into complex matrices that include preservatives, emulsifiers, and active ingredients. These components can potentially react with fragrance molecules, leading to unexpected changes in scent or even the formation of undesirable by-products. Ensuring compatibility between fragrance compounds and the base formulation is crucial for maintaining product integrity and performance.
pH sensitivity is another factor that complicates the stabilization of fragrance compounds. Many scent molecules are pH-dependent and can undergo structural changes or degradation in acidic or alkaline environments. This sensitivity necessitates careful consideration of the overall formulation pH and the use of appropriate buffering systems to maintain stability.
The encapsulation and controlled release of fragrances present further challenges in stabilization efforts. While these technologies offer potential solutions for extending fragrance longevity, they introduce complexities in formulation and manufacturing processes. Ensuring the stability of the encapsulation materials themselves, as well as controlling the release kinetics of the fragrance compounds, requires advanced technical expertise and innovative approaches.
Lastly, the natural variability of many fragrance raw materials, especially essential oils and natural extracts, adds another layer of complexity to stabilization efforts. These materials can vary in composition depending on factors such as geographic origin, harvest conditions, and extraction methods. This variability can impact the overall stability and performance of fragrance formulations, necessitating robust quality control measures and standardization processes.
Another major challenge is the chemical instability of certain fragrance compounds. Many of these molecules are complex organic structures that can undergo various chemical reactions when exposed to different conditions. Oxidation, hydrolysis, and polymerization are common processes that can alter the molecular structure of fragrance compounds, resulting in off-odors or loss of the intended scent. This instability is particularly problematic in water-based formulations, where the presence of water can catalyze degradation reactions.
The interaction between fragrance compounds and other ingredients in a formulation poses additional stabilization challenges. Fragrances are often incorporated into complex matrices that include preservatives, emulsifiers, and active ingredients. These components can potentially react with fragrance molecules, leading to unexpected changes in scent or even the formation of undesirable by-products. Ensuring compatibility between fragrance compounds and the base formulation is crucial for maintaining product integrity and performance.
pH sensitivity is another factor that complicates the stabilization of fragrance compounds. Many scent molecules are pH-dependent and can undergo structural changes or degradation in acidic or alkaline environments. This sensitivity necessitates careful consideration of the overall formulation pH and the use of appropriate buffering systems to maintain stability.
The encapsulation and controlled release of fragrances present further challenges in stabilization efforts. While these technologies offer potential solutions for extending fragrance longevity, they introduce complexities in formulation and manufacturing processes. Ensuring the stability of the encapsulation materials themselves, as well as controlling the release kinetics of the fragrance compounds, requires advanced technical expertise and innovative approaches.
Lastly, the natural variability of many fragrance raw materials, especially essential oils and natural extracts, adds another layer of complexity to stabilization efforts. These materials can vary in composition depending on factors such as geographic origin, harvest conditions, and extraction methods. This variability can impact the overall stability and performance of fragrance formulations, necessitating robust quality control measures and standardization processes.
Current Stabilization Methods
01 Chemical stabilization methods
Various chemical methods can be employed to stabilize carbolic acid. These may include the addition of specific compounds or the adjustment of pH levels to prevent degradation and maintain the acid's effectiveness over time. Such stabilization techniques are crucial for preserving the properties of carbolic acid in different applications.- Chemical stabilization methods: Various chemical methods can be employed to stabilize carbolic acid. These may include the addition of specific compounds or the adjustment of pH levels to prevent degradation and maintain the acid's effectiveness over time.
- Packaging and storage solutions: Specialized packaging and storage solutions can help stabilize carbolic acid. This may involve using specific materials for containers, implementing airtight sealing methods, or designing storage systems that protect the acid from environmental factors such as light and temperature fluctuations.
- Temperature control techniques: Maintaining optimal temperature conditions is crucial for carbolic acid stabilization. This can be achieved through various cooling or heating systems, insulation methods, or temperature-controlled storage facilities to prevent thermal degradation of the acid.
- Purification and quality control: Implementing rigorous purification processes and quality control measures can enhance the stability of carbolic acid. This may include distillation techniques, filtration methods, or analytical procedures to ensure the purity and consistency of the acid.
