Redox Kinetics and Mass Transport Optimization
OCT 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Redox Kinetics Background and Research Objectives
Redox reactions have been a cornerstone of electrochemical systems since the early 19th century, with foundational work by pioneers such as Michael Faraday establishing the fundamental principles of electron transfer. Over the past decades, redox kinetics has evolved from basic theoretical frameworks to sophisticated models incorporating multiple variables affecting reaction rates at electrode interfaces. The field has seen accelerated development with the advent of advanced analytical techniques and computational methods, enabling researchers to probe reaction mechanisms at unprecedented temporal and spatial resolutions.
The evolution of redox kinetics understanding has been closely tied to technological advancements in energy storage, catalysis, and sensing applications. From the Butler-Volmer equation to Marcus theory, theoretical frameworks have progressively refined our ability to predict and control electron transfer processes. Recent trends indicate a shift toward multi-scale modeling approaches that bridge molecular-level phenomena with macroscopic performance metrics, particularly important for complex systems with coupled chemical and transport processes.
Our technical objectives in this research focus on elucidating the fundamental mechanisms governing redox reactions under various operating conditions while developing optimized strategies for mass transport enhancement. Specifically, we aim to quantify the relationship between electrode architecture and reaction kinetics, identify rate-limiting steps in complex redox systems, and establish predictive models that can guide material design for targeted applications.
The research seeks to address several critical knowledge gaps, including the influence of nanoscale structural features on local reaction environments, the interplay between homogeneous and heterogeneous electron transfer processes, and the development of universal descriptors for redox activity across diverse material classes. By resolving these questions, we anticipate enabling significant improvements in the efficiency and performance of electrochemical technologies.
A key objective is to develop methodologies that decouple mass transport limitations from intrinsic kinetic parameters, allowing for more accurate determination of fundamental reaction constants. This separation is essential for designing systems where either kinetics or transport can be selectively enhanced based on application requirements. Additionally, we aim to establish standardized protocols for measuring and reporting redox kinetics that facilitate meaningful comparisons across different research groups and experimental conditions.
The ultimate goal of this research is to translate fundamental understanding into practical design principles for next-generation electrochemical systems with optimized redox kinetics and mass transport characteristics. Success in this endeavor would significantly impact fields ranging from grid-scale energy storage to point-of-care diagnostic devices, where efficient electron transfer processes are critical performance determinants.
The evolution of redox kinetics understanding has been closely tied to technological advancements in energy storage, catalysis, and sensing applications. From the Butler-Volmer equation to Marcus theory, theoretical frameworks have progressively refined our ability to predict and control electron transfer processes. Recent trends indicate a shift toward multi-scale modeling approaches that bridge molecular-level phenomena with macroscopic performance metrics, particularly important for complex systems with coupled chemical and transport processes.
Our technical objectives in this research focus on elucidating the fundamental mechanisms governing redox reactions under various operating conditions while developing optimized strategies for mass transport enhancement. Specifically, we aim to quantify the relationship between electrode architecture and reaction kinetics, identify rate-limiting steps in complex redox systems, and establish predictive models that can guide material design for targeted applications.
The research seeks to address several critical knowledge gaps, including the influence of nanoscale structural features on local reaction environments, the interplay between homogeneous and heterogeneous electron transfer processes, and the development of universal descriptors for redox activity across diverse material classes. By resolving these questions, we anticipate enabling significant improvements in the efficiency and performance of electrochemical technologies.
A key objective is to develop methodologies that decouple mass transport limitations from intrinsic kinetic parameters, allowing for more accurate determination of fundamental reaction constants. This separation is essential for designing systems where either kinetics or transport can be selectively enhanced based on application requirements. Additionally, we aim to establish standardized protocols for measuring and reporting redox kinetics that facilitate meaningful comparisons across different research groups and experimental conditions.
The ultimate goal of this research is to translate fundamental understanding into practical design principles for next-generation electrochemical systems with optimized redox kinetics and mass transport characteristics. Success in this endeavor would significantly impact fields ranging from grid-scale energy storage to point-of-care diagnostic devices, where efficient electron transfer processes are critical performance determinants.
Market Applications and Demand Analysis
The redox kinetics and mass transport optimization market is experiencing significant growth driven by the increasing demand for energy storage solutions, particularly in renewable energy integration. As global energy systems transition toward cleaner alternatives, the need for efficient energy storage technologies has become paramount. The global energy storage market, where redox kinetics plays a crucial role, is projected to grow at a compound annual growth rate of 20-25% through 2030, reaching hundreds of billions in market value.
The electrochemical energy storage sector, including redox flow batteries, lithium-ion batteries, and fuel cells, represents the largest application segment for redox kinetics research. These technologies are essential for grid-scale energy storage, which is necessary to address the intermittency challenges of renewable energy sources like solar and wind. Utility companies and grid operators are increasingly investing in advanced energy storage solutions to enhance grid stability and resilience.
Beyond energy storage, industrial catalysis represents another significant market for redox kinetics optimization. The chemical manufacturing industry relies heavily on catalytic processes where electron transfer and mass transport phenomena are fundamental. Optimized redox systems can significantly reduce energy consumption and increase yield in various industrial processes, including petroleum refining, pharmaceutical manufacturing, and fine chemical production.
The environmental remediation sector also presents substantial market opportunities. Advanced oxidation processes utilizing optimized redox reactions are increasingly employed for wastewater treatment, soil remediation, and air purification. As environmental regulations become more stringent globally, the demand for efficient remediation technologies continues to rise.
The healthcare and biomedical sectors are emerging markets for redox kinetics applications. Biosensors, drug delivery systems, and diagnostic tools often rely on controlled redox reactions. The global biosensors market alone is growing rapidly, with applications ranging from glucose monitoring to pathogen detection.
Market analysis indicates that companies are increasingly focused on developing proprietary technologies that optimize mass transport in redox systems. This trend is particularly evident in the competitive landscape of flow battery manufacturers, where enhanced mass transport can significantly improve power density and overall system performance.
Regional market assessment shows that Asia-Pacific, particularly China, Japan, and South Korea, leads in commercial applications of advanced redox technologies, while North America and Europe dominate in research and development activities. Government incentives for clean energy technologies in these regions are further accelerating market growth and technological innovation in redox kinetics optimization.
The electrochemical energy storage sector, including redox flow batteries, lithium-ion batteries, and fuel cells, represents the largest application segment for redox kinetics research. These technologies are essential for grid-scale energy storage, which is necessary to address the intermittency challenges of renewable energy sources like solar and wind. Utility companies and grid operators are increasingly investing in advanced energy storage solutions to enhance grid stability and resilience.
Beyond energy storage, industrial catalysis represents another significant market for redox kinetics optimization. The chemical manufacturing industry relies heavily on catalytic processes where electron transfer and mass transport phenomena are fundamental. Optimized redox systems can significantly reduce energy consumption and increase yield in various industrial processes, including petroleum refining, pharmaceutical manufacturing, and fine chemical production.
The environmental remediation sector also presents substantial market opportunities. Advanced oxidation processes utilizing optimized redox reactions are increasingly employed for wastewater treatment, soil remediation, and air purification. As environmental regulations become more stringent globally, the demand for efficient remediation technologies continues to rise.
The healthcare and biomedical sectors are emerging markets for redox kinetics applications. Biosensors, drug delivery systems, and diagnostic tools often rely on controlled redox reactions. The global biosensors market alone is growing rapidly, with applications ranging from glucose monitoring to pathogen detection.
Market analysis indicates that companies are increasingly focused on developing proprietary technologies that optimize mass transport in redox systems. This trend is particularly evident in the competitive landscape of flow battery manufacturers, where enhanced mass transport can significantly improve power density and overall system performance.
Regional market assessment shows that Asia-Pacific, particularly China, Japan, and South Korea, leads in commercial applications of advanced redox technologies, while North America and Europe dominate in research and development activities. Government incentives for clean energy technologies in these regions are further accelerating market growth and technological innovation in redox kinetics optimization.
Current Challenges in Redox Kinetics and Mass Transport
Despite significant advancements in redox chemistry and mass transport phenomena, several critical challenges continue to impede progress in this field. The complexity of electron transfer kinetics at electrode-electrolyte interfaces remains a fundamental obstacle, particularly in systems with multiple redox species or complex reaction mechanisms. Current analytical models often fail to accurately predict reaction rates under non-ideal conditions, such as high current densities or in the presence of competing side reactions.
Mass transport limitations represent another significant barrier, especially in energy storage and conversion devices. The movement of reactants to and products from reaction sites frequently becomes rate-limiting, reducing overall system efficiency. This challenge is particularly pronounced in porous electrode structures where diffusion pathways are tortuous and variable. Conventional approaches to enhance mass transport often compromise other system parameters, creating difficult engineering trade-offs.
The coupling between redox kinetics and mass transport presents perhaps the most formidable challenge. These phenomena do not operate independently but rather influence each other in complex, often non-linear ways. For instance, concentration gradients arising from mass transport limitations can significantly alter local reaction kinetics, while reaction rates affect the concentration profiles that drive diffusion. Current modeling approaches frequently treat these phenomena separately, leading to inaccurate predictions in real-world applications.
Scale-up issues further complicate matters, as laboratory-optimized systems often perform poorly when implemented at industrial scales. The behavior of redox systems can change dramatically with increasing dimensions due to emergent transport phenomena and heat management challenges. This scale-dependence is particularly problematic for emerging technologies like flow batteries and industrial electrolysis.
Material stability under redox conditions represents another persistent challenge. Electrode materials and catalysts frequently degrade during operation, particularly under extreme pH conditions or high potential regions. This degradation alters surface properties, affecting both kinetics and mass transport characteristics over time. The development of stable materials that maintain consistent performance remains elusive for many applications.
Measurement and characterization techniques also present limitations. Current methods often cannot resolve spatial and temporal variations in redox processes with sufficient precision, particularly in complex, heterogeneous systems. This diagnostic gap hinders the development of more sophisticated understanding and optimization strategies. Advanced operando techniques are emerging but remain specialized and not widely accessible.
Mass transport limitations represent another significant barrier, especially in energy storage and conversion devices. The movement of reactants to and products from reaction sites frequently becomes rate-limiting, reducing overall system efficiency. This challenge is particularly pronounced in porous electrode structures where diffusion pathways are tortuous and variable. Conventional approaches to enhance mass transport often compromise other system parameters, creating difficult engineering trade-offs.
The coupling between redox kinetics and mass transport presents perhaps the most formidable challenge. These phenomena do not operate independently but rather influence each other in complex, often non-linear ways. For instance, concentration gradients arising from mass transport limitations can significantly alter local reaction kinetics, while reaction rates affect the concentration profiles that drive diffusion. Current modeling approaches frequently treat these phenomena separately, leading to inaccurate predictions in real-world applications.
Scale-up issues further complicate matters, as laboratory-optimized systems often perform poorly when implemented at industrial scales. The behavior of redox systems can change dramatically with increasing dimensions due to emergent transport phenomena and heat management challenges. This scale-dependence is particularly problematic for emerging technologies like flow batteries and industrial electrolysis.
Material stability under redox conditions represents another persistent challenge. Electrode materials and catalysts frequently degrade during operation, particularly under extreme pH conditions or high potential regions. This degradation alters surface properties, affecting both kinetics and mass transport characteristics over time. The development of stable materials that maintain consistent performance remains elusive for many applications.
Measurement and characterization techniques also present limitations. Current methods often cannot resolve spatial and temporal variations in redox processes with sufficient precision, particularly in complex, heterogeneous systems. This diagnostic gap hinders the development of more sophisticated understanding and optimization strategies. Advanced operando techniques are emerging but remain specialized and not widely accessible.
Contemporary Methodologies for Mass Transport Optimization
01 Electrochemical cell design for redox reactions
Electrochemical cells are designed to optimize redox reaction kinetics by controlling electrode materials, electrolyte composition, and cell configuration. These designs focus on enhancing electron transfer rates and reducing mass transport limitations at electrode interfaces. Advanced cell architectures incorporate features that minimize internal resistance and maximize active surface area, thereby improving overall reaction efficiency and power output.- Electrochemical cell design for redox reactions: Electrochemical cells are designed to optimize redox reaction kinetics by controlling electrode materials, electrolyte composition, and cell configuration. These designs focus on enhancing electron transfer rates at electrode surfaces while managing mass transport limitations. Advanced cell architectures incorporate features to minimize concentration polarization and improve reaction efficiency through strategic placement of electrodes and optimized flow patterns.
