Sodium silicate reinforced hydrogel for tissue engineering
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogel Development for Tissue Engineering
Hydrogel development for tissue engineering has seen significant advancements in recent years, with a particular focus on enhancing the mechanical properties and biocompatibility of these materials. The incorporation of sodium silicate into hydrogels represents a promising approach to address some of the key challenges in this field.
Hydrogels are three-dimensional networks of hydrophilic polymers that can absorb and retain large amounts of water. Their structural similarity to the extracellular matrix makes them ideal candidates for tissue engineering applications. However, traditional hydrogels often lack the necessary mechanical strength and durability required for load-bearing tissues.
The introduction of sodium silicate as a reinforcing agent in hydrogels has emerged as a potential solution to overcome these limitations. Sodium silicate, also known as water glass, is an inorganic compound that can form strong chemical bonds with the polymer network of hydrogels. This interaction results in improved mechanical properties, including increased compressive strength, tensile strength, and elastic modulus.
One of the key advantages of sodium silicate reinforced hydrogels is their enhanced biocompatibility. The silicate ions released from the hydrogel network can stimulate cell proliferation and differentiation, promoting tissue regeneration. Additionally, these ions can induce the formation of hydroxyapatite, a mineral component of bone, making these hydrogels particularly suitable for bone tissue engineering applications.
Recent research has focused on optimizing the composition and fabrication methods of sodium silicate reinforced hydrogels. Various techniques, such as in situ polymerization, freeze-drying, and 3D printing, have been explored to create hydrogels with tailored properties for specific tissue engineering applications. The ability to control the porosity, degradation rate, and mechanical properties of these hydrogels allows for the development of scaffolds that closely mimic the native tissue environment.
Furthermore, the incorporation of sodium silicate into hydrogels has opened up new possibilities for creating smart, responsive materials. These hydrogels can be designed to respond to external stimuli such as pH, temperature, or mechanical stress, enabling controlled release of bioactive molecules or dynamic changes in scaffold properties to guide tissue regeneration.
As research in this field progresses, there is a growing interest in developing multifunctional sodium silicate reinforced hydrogels that combine mechanical strength with other desirable properties, such as antimicrobial activity or electrical conductivity. These advanced materials hold great promise for addressing complex tissue engineering challenges and enabling the development of more effective regenerative therapies.
Hydrogels are three-dimensional networks of hydrophilic polymers that can absorb and retain large amounts of water. Their structural similarity to the extracellular matrix makes them ideal candidates for tissue engineering applications. However, traditional hydrogels often lack the necessary mechanical strength and durability required for load-bearing tissues.
The introduction of sodium silicate as a reinforcing agent in hydrogels has emerged as a potential solution to overcome these limitations. Sodium silicate, also known as water glass, is an inorganic compound that can form strong chemical bonds with the polymer network of hydrogels. This interaction results in improved mechanical properties, including increased compressive strength, tensile strength, and elastic modulus.
One of the key advantages of sodium silicate reinforced hydrogels is their enhanced biocompatibility. The silicate ions released from the hydrogel network can stimulate cell proliferation and differentiation, promoting tissue regeneration. Additionally, these ions can induce the formation of hydroxyapatite, a mineral component of bone, making these hydrogels particularly suitable for bone tissue engineering applications.
Recent research has focused on optimizing the composition and fabrication methods of sodium silicate reinforced hydrogels. Various techniques, such as in situ polymerization, freeze-drying, and 3D printing, have been explored to create hydrogels with tailored properties for specific tissue engineering applications. The ability to control the porosity, degradation rate, and mechanical properties of these hydrogels allows for the development of scaffolds that closely mimic the native tissue environment.
Furthermore, the incorporation of sodium silicate into hydrogels has opened up new possibilities for creating smart, responsive materials. These hydrogels can be designed to respond to external stimuli such as pH, temperature, or mechanical stress, enabling controlled release of bioactive molecules or dynamic changes in scaffold properties to guide tissue regeneration.
As research in this field progresses, there is a growing interest in developing multifunctional sodium silicate reinforced hydrogels that combine mechanical strength with other desirable properties, such as antimicrobial activity or electrical conductivity. These advanced materials hold great promise for addressing complex tissue engineering challenges and enabling the development of more effective regenerative therapies.
Market Analysis for Biomedical Hydrogels
The biomedical hydrogel market has been experiencing significant growth in recent years, driven by the increasing demand for advanced tissue engineering solutions and regenerative medicine applications. Sodium silicate reinforced hydrogels, in particular, have garnered attention due to their enhanced mechanical properties and biocompatibility, making them promising candidates for various tissue engineering applications.
The global market for biomedical hydrogels is projected to reach substantial value in the coming years, with a compound annual growth rate (CAGR) outpacing many other segments in the healthcare industry. This growth is primarily attributed to the rising prevalence of chronic diseases, an aging population, and the increasing adoption of minimally invasive surgical procedures.
Tissue engineering applications represent a significant portion of the biomedical hydrogel market, with sodium silicate reinforced hydrogels showing particular promise in this area. These advanced materials offer improved structural integrity and cell adhesion properties, making them ideal for applications such as wound healing, cartilage regeneration, and bone tissue engineering.
The market for sodium silicate reinforced hydrogels in tissue engineering is expected to witness robust growth, driven by ongoing research and development activities, as well as increasing investments in regenerative medicine. Key market players are focusing on developing innovative products and expanding their product portfolios to gain a competitive edge in this rapidly evolving market.
Geographically, North America currently dominates the biomedical hydrogel market, followed by Europe and Asia-Pacific. However, emerging economies in Asia-Pacific and Latin America are expected to present lucrative growth opportunities due to improving healthcare infrastructure and increasing healthcare expenditure.
Despite the positive outlook, the market faces challenges such as stringent regulatory requirements and the high cost of advanced hydrogel-based products. These factors may hinder market growth to some extent, particularly in developing regions.
In conclusion, the market analysis for biomedical hydrogels, particularly sodium silicate reinforced hydrogels for tissue engineering, indicates a promising future with significant growth potential. As research continues to advance and new applications emerge, this market segment is poised to play a crucial role in the broader field of regenerative medicine and tissue engineering.
The global market for biomedical hydrogels is projected to reach substantial value in the coming years, with a compound annual growth rate (CAGR) outpacing many other segments in the healthcare industry. This growth is primarily attributed to the rising prevalence of chronic diseases, an aging population, and the increasing adoption of minimally invasive surgical procedures.
Tissue engineering applications represent a significant portion of the biomedical hydrogel market, with sodium silicate reinforced hydrogels showing particular promise in this area. These advanced materials offer improved structural integrity and cell adhesion properties, making them ideal for applications such as wound healing, cartilage regeneration, and bone tissue engineering.
The market for sodium silicate reinforced hydrogels in tissue engineering is expected to witness robust growth, driven by ongoing research and development activities, as well as increasing investments in regenerative medicine. Key market players are focusing on developing innovative products and expanding their product portfolios to gain a competitive edge in this rapidly evolving market.
Geographically, North America currently dominates the biomedical hydrogel market, followed by Europe and Asia-Pacific. However, emerging economies in Asia-Pacific and Latin America are expected to present lucrative growth opportunities due to improving healthcare infrastructure and increasing healthcare expenditure.
Despite the positive outlook, the market faces challenges such as stringent regulatory requirements and the high cost of advanced hydrogel-based products. These factors may hinder market growth to some extent, particularly in developing regions.
In conclusion, the market analysis for biomedical hydrogels, particularly sodium silicate reinforced hydrogels for tissue engineering, indicates a promising future with significant growth potential. As research continues to advance and new applications emerge, this market segment is poised to play a crucial role in the broader field of regenerative medicine and tissue engineering.
Current Challenges in Hydrogel Reinforcement
Despite significant advancements in hydrogel technology, several challenges persist in the reinforcement of hydrogels for tissue engineering applications, particularly when using sodium silicate as a reinforcing agent. One of the primary obstacles is achieving an optimal balance between mechanical strength and biocompatibility. While sodium silicate can enhance the structural integrity of hydrogels, excessive concentrations may compromise cell viability and tissue integration.
Another critical challenge lies in controlling the gelation kinetics and crosslinking density of sodium silicate-reinforced hydrogels. The rapid gelation process of sodium silicate can lead to inhomogeneous structures and poor reproducibility, making it difficult to create consistent and predictable hydrogel properties. This inconsistency can significantly impact the hydrogel's performance in tissue engineering applications, where precise control over mechanical and biological properties is crucial.
The long-term stability of sodium silicate-reinforced hydrogels in physiological conditions remains a concern. The gradual dissolution of silicate ions in aqueous environments can lead to changes in the hydrogel's mechanical properties over time, potentially compromising its structural integrity and functionality within the engineered tissue constructs.
Furthermore, the integration of sodium silicate into hydrogels while maintaining their porosity and permeability presents a significant challenge. The reinforcement process must not compromise the hydrogel's ability to facilitate nutrient diffusion and waste removal, which are essential for cell survival and tissue growth.
The biocompatibility and potential toxicity of sodium silicate-reinforced hydrogels require thorough investigation. While silicate-based materials have shown promise in various biomedical applications, the long-term effects of silicate ions on cellular behavior, differentiation, and tissue formation need to be carefully evaluated to ensure the safety and efficacy of these reinforced hydrogels in tissue engineering.
Another challenge is the development of scalable and reproducible manufacturing processes for sodium silicate-reinforced hydrogels. Current methods often face difficulties in maintaining consistent properties when scaling up from laboratory to industrial production, which is crucial for the widespread adoption of these materials in clinical applications.
Lastly, the optimization of sodium silicate reinforcement for specific tissue types and applications remains a complex task. Different tissues require varying mechanical properties and degradation profiles, necessitating tailored approaches to hydrogel reinforcement that can meet the diverse needs of various tissue engineering applications.
Another critical challenge lies in controlling the gelation kinetics and crosslinking density of sodium silicate-reinforced hydrogels. The rapid gelation process of sodium silicate can lead to inhomogeneous structures and poor reproducibility, making it difficult to create consistent and predictable hydrogel properties. This inconsistency can significantly impact the hydrogel's performance in tissue engineering applications, where precise control over mechanical and biological properties is crucial.
The long-term stability of sodium silicate-reinforced hydrogels in physiological conditions remains a concern. The gradual dissolution of silicate ions in aqueous environments can lead to changes in the hydrogel's mechanical properties over time, potentially compromising its structural integrity and functionality within the engineered tissue constructs.
Furthermore, the integration of sodium silicate into hydrogels while maintaining their porosity and permeability presents a significant challenge. The reinforcement process must not compromise the hydrogel's ability to facilitate nutrient diffusion and waste removal, which are essential for cell survival and tissue growth.
The biocompatibility and potential toxicity of sodium silicate-reinforced hydrogels require thorough investigation. While silicate-based materials have shown promise in various biomedical applications, the long-term effects of silicate ions on cellular behavior, differentiation, and tissue formation need to be carefully evaluated to ensure the safety and efficacy of these reinforced hydrogels in tissue engineering.
Another challenge is the development of scalable and reproducible manufacturing processes for sodium silicate-reinforced hydrogels. Current methods often face difficulties in maintaining consistent properties when scaling up from laboratory to industrial production, which is crucial for the widespread adoption of these materials in clinical applications.
Lastly, the optimization of sodium silicate reinforcement for specific tissue types and applications remains a complex task. Different tissues require varying mechanical properties and degradation profiles, necessitating tailored approaches to hydrogel reinforcement that can meet the diverse needs of various tissue engineering applications.