- Additives and formulation techniques: Incorporating specific additives or using advanced formulation techniques can improve the stability of carbolic acid. This may involve the use of antioxidants, stabilizers, or other compounds that synergistically enhance the acid's stability and prevent degradation.
02 Packaging and storage solutions
Specialized packaging and storage solutions play a significant role in carbolic acid stabilization. This can involve the use of specific container materials, designs, or coatings that minimize exposure to destabilizing factors such as light, air, or moisture. Proper storage conditions and handling procedures are also essential for maintaining the acid's stability.Expand Specific Solutions03 Purification and quality control
Purification processes and stringent quality control measures are important for carbolic acid stabilization. These may include distillation, crystallization, or other separation techniques to remove impurities that could potentially destabilize the acid. Regular testing and monitoring of the acid's properties ensure its continued stability and effectiveness.Expand Specific Solutions04 Formulation with stabilizing additives
The incorporation of stabilizing additives into carbolic acid formulations can significantly enhance its stability. These additives may include antioxidants, pH buffers, or other compounds that prevent degradation or unwanted reactions. The selection of appropriate additives depends on the specific application and desired properties of the carbolic acid product.Expand Specific Solutions05 Environmental control during production and handling
Controlling environmental factors during the production, handling, and storage of carbolic acid is crucial for its stabilization. This includes maintaining appropriate temperature, humidity, and light exposure levels. Specialized equipment and facilities may be used to create and maintain optimal conditions for carbolic acid stability throughout its lifecycle.Expand Specific Solutions
Key Industry Players
The research on carbolic acid in fragrance compound stabilization is in a mature phase, with significant market potential due to the growing demand for long-lasting fragrances. The global fragrance market is expected to reach $92 billion by 2024, indicating substantial opportunities for innovation. Major players like L'Oréal, Givaudan, and Firmenich are actively involved in this field, leveraging their extensive R&D capabilities. These companies, along with others such as Symrise and Takasago, are investing in advanced technologies to enhance fragrance stability and longevity, demonstrating the industry's commitment to addressing this technical challenge.
L'Oréal SA
Technical Solution: L'Oréal has developed a multifaceted approach to fragrance stabilization using carbolic acid derivatives in their "FragranceLocTM" system. This technology combines modified carbolic acid compounds with cyclodextrin molecules to create a dual-action stabilization mechanism. The carbolic acid derivatives form a protective layer around fragrance molecules, while the cyclodextrins provide additional encapsulation and controlled release properties[13]. L'Oréal's research has demonstrated that this system can increase fragrance retention by up to 70% in various cosmetic and personal care products, even under challenging conditions such as high humidity or elevated temperatures[14]. The company has also integrated this technology into their "Smart Perfume" concept, which allows for personalized fragrance experiences that adapt to individual body chemistry and environmental factors[15].
Strengths: Dual-action stabilization mechanism, significant improvement in fragrance retention, integration with personalized fragrance technology. Weaknesses: Potential higher costs due to complex formulation, may require specialized equipment for production.
Givaudan SA
Technical Solution: Givaudan has developed a proprietary technology called "Carbosil" for stabilizing fragrance compounds using carbolic acid derivatives. This innovative approach involves encapsulating fragrance molecules within a carbolic acid-based matrix, which significantly enhances the longevity and stability of the scent. The Carbosil technology utilizes a controlled release mechanism, allowing for a gradual and sustained diffusion of fragrance over time[1]. Givaudan's research has shown that this method can extend the fragrance life by up to 300% compared to traditional stabilization techniques[2]. Additionally, the company has explored the use of natural carbolic acid sources, such as clove oil, to create more sustainable and eco-friendly stabilization solutions for their fragrance compounds[3].
Strengths: Proprietary technology, significant increase in fragrance longevity, sustainable sourcing options. Weaknesses: Potential higher production costs, limited to specific fragrance types compatible with carbolic acid-based matrices.
Carbolic Acid Innovations
Stabilized fragrance compositions and uses thereof
PatentWO2024074323A1
Innovation
- A fragrance composition comprising a fragrance oil and a stabilizing mixture with a combination of antioxidants, metal chelators, and pH regulators, formulated to maintain oxidative stability, where the stabilizing ingredients do not have the same function, and are used in consumer products to prevent olfactive deviation and chemical degradation, while being environmentally friendly and compatible with natural sources.
Fragranced cosmetic composition comprising an organic acid, a natural anthocyani(DI)n dye and a fragrancing material, and process for treating keratin materials and/or clothing using the composition
PatentWO2022144369A1
Innovation
- A fragranced composition comprising an organic acid, a natural or water-soluble anthocyanin dye, and a fragrancing substance, where the organic acid stabilizes the anthocyanin dye, maintaining color and odor stability over time, even under accelerated aging conditions and light exposure, while adhering to green chemistry principles.
Regulatory Compliance
The regulatory landscape surrounding the use of carbolic acid (phenol) in fragrance compounds is complex and multifaceted, requiring careful consideration by manufacturers and researchers. In the United States, the Food and Drug Administration (FDA) regulates the use of phenol in cosmetics and personal care products, including fragrances. The FDA has established specific limits on the concentration of phenol in these products, typically not exceeding 1% in rinse-off products and 0.5% in leave-on products.
The European Union, through its Cosmetic Regulation (EC) No. 1223/2009, has imposed stricter controls on phenol usage. The EU has classified phenol as a Category 1B substance under the Classification, Labelling and Packaging (CLP) Regulation, indicating its potential carcinogenicity. As a result, its use in cosmetic products is heavily restricted, with specific concentration limits and labeling requirements.
Globally, the International Fragrance Association (IFRA) provides guidelines for the safe use of fragrance ingredients, including phenol. IFRA's standards are widely adopted by the fragrance industry and often serve as a benchmark for regulatory compliance. These standards specify maximum concentrations for phenol in various product categories, taking into account potential skin sensitization and other safety concerns.
Manufacturers must also comply with workplace safety regulations when handling phenol during the production process. In the United States, the Occupational Safety and Health Administration (OSHA) has established permissible exposure limits (PELs) for phenol in the workplace. Similarly, the European Chemicals Agency (ECHA) provides guidance on safe handling and exposure limits under the REACH regulation.
Environmental regulations also play a crucial role in the use of phenol in fragrance stabilization. Many countries have implemented strict controls on the release of phenol into the environment due to its potential toxicity to aquatic life. Manufacturers must adhere to local and national environmental protection laws regarding waste disposal and emissions.
As research on carbolic acid in fragrance stabilization progresses, it is essential for researchers and manufacturers to stay abreast of evolving regulations. This includes monitoring changes in permitted concentrations, labeling requirements, and emerging scientific data on the safety and environmental impact of phenol. Compliance with these regulations not only ensures legal operation but also contributes to consumer safety and environmental protection in the fragrance industry.
The European Union, through its Cosmetic Regulation (EC) No. 1223/2009, has imposed stricter controls on phenol usage. The EU has classified phenol as a Category 1B substance under the Classification, Labelling and Packaging (CLP) Regulation, indicating its potential carcinogenicity. As a result, its use in cosmetic products is heavily restricted, with specific concentration limits and labeling requirements.
Globally, the International Fragrance Association (IFRA) provides guidelines for the safe use of fragrance ingredients, including phenol. IFRA's standards are widely adopted by the fragrance industry and often serve as a benchmark for regulatory compliance. These standards specify maximum concentrations for phenol in various product categories, taking into account potential skin sensitization and other safety concerns.
Manufacturers must also comply with workplace safety regulations when handling phenol during the production process. In the United States, the Occupational Safety and Health Administration (OSHA) has established permissible exposure limits (PELs) for phenol in the workplace. Similarly, the European Chemicals Agency (ECHA) provides guidance on safe handling and exposure limits under the REACH regulation.
Environmental regulations also play a crucial role in the use of phenol in fragrance stabilization. Many countries have implemented strict controls on the release of phenol into the environment due to its potential toxicity to aquatic life. Manufacturers must adhere to local and national environmental protection laws regarding waste disposal and emissions.