- Modeling and simulation of redox kinetics: Computational methods are employed to model redox reaction kinetics and mass transport phenomena. These models incorporate parameters such as diffusion coefficients, reaction rate constants, and electrode surface properties to predict system behavior. Simulation techniques help optimize reaction conditions by analyzing the interplay between electron transfer kinetics and mass transport limitations, enabling researchers to design more efficient redox systems without extensive experimental testing.
- Catalysts for enhancing redox reaction rates: Specialized catalysts are developed to accelerate redox reactions by lowering activation energy barriers and providing alternative reaction pathways. These catalysts can be homogeneous or heterogeneous, with the latter often designed as nanostructured materials to maximize surface area and active sites. By enhancing reaction kinetics, these catalysts help overcome mass transport limitations and improve overall system efficiency in applications ranging from fuel cells to industrial chemical processes.
- Mass transport enhancement techniques: Various methods are employed to enhance mass transport in redox reaction systems, including forced convection, ultrasonic agitation, and microfluidic channel designs. These techniques aim to reduce diffusion layer thickness and increase the rate at which reactants reach electrode surfaces. By addressing mass transport limitations, these approaches allow redox reactions to proceed at rates closer to their kinetic limits, improving overall system performance and energy efficiency.
- Redox flow battery optimization: Redox flow batteries represent a significant application of redox reaction kinetics and mass transport principles. Optimization strategies focus on electrolyte composition, membrane properties, and flow field designs to enhance energy density and power output. Advanced flow battery systems incorporate electrode materials with high catalytic activity and employ flow patterns that minimize concentration polarization, addressing both kinetic and mass transport aspects of battery performance.
02 Modeling and simulation of redox kinetics
Computational methods are employed to model redox reaction kinetics and mass transport phenomena. These models incorporate parameters such as diffusion coefficients, reaction rate constants, and electrode geometry to predict system behavior under various conditions. Simulation techniques help optimize reaction conditions, identify rate-limiting steps, and guide experimental design for improved performance in applications ranging from energy storage to chemical synthesis.Expand Specific Solutions03 Catalysts for enhancing redox reaction rates
Catalytic materials are developed to accelerate redox reactions by providing alternative reaction pathways with lower activation energies. These catalysts can be homogeneous or heterogeneous, and their design focuses on maximizing active sites, stability, and selectivity. Novel catalyst formulations incorporate nanomaterials, metal complexes, or enzyme-inspired structures to enhance electron transfer kinetics while minimizing mass transport limitations.Expand Specific Solutions04 Mass transport enhancement in redox systems
Techniques to improve mass transport in redox reaction systems include optimized flow field designs, porous electrode structures, and advanced mixing methods. These approaches aim to reduce concentration gradients and enhance the delivery of reactants to active sites. Strategies such as forced convection, ultrasonic agitation, and microfluidic channel designs help overcome diffusion limitations that would otherwise restrict reaction rates in electrochemical and chemical redox processes.Expand Specific Solutions05 Redox flow battery technology
Redox flow batteries utilize controlled redox reactions with liquid electrolytes containing active species that can be oxidized and reduced. These systems separate power and energy capacity, allowing for flexible scaling. The performance of these batteries depends on electrolyte composition, membrane properties, and electrode design, all of which affect reaction kinetics and mass transport. Innovations focus on improving energy density, cycle life, and system efficiency through advanced materials and cell architectures.Expand Specific Solutions
Leading Research Institutions and Industry Players
The redox kinetics and mass transport optimization field is currently in a growth phase, characterized by increasing research intensity and technological applications. The market is expanding rapidly, driven by energy storage, fuel cells, and electrochemical process optimization demands. While the technology shows promising maturity in academic settings, with Beijing Jiaotong University, Southwest Jiaotong University, and Wuhan University leading fundamental research, industrial implementation varies. Companies like Toshiba, Hitachi, and Toyota are advancing practical applications, particularly in energy systems and automotive sectors. Siemens and ExxonMobil are developing industrial-scale solutions, while emerging players like Impargo focus on specialized transport optimization applications, creating a competitive landscape balancing established corporations and innovative newcomers.
Hitachi Ltd.
Technical Solution: Hitachi has developed advanced redox flow battery systems with optimized mass transport characteristics for grid-scale energy storage applications. Their technology incorporates innovative electrode materials with controlled surface chemistry and optimized microstructure to enhance reaction kinetics at the electrode-electrolyte interface. Hitachi's approach includes computational fluid dynamics modeling to design flow field geometries that minimize pressure drop while maximizing electrolyte distribution uniformity. They've implemented novel membrane technologies with tailored ion selectivity and conductivity properties, reducing crossover effects while maintaining high ionic flux. Their systems feature adaptive flow control algorithms that adjust electrolyte circulation rates based on real-time monitoring of concentration gradients and reaction rates, ensuring optimal mass transport conditions across varying charge/discharge cycles. This comprehensive approach has enabled Hitachi to achieve energy efficiencies exceeding 80% in their redox flow systems, with significantly improved power density compared to conventional designs.
Strengths: Holistic system-level optimization that addresses multiple aspects of mass transport limitations simultaneously, resulting in commercially viable large-scale energy storage solutions. Weakness: Higher system complexity requires sophisticated control systems and may present maintenance challenges in certain deployment scenarios.
Toyota Motor Corp.
Technical Solution: Toyota has developed innovative fuel cell technologies with advanced approaches to redox kinetics and mass transport optimization. Their research focuses on enhancing oxygen reduction reaction (ORR) kinetics at the cathode while optimizing hydrogen oxidation at the anode. Toyota's proprietary catalyst designs feature controlled particle size distribution and novel support materials that maximize triple-phase boundary regions, significantly improving reaction rates. They've implemented sophisticated gas diffusion layers with gradient porosity structures that optimize reactant transport while effectively managing water removal—a critical factor in maintaining consistent performance. Their systems incorporate computational models that predict local current density distributions and identify potential mass transport limitations under various operating conditions. Toyota has also pioneered membrane electrode assemblies with optimized ionomer distributions that enhance proton conductivity while minimizing mass transport resistance. These technologies have enabled Toyota to achieve power densities exceeding 4 W/cm² in their latest fuel cell stacks, representing a significant advancement over previous generations.
Strengths: Exceptional integration of materials science with system engineering, resulting in commercially viable fuel cell vehicles with industry-leading power density and durability. Weakness: Solutions are highly specialized for automotive applications and may require significant adaptation for other applications.
Key Scientific Breakthroughs in Redox Reaction Mechanisms
Novel-architecture electrodes with enhanced mass transport for high-efficiency and low-cost hydrogen energy
PatentWO2023023093A1
Innovation
- A fluid flow assembly with a bipolar plate flow field and a liquid/gas diffusion layer featuring micro-patterned pores that are unobstructed by the bipolar plate lands, including additional micro channels for enhanced in-plane and through-plane transport, such as pin-type and wedge-shaped designs, to improve reactant and product transport.
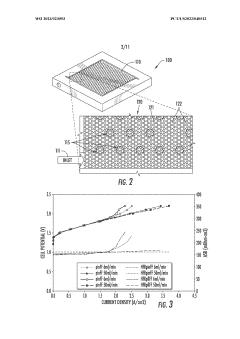
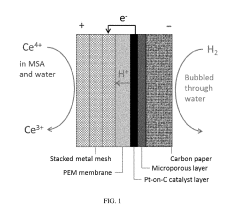
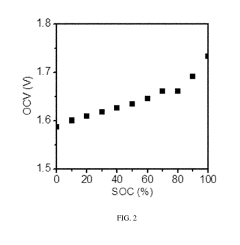
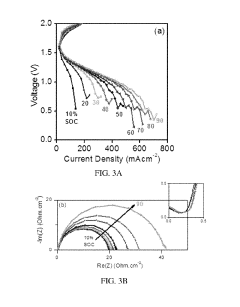
Optimization of the cerium-hydrogen redox flow cell
PatentActiveUS10424804B2
Innovation
- The implementation of a Ce—H2 redox flow cell with a 3-dimensional porous positive electrode, nanostructured thin-film platinum catalyst layer, and optimized electrolyte composition of 0.6 M Cerium and 5 M MSA, operated at elevated temperatures, and utilizing a pre-boiled membrane to minimize crossover and maximize energy efficiency.
Computational Modeling Approaches for Redox Systems
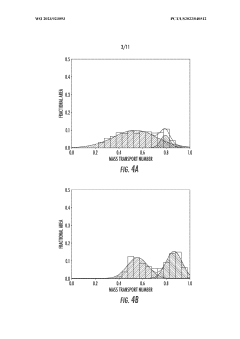
Computational modeling has emerged as a powerful tool for understanding and optimizing redox systems across various applications. These modeling approaches span multiple scales, from quantum mechanical calculations that capture electronic structure and reaction mechanisms to continuum models that describe mass transport phenomena in complex electrochemical systems.
Density Functional Theory (DFT) calculations represent the foundation of computational approaches for redox systems, enabling researchers to investigate electron transfer processes at the atomic level. These calculations provide insights into reaction energetics, activation barriers, and the electronic properties of redox-active species. Recent advancements in DFT methodologies have improved accuracy in predicting redox potentials and reaction mechanisms, particularly for transition metal complexes and organic redox mediators.
Molecular dynamics (MD) simulations complement quantum mechanical approaches by modeling the dynamic behavior of redox species in solution. These simulations capture solvent effects, diffusion processes, and conformational changes that influence redox kinetics. The integration of reactive force fields has further enhanced MD capabilities, allowing for the simulation of bond breaking and formation during redox reactions while maintaining computational efficiency.
Multiphysics models have gained prominence for system-level analysis of redox processes. These models couple electrochemistry with fluid dynamics, heat transfer, and mass transport to simulate complex electrochemical devices such as flow batteries, fuel cells, and electrolyzers. Commercial software packages like COMSOL Multiphysics and ANSYS Fluent provide platforms for implementing these coupled models, enabling optimization of electrode geometries, flow field designs, and operating conditions.
Machine learning approaches are increasingly being applied to redox systems, offering new pathways for accelerated materials discovery and process optimization. Neural networks trained on experimental and computational data can predict redox properties of novel compounds, while genetic algorithms optimize complex multivariable systems. These data-driven methods complement traditional physics-based models, particularly when dealing with systems too complex for ab initio calculations.
Digital twins represent the frontier of computational modeling for redox systems, creating virtual replicas of physical devices that update in real-time based on operational data. This approach enables predictive maintenance, performance optimization, and failure analysis for redox-based technologies in industrial settings. The integration of physics-based models with real-time data streams provides unprecedented capabilities for monitoring and controlling redox processes across scales.
Density Functional Theory (DFT) calculations represent the foundation of computational approaches for redox systems, enabling researchers to investigate electron transfer processes at the atomic level. These calculations provide insights into reaction energetics, activation barriers, and the electronic properties of redox-active species. Recent advancements in DFT methodologies have improved accuracy in predicting redox potentials and reaction mechanisms, particularly for transition metal complexes and organic redox mediators.
Molecular dynamics (MD) simulations complement quantum mechanical approaches by modeling the dynamic behavior of redox species in solution. These simulations capture solvent effects, diffusion processes, and conformational changes that influence redox kinetics. The integration of reactive force fields has further enhanced MD capabilities, allowing for the simulation of bond breaking and formation during redox reactions while maintaining computational efficiency.
Multiphysics models have gained prominence for system-level analysis of redox processes. These models couple electrochemistry with fluid dynamics, heat transfer, and mass transport to simulate complex electrochemical devices such as flow batteries, fuel cells, and electrolyzers. Commercial software packages like COMSOL Multiphysics and ANSYS Fluent provide platforms for implementing these coupled models, enabling optimization of electrode geometries, flow field designs, and operating conditions.
Machine learning approaches are increasingly being applied to redox systems, offering new pathways for accelerated materials discovery and process optimization. Neural networks trained on experimental and computational data can predict redox properties of novel compounds, while genetic algorithms optimize complex multivariable systems. These data-driven methods complement traditional physics-based models, particularly when dealing with systems too complex for ab initio calculations.
Digital twins represent the frontier of computational modeling for redox systems, creating virtual replicas of physical devices that update in real-time based on operational data. This approach enables predictive maintenance, performance optimization, and failure analysis for redox-based technologies in industrial settings. The integration of physics-based models with real-time data streams provides unprecedented capabilities for monitoring and controlling redox processes across scales.
Environmental Impact and Sustainability Considerations
The optimization of redox kinetics and mass transport processes carries significant environmental implications that must be carefully considered in sustainable technology development. These processes, fundamental to numerous industrial applications including energy storage systems, catalytic converters, and water treatment facilities, can either contribute to environmental degradation or promote sustainability depending on their implementation.