Sodium Silicate Reinforcement Techniques
01 Incorporation of sodium silicate in hydrogels
Sodium silicate is incorporated into hydrogel formulations to enhance their mechanical properties. The addition of sodium silicate can improve the strength, stiffness, and durability of the hydrogel structure. This reinforcement technique is particularly useful in applications requiring robust hydrogel materials.- Incorporation of sodium silicate in hydrogels: Sodium silicate is incorporated into hydrogel formulations to enhance their mechanical properties. The addition of sodium silicate can improve the strength, durability, and stability of the hydrogel structure. This reinforcement technique is particularly useful in applications requiring robust hydrogel materials.
- Optimization of sodium silicate concentration: The concentration of sodium silicate in hydrogels is optimized to achieve desired mechanical properties. Different ratios of sodium silicate to other hydrogel components are explored to find the optimal balance between reinforcement and maintaining the hydrogel's inherent characteristics, such as flexibility and water retention.
- Crosslinking mechanisms with sodium silicate: Various crosslinking mechanisms involving sodium silicate are investigated to enhance the mechanical properties of hydrogels. These may include chemical crosslinking, physical entanglement, or ionic interactions between sodium silicate and other hydrogel components, resulting in improved strength and stability.
- Composite hydrogels with sodium silicate: Composite hydrogels are developed by combining sodium silicate with other reinforcing materials or polymers. This approach aims to create synergistic effects, enhancing mechanical properties such as tensile strength, compressive strength, and elasticity beyond what can be achieved with sodium silicate alone.
- Characterization of mechanical properties: Various techniques and methods are employed to characterize the mechanical properties of sodium silicate reinforced hydrogels. These may include tensile testing, compression testing, rheological measurements, and microscopic analysis to evaluate the impact of sodium silicate on the hydrogel's structure and performance.
02 Optimization of sodium silicate concentration
The concentration of sodium silicate in hydrogels is optimized to achieve desired mechanical properties. Different ratios of sodium silicate to hydrogel components are explored to find the optimal balance between reinforcement and maintaining the hydrogel's inherent characteristics, such as water retention and flexibility.Expand Specific Solutions03 Cross-linking mechanisms with sodium silicate
Sodium silicate participates in cross-linking mechanisms within the hydrogel network. This cross-linking enhances the mechanical strength and stability of the hydrogel. The interaction between sodium silicate and other hydrogel components creates a more robust three-dimensional structure.Expand Specific Solutions04 Impact on hydrogel porosity and water absorption
The addition of sodium silicate affects the porosity and water absorption properties of hydrogels. While enhancing mechanical strength, it can also influence the hydrogel's ability to absorb and retain water. Balancing these properties is crucial for applications requiring both strength and water retention capabilities.Expand Specific Solutions05 Applications of sodium silicate reinforced hydrogels
Sodium silicate reinforced hydrogels find applications in various fields due to their enhanced mechanical properties. These include biomedical applications, tissue engineering, drug delivery systems, and industrial uses where both strength and hydrogel characteristics are required. The improved mechanical properties expand the potential uses of these materials.Expand Specific Solutions
Key Players in Tissue Engineering Materials
The research on sodium silicate reinforced hydrogel for tissue engineering is in an emerging stage, with growing market potential due to increasing applications in regenerative medicine. The global tissue engineering market is expected to reach $28.9 billion by 2027, driven by advancements in biomaterials. While the technology is still developing, several key players are making significant progress. Companies like Suzhou Institute of Nano-Tech & Nano-Bionics (SINANO), Sichuan University, and Guangzhou Beogene Biotech Co., Ltd. are at the forefront of research and development in this field, focusing on improving hydrogel properties and biocompatibility for tissue engineering applications.