As research on carbolic acid in fragrance stabilization progresses, it is essential for researchers and manufacturers to stay abreast of evolving regulations. This includes monitoring changes in permitted concentrations, labeling requirements, and emerging scientific data on the safety and environmental impact of phenol. Compliance with these regulations not only ensures legal operation but also contributes to consumer safety and environmental protection in the fragrance industry.
Environmental Impact
The use of carbolic acid in the stabilization of fragrance compounds raises significant environmental concerns that warrant careful consideration. The production, application, and disposal of carbolic acid can have far-reaching impacts on ecosystems and human health if not properly managed.
One of the primary environmental issues associated with carbolic acid is its potential for water pollution. When released into aquatic environments, carbolic acid can be toxic to fish and other aquatic organisms, even at relatively low concentrations. It can disrupt the delicate balance of aquatic ecosystems, leading to long-term ecological damage. Furthermore, carbolic acid can contaminate groundwater sources, posing risks to both wildlife and human populations that rely on these water resources.
Air pollution is another environmental concern related to carbolic acid use in fragrance stabilization. During the manufacturing process and application of carbolic acid-based stabilizers, volatile organic compounds (VOCs) may be released into the atmosphere. These emissions can contribute to the formation of ground-level ozone and smog, which have detrimental effects on air quality and human respiratory health.
The persistence of carbolic acid in the environment is also a significant issue. Unlike some other organic compounds, carbolic acid does not readily biodegrade, meaning it can accumulate in soil and sediments over time. This persistence can lead to long-term contamination of ecosystems and potentially enter the food chain, affecting various trophic levels.
From a waste management perspective, the disposal of products containing carbolic acid-stabilized fragrances presents challenges. Improper disposal can lead to soil contamination and leaching into water systems. Additionally, the incineration of such products may release harmful substances into the air, further contributing to environmental pollution.
To mitigate these environmental impacts, research efforts are focusing on developing more eco-friendly alternatives to carbolic acid for fragrance stabilization. These include exploring natural stabilizers derived from plant sources, as well as investigating synthetic compounds with lower environmental persistence and toxicity profiles.
Regulatory bodies worldwide are also implementing stricter guidelines for the use and disposal of carbolic acid and related compounds. These regulations aim to minimize environmental exposure and promote more sustainable practices in the fragrance industry. As a result, companies are increasingly investing in green chemistry initiatives to develop environmentally benign stabilization methods that maintain fragrance integrity without compromising ecological health.
One of the primary environmental issues associated with carbolic acid is its potential for water pollution. When released into aquatic environments, carbolic acid can be toxic to fish and other aquatic organisms, even at relatively low concentrations. It can disrupt the delicate balance of aquatic ecosystems, leading to long-term ecological damage. Furthermore, carbolic acid can contaminate groundwater sources, posing risks to both wildlife and human populations that rely on these water resources.
Air pollution is another environmental concern related to carbolic acid use in fragrance stabilization. During the manufacturing process and application of carbolic acid-based stabilizers, volatile organic compounds (VOCs) may be released into the atmosphere. These emissions can contribute to the formation of ground-level ozone and smog, which have detrimental effects on air quality and human respiratory health.
The persistence of carbolic acid in the environment is also a significant issue. Unlike some other organic compounds, carbolic acid does not readily biodegrade, meaning it can accumulate in soil and sediments over time. This persistence can lead to long-term contamination of ecosystems and potentially enter the food chain, affecting various trophic levels.
From a waste management perspective, the disposal of products containing carbolic acid-stabilized fragrances presents challenges. Improper disposal can lead to soil contamination and leaching into water systems. Additionally, the incineration of such products may release harmful substances into the air, further contributing to environmental pollution.
To mitigate these environmental impacts, research efforts are focusing on developing more eco-friendly alternatives to carbolic acid for fragrance stabilization. These include exploring natural stabilizers derived from plant sources, as well as investigating synthetic compounds with lower environmental persistence and toxicity profiles.
Regulatory bodies worldwide are also implementing stricter guidelines for the use and disposal of carbolic acid and related compounds. These regulations aim to minimize environmental exposure and promote more sustainable practices in the fragrance industry. As a result, companies are increasingly investing in green chemistry initiatives to develop environmentally benign stabilization methods that maintain fragrance integrity without compromising ecological health.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!