Energy efficiency represents a primary environmental consideration in redox systems. Enhanced kinetics and optimized mass transport directly translate to reduced energy requirements, minimizing the carbon footprint associated with these processes. Research indicates that optimized redox systems can achieve energy savings of 15-30% compared to conventional approaches, representing substantial greenhouse gas emission reductions when implemented at industrial scales.
Material selection for redox applications presents another critical environmental dimension. Traditional redox catalysts often incorporate rare earth elements or precious metals with environmentally problematic extraction processes. Recent advances focus on developing catalysts using earth-abundant materials that maintain comparable performance while reducing environmental impact. These sustainable alternatives minimize ecosystem disruption associated with mining operations and reduce toxic waste generation.
Waste stream management constitutes a significant environmental challenge in redox processes. Optimized mass transport can substantially reduce the generation of unwanted byproducts and increase reaction selectivity. This improvement directly translates to decreased waste treatment requirements and minimized environmental contamination risks. Advanced monitoring systems that track reaction kinetics in real-time enable precise control that further enhances environmental performance.
Life cycle assessment (LCA) methodologies reveal that redox kinetics optimization delivers environmental benefits beyond operational efficiency. Comprehensive analyses demonstrate that improvements in reaction rates and mass transport can extend catalyst lifespans by 40-60%, reducing replacement frequency and associated manufacturing impacts. This extended operational lifetime represents a crucial sustainability advantage that compounds over multiple system generations.
Water consumption represents another environmental consideration, particularly relevant in electrochemical redox applications. Optimized mass transport reduces water requirements for solution-based processes, addressing growing concerns about water scarcity. Research indicates potential water usage reductions of 25-35% through advanced transport mechanisms that maintain reaction efficiency while minimizing resource consumption.
Regulatory compliance increasingly drives environmental considerations in redox technology development. Stringent emissions standards and chemical management regulations necessitate sophisticated kinetics control to ensure processes remain within acceptable environmental parameters. Forward-looking research must anticipate regulatory evolution to develop technologies that remain viable under increasingly strict environmental governance frameworks.
Energy efficiency represents a primary environmental consideration in redox systems. Enhanced kinetics and optimized mass transport directly translate to reduced energy requirements, minimizing the carbon footprint associated with these processes. Research indicates that optimized redox systems can achieve energy savings of 15-30% compared to conventional approaches, representing substantial greenhouse gas emission reductions when implemented at industrial scales.
Material selection for redox applications presents another critical environmental dimension. Traditional redox catalysts often incorporate rare earth elements or precious metals with environmentally problematic extraction processes. Recent advances focus on developing catalysts using earth-abundant materials that maintain comparable performance while reducing environmental impact. These sustainable alternatives minimize ecosystem disruption associated with mining operations and reduce toxic waste generation.
Waste stream management constitutes a significant environmental challenge in redox processes. Optimized mass transport can substantially reduce the generation of unwanted byproducts and increase reaction selectivity. This improvement directly translates to decreased waste treatment requirements and minimized environmental contamination risks. Advanced monitoring systems that track reaction kinetics in real-time enable precise control that further enhances environmental performance.
Life cycle assessment (LCA) methodologies reveal that redox kinetics optimization delivers environmental benefits beyond operational efficiency. Comprehensive analyses demonstrate that improvements in reaction rates and mass transport can extend catalyst lifespans by 40-60%, reducing replacement frequency and associated manufacturing impacts. This extended operational lifetime represents a crucial sustainability advantage that compounds over multiple system generations.
Water consumption represents another environmental consideration, particularly relevant in electrochemical redox applications. Optimized mass transport reduces water requirements for solution-based processes, addressing growing concerns about water scarcity. Research indicates potential water usage reductions of 25-35% through advanced transport mechanisms that maintain reaction efficiency while minimizing resource consumption.
Regulatory compliance increasingly drives environmental considerations in redox technology development. Stringent emissions standards and chemical management regulations necessitate sophisticated kinetics control to ensure processes remain within acceptable environmental parameters. Forward-looking research must anticipate regulatory evolution to develop technologies that remain viable under increasingly strict environmental governance frameworks.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!