Sichuan University
Technical Solution: Sichuan University has developed a novel sodium silicate reinforced hydrogel for tissue engineering applications. Their approach involves incorporating sodium silicate nanoparticles into a polymer matrix to enhance mechanical properties and biocompatibility. The resulting hydrogel demonstrates improved compressive strength and elasticity, making it suitable for load-bearing tissue applications[1]. Additionally, the sodium silicate component promotes cell adhesion and proliferation, enhancing the scaffold's ability to support tissue growth[2]. The university has also explored the use of this hydrogel in 3D bioprinting, allowing for the creation of complex tissue-like structures with precise control over porosity and architecture[3].
Strengths: Enhanced mechanical properties, improved biocompatibility, and versatility in fabrication methods. Weaknesses: Potential long-term stability issues and need for further in vivo studies to validate clinical efficacy.
Dalian University of Technology
Technical Solution: Dalian University of Technology has made significant advancements in sodium silicate reinforced hydrogels for tissue engineering. Their research focuses on developing a multi-functional hydrogel system that combines the mechanical strength of sodium silicate with the bioactivity of natural polymers. The team has successfully created a composite hydrogel using a unique crosslinking method that allows for controlled degradation rates[4]. This hydrogel exhibits excellent cell encapsulation properties and promotes the differentiation of mesenchymal stem cells into osteoblasts, making it particularly suitable for bone tissue engineering[5]. Furthermore, they have incorporated growth factors into the hydrogel matrix, enabling sustained release and enhanced tissue regeneration[6].
Strengths: Controlled degradation, excellent cell encapsulation, and promotion of osteogenic differentiation. Weaknesses: Complexity in manufacturing process and potential challenges in scaling up production.
Innovations in Hydrogel Mechanical Properties
Highly strong and tough photo-crosslinked hydrogel material and its preparation and application
PatentActiveUS20230130864A1
Innovation
- A highly strong and tough photo-crosslinked hydrogel material is developed by cross-linking photosensitive groups between polymer derivatives, specifically using o-nitrobenzyl phototrigger modified polymer derivatives, double bond groups modified polymer derivatives, and photoinitiators to enhance mechanical properties through improved load transfer and polymerization processes.
A hydrogel
PatentWO2025071518A1
Innovation
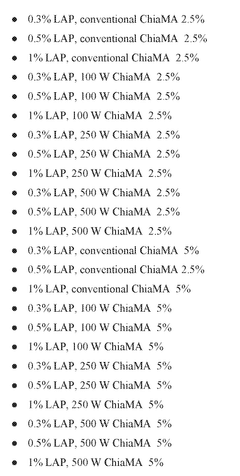
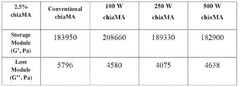
- The development of hydrogels through the methacrylation of chia seed mucilage using both conventional and microwave-assisted methods, which enhances mechanical stability, reduces microbial contamination risks, and promotes cell adhesion and tissue regeneration.
Biocompatibility and Safety Considerations
Biocompatibility and safety considerations are paramount in the development of sodium silicate reinforced hydrogels for tissue engineering applications. The integration of these materials into biological systems necessitates a comprehensive evaluation of their interactions with living tissues and potential long-term effects on the host organism.
One of the primary concerns in utilizing sodium silicate reinforced hydrogels is their potential to elicit an immune response. The immune system's reaction to these materials can significantly impact their efficacy and safety in tissue engineering applications. Researchers must carefully assess the hydrogel's ability to avoid triggering excessive inflammation or adverse immune reactions, which could compromise the intended therapeutic outcomes.
The degradation profile of sodium silicate reinforced hydrogels is another critical aspect of their biocompatibility. As these materials are designed to support tissue regeneration, it is essential that they degrade at a rate that complements the natural healing process. The breakdown products of the hydrogel should be non-toxic and easily metabolized or excreted by the body, preventing any accumulation that could lead to adverse effects.
Cytotoxicity testing is a fundamental step in evaluating the safety of these hydrogels. In vitro studies using relevant cell lines can provide valuable insights into the material's potential to cause cell death or impair cellular functions. These tests should assess both direct contact with the hydrogel and the effects of its leachable components on cell viability, proliferation, and differentiation.
The mechanical properties of sodium silicate reinforced hydrogels must also be considered from a safety perspective. While the reinforcement aims to enhance the hydrogel's strength and stability, it is crucial to ensure that these improved mechanical characteristics do not lead to tissue damage or irritation. The hydrogel should mimic the mechanical properties of the target tissue as closely as possible to promote proper integration and function.
Long-term biocompatibility studies are essential to understand the potential chronic effects of sodium silicate reinforced hydrogels. Animal models can provide valuable information on the material's performance over extended periods, including its integration with surrounding tissues, potential for fibrosis or encapsulation, and any systemic effects that may not be apparent in short-term studies.
The potential for silicon accumulation in tissues is a specific concern for sodium silicate reinforced hydrogels. While silicon is generally considered to have low toxicity, the long-term effects of localized silicon release from degrading hydrogels must be thoroughly investigated. Researchers should monitor silicon levels in various organs and assess any potential impact on normal physiological functions.
In conclusion, ensuring the biocompatibility and safety of sodium silicate reinforced hydrogels for tissue engineering requires a multifaceted approach. Comprehensive in vitro and in vivo studies, coupled with long-term follow-up, are necessary to establish the safety profile of these materials before their clinical application. As the field advances, continuous monitoring and refinement of safety protocols will be crucial to maximize the potential of these innovative biomaterials while minimizing risks to patients.
One of the primary concerns in utilizing sodium silicate reinforced hydrogels is their potential to elicit an immune response. The immune system's reaction to these materials can significantly impact their efficacy and safety in tissue engineering applications. Researchers must carefully assess the hydrogel's ability to avoid triggering excessive inflammation or adverse immune reactions, which could compromise the intended therapeutic outcomes.
The degradation profile of sodium silicate reinforced hydrogels is another critical aspect of their biocompatibility. As these materials are designed to support tissue regeneration, it is essential that they degrade at a rate that complements the natural healing process. The breakdown products of the hydrogel should be non-toxic and easily metabolized or excreted by the body, preventing any accumulation that could lead to adverse effects.
Cytotoxicity testing is a fundamental step in evaluating the safety of these hydrogels. In vitro studies using relevant cell lines can provide valuable insights into the material's potential to cause cell death or impair cellular functions. These tests should assess both direct contact with the hydrogel and the effects of its leachable components on cell viability, proliferation, and differentiation.
The mechanical properties of sodium silicate reinforced hydrogels must also be considered from a safety perspective. While the reinforcement aims to enhance the hydrogel's strength and stability, it is crucial to ensure that these improved mechanical characteristics do not lead to tissue damage or irritation. The hydrogel should mimic the mechanical properties of the target tissue as closely as possible to promote proper integration and function.
Long-term biocompatibility studies are essential to understand the potential chronic effects of sodium silicate reinforced hydrogels. Animal models can provide valuable information on the material's performance over extended periods, including its integration with surrounding tissues, potential for fibrosis or encapsulation, and any systemic effects that may not be apparent in short-term studies.
The potential for silicon accumulation in tissues is a specific concern for sodium silicate reinforced hydrogels. While silicon is generally considered to have low toxicity, the long-term effects of localized silicon release from degrading hydrogels must be thoroughly investigated. Researchers should monitor silicon levels in various organs and assess any potential impact on normal physiological functions.
In conclusion, ensuring the biocompatibility and safety of sodium silicate reinforced hydrogels for tissue engineering requires a multifaceted approach. Comprehensive in vitro and in vivo studies, coupled with long-term follow-up, are necessary to establish the safety profile of these materials before their clinical application. As the field advances, continuous monitoring and refinement of safety protocols will be crucial to maximize the potential of these innovative biomaterials while minimizing risks to patients.
Regulatory Pathway for Biomaterials
The regulatory pathway for biomaterials used in tissue engineering, such as sodium silicate reinforced hydrogels, is complex and multifaceted. In the United States, these materials typically fall under the jurisdiction of the Food and Drug Administration (FDA), specifically within the Center for Devices and Radiological Health (CDRH). The regulatory classification of these biomaterials depends on their intended use, mechanism of action, and risk profile.
For sodium silicate reinforced hydrogels intended for tissue engineering applications, the regulatory pathway would likely begin with determining the appropriate product classification. These materials may be classified as medical devices, combination products, or in some cases, as biologics. The classification will significantly impact the regulatory requirements and approval process.
If classified as a medical device, the hydrogel would likely be considered a Class III device due to its implantable nature and potential high risk. This classification would require a Premarket Approval (PMA) application, which involves extensive preclinical and clinical testing to demonstrate safety and efficacy. The PMA process is rigorous and typically takes several years to complete.
Alternatively, if the hydrogel is considered a combination product, incorporating both device and biologic components, the regulatory pathway may involve multiple FDA centers. In this case, the Office of Combination Products (OCP) would determine the lead center based on the primary mode of action of the product.
The regulatory process would involve several key steps. Initially, researchers would need to conduct extensive in vitro and in vivo studies to characterize the material's properties, biocompatibility, and potential toxicity. This would be followed by preclinical animal studies to assess safety and preliminary efficacy. Once sufficient preclinical data is gathered, an Investigational Device Exemption (IDE) application would be submitted to the FDA to obtain approval for initiating human clinical trials.
Clinical trials for tissue engineering biomaterials typically involve multiple phases, starting with small-scale safety studies and progressing to larger efficacy trials. Throughout this process, Good Laboratory Practices (GLP) and Good Manufacturing Practices (GMP) must be adhered to, ensuring the quality and consistency of the product.
In addition to FDA regulations, researchers must also consider international standards such as those set by the International Organization for Standardization (ISO) and the American Society for Testing and Materials (ASTM). These standards provide guidelines for material characterization, biocompatibility testing, and manufacturing processes.
For sodium silicate reinforced hydrogels intended for tissue engineering applications, the regulatory pathway would likely begin with determining the appropriate product classification. These materials may be classified as medical devices, combination products, or in some cases, as biologics. The classification will significantly impact the regulatory requirements and approval process.
If classified as a medical device, the hydrogel would likely be considered a Class III device due to its implantable nature and potential high risk. This classification would require a Premarket Approval (PMA) application, which involves extensive preclinical and clinical testing to demonstrate safety and efficacy. The PMA process is rigorous and typically takes several years to complete.
Alternatively, if the hydrogel is considered a combination product, incorporating both device and biologic components, the regulatory pathway may involve multiple FDA centers. In this case, the Office of Combination Products (OCP) would determine the lead center based on the primary mode of action of the product.
The regulatory process would involve several key steps. Initially, researchers would need to conduct extensive in vitro and in vivo studies to characterize the material's properties, biocompatibility, and potential toxicity. This would be followed by preclinical animal studies to assess safety and preliminary efficacy. Once sufficient preclinical data is gathered, an Investigational Device Exemption (IDE) application would be submitted to the FDA to obtain approval for initiating human clinical trials.
Clinical trials for tissue engineering biomaterials typically involve multiple phases, starting with small-scale safety studies and progressing to larger efficacy trials. Throughout this process, Good Laboratory Practices (GLP) and Good Manufacturing Practices (GMP) must be adhered to, ensuring the quality and consistency of the product.
In addition to FDA regulations, researchers must also consider international standards such as those set by the International Organization for Standardization (ISO) and the American Society for Testing and Materials (ASTM). These standards provide guidelines for material characterization, biocompatibility testing, and manufacturing processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!