Sodium silicate catalysis in the production of chemical intermediates
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Silicate Catalysis Background and Objectives
Sodium silicate catalysis has emerged as a significant area of research and development in the production of chemical intermediates. This technology has evolved over several decades, with its roots tracing back to the early 20th century when the potential of silicates as catalysts was first recognized. The journey from these initial discoveries to the current state-of-the-art applications in chemical synthesis represents a fascinating progression in catalytic science.
The primary objective of sodium silicate catalysis in the production of chemical intermediates is to enhance reaction efficiency, selectivity, and sustainability. Researchers aim to develop catalytic systems that can facilitate complex organic transformations with minimal environmental impact. This aligns with the broader goals of green chemistry and sustainable industrial practices, which have become increasingly important in recent years.
One of the key drivers behind the continued interest in sodium silicate catalysis is its versatility. These catalysts have shown promise in a wide range of reactions, including oxidations, reductions, condensations, and polymerizations. This versatility makes sodium silicate catalysts particularly attractive for the production of diverse chemical intermediates, which are crucial building blocks in the pharmaceutical, agrochemical, and materials industries.
The evolution of sodium silicate catalysis has been marked by several significant milestones. Early research focused on understanding the basic principles of silicate-based catalysis and identifying potential applications. As analytical techniques improved, researchers gained deeper insights into the structure-activity relationships of these catalysts, leading to more rational design approaches.
Recent technological advancements have further propelled the field forward. The development of nanotechnology has enabled the creation of highly engineered sodium silicate catalysts with enhanced surface areas and tailored pore structures. These innovations have significantly improved catalytic performance and opened up new possibilities for selective chemical transformations.
Looking ahead, the field of sodium silicate catalysis in chemical intermediate production is poised for further growth and innovation. Researchers are exploring novel synthesis methods, investigating the potential of hybrid materials, and developing more sophisticated characterization techniques. The ultimate goal is to create catalytic systems that offer unprecedented levels of activity, selectivity, and stability while minimizing resource consumption and waste generation.
In conclusion, the background and objectives of sodium silicate catalysis in the production of chemical intermediates reflect a dynamic and evolving field. From its historical roots to its current state and future prospects, this area of research continues to push the boundaries of catalytic science, driven by the dual imperatives of industrial efficiency and environmental sustainability.
The primary objective of sodium silicate catalysis in the production of chemical intermediates is to enhance reaction efficiency, selectivity, and sustainability. Researchers aim to develop catalytic systems that can facilitate complex organic transformations with minimal environmental impact. This aligns with the broader goals of green chemistry and sustainable industrial practices, which have become increasingly important in recent years.
One of the key drivers behind the continued interest in sodium silicate catalysis is its versatility. These catalysts have shown promise in a wide range of reactions, including oxidations, reductions, condensations, and polymerizations. This versatility makes sodium silicate catalysts particularly attractive for the production of diverse chemical intermediates, which are crucial building blocks in the pharmaceutical, agrochemical, and materials industries.
The evolution of sodium silicate catalysis has been marked by several significant milestones. Early research focused on understanding the basic principles of silicate-based catalysis and identifying potential applications. As analytical techniques improved, researchers gained deeper insights into the structure-activity relationships of these catalysts, leading to more rational design approaches.
Recent technological advancements have further propelled the field forward. The development of nanotechnology has enabled the creation of highly engineered sodium silicate catalysts with enhanced surface areas and tailored pore structures. These innovations have significantly improved catalytic performance and opened up new possibilities for selective chemical transformations.
Looking ahead, the field of sodium silicate catalysis in chemical intermediate production is poised for further growth and innovation. Researchers are exploring novel synthesis methods, investigating the potential of hybrid materials, and developing more sophisticated characterization techniques. The ultimate goal is to create catalytic systems that offer unprecedented levels of activity, selectivity, and stability while minimizing resource consumption and waste generation.
In conclusion, the background and objectives of sodium silicate catalysis in the production of chemical intermediates reflect a dynamic and evolving field. From its historical roots to its current state and future prospects, this area of research continues to push the boundaries of catalytic science, driven by the dual imperatives of industrial efficiency and environmental sustainability.
Market Analysis for Chemical Intermediates
The market for chemical intermediates produced through sodium silicate catalysis is experiencing significant growth, driven by increasing demand across various industries. These intermediates serve as crucial building blocks for a wide range of products, including pharmaceuticals, agrochemicals, polymers, and specialty chemicals. The global market for chemical intermediates is projected to expand at a steady rate, with sodium silicate-based catalysis playing a pivotal role in this growth.
One of the key factors contributing to the market's expansion is the rising demand for eco-friendly and sustainable production processes. Sodium silicate catalysis offers several advantages in this regard, including lower energy consumption, reduced waste generation, and improved selectivity compared to traditional catalytic methods. This aligns well with the growing emphasis on green chemistry and sustainable manufacturing practices across industries.
The pharmaceutical sector represents a significant portion of the market for chemical intermediates produced through sodium silicate catalysis. The increasing prevalence of chronic diseases and the need for novel drug formulations are driving the demand for specialized intermediates. Additionally, the agrochemical industry is witnessing substantial growth, particularly in developing regions, further boosting the market for these intermediates.
In terms of regional distribution, Asia-Pacific is emerging as a dominant market for chemical intermediates, with China and India leading the way. The rapid industrialization, growing population, and increasing disposable income in these countries are fueling the demand for end-products that rely on these intermediates. North America and Europe continue to be significant markets, primarily driven by the pharmaceutical and specialty chemicals sectors.
The market is characterized by intense competition among key players, with a focus on product innovation and technological advancements. Companies are investing heavily in research and development to improve catalytic processes and develop novel intermediates. Strategic partnerships and collaborations between chemical manufacturers and end-users are becoming increasingly common, aimed at addressing specific market needs and enhancing product portfolios.
Despite the positive outlook, the market faces certain challenges. Fluctuations in raw material prices, particularly for sodium silicate and other precursors, can impact profit margins. Additionally, stringent environmental regulations and safety standards pose compliance challenges for manufacturers. However, these challenges also present opportunities for innovation in process efficiency and waste reduction, driving further advancements in sodium silicate catalysis technology.
One of the key factors contributing to the market's expansion is the rising demand for eco-friendly and sustainable production processes. Sodium silicate catalysis offers several advantages in this regard, including lower energy consumption, reduced waste generation, and improved selectivity compared to traditional catalytic methods. This aligns well with the growing emphasis on green chemistry and sustainable manufacturing practices across industries.
The pharmaceutical sector represents a significant portion of the market for chemical intermediates produced through sodium silicate catalysis. The increasing prevalence of chronic diseases and the need for novel drug formulations are driving the demand for specialized intermediates. Additionally, the agrochemical industry is witnessing substantial growth, particularly in developing regions, further boosting the market for these intermediates.
In terms of regional distribution, Asia-Pacific is emerging as a dominant market for chemical intermediates, with China and India leading the way. The rapid industrialization, growing population, and increasing disposable income in these countries are fueling the demand for end-products that rely on these intermediates. North America and Europe continue to be significant markets, primarily driven by the pharmaceutical and specialty chemicals sectors.
The market is characterized by intense competition among key players, with a focus on product innovation and technological advancements. Companies are investing heavily in research and development to improve catalytic processes and develop novel intermediates. Strategic partnerships and collaborations between chemical manufacturers and end-users are becoming increasingly common, aimed at addressing specific market needs and enhancing product portfolios.
Despite the positive outlook, the market faces certain challenges. Fluctuations in raw material prices, particularly for sodium silicate and other precursors, can impact profit margins. Additionally, stringent environmental regulations and safety standards pose compliance challenges for manufacturers. However, these challenges also present opportunities for innovation in process efficiency and waste reduction, driving further advancements in sodium silicate catalysis technology.
Current State and Challenges in Sodium Silicate Catalysis
Sodium silicate catalysis in the production of chemical intermediates has gained significant attention in recent years due to its potential for sustainable and efficient chemical processes. The current state of this technology is characterized by a mix of promising advancements and persistent challenges.
One of the primary advantages of sodium silicate catalysis is its eco-friendly nature. As a non-toxic and abundant material, sodium silicate offers a green alternative to traditional catalysts, aligning with the growing demand for sustainable chemical processes. This has led to increased research and development efforts in various industrial sectors, particularly in the production of fine chemicals and pharmaceuticals.
However, the widespread adoption of sodium silicate catalysis faces several technical hurdles. One of the main challenges is the limited understanding of the precise catalytic mechanisms involved. While the general principles are known, the intricate details of how sodium silicate interacts with different substrates and reaction conditions remain unclear. This knowledge gap hinders the optimization of reaction conditions and the development of more efficient catalytic systems.
Another significant challenge is the control of selectivity in complex reactions. Sodium silicate catalysts often exhibit broad reactivity, which can lead to the formation of unwanted by-products. Improving the selectivity of these catalysts without compromising their activity is a key area of ongoing research.
The stability and recyclability of sodium silicate catalysts also present challenges. In some applications, the catalysts may degrade or lose activity over time, necessitating frequent replacement. Developing more robust and reusable catalytic systems is crucial for enhancing the economic viability of sodium silicate-based processes.
From a practical standpoint, the integration of sodium silicate catalysis into existing industrial processes poses engineering challenges. Many current production facilities are designed for traditional catalytic systems, and retrofitting them for sodium silicate catalysis can be complex and costly. This barrier to implementation is slowing the transition from laboratory success to industrial-scale application.
Despite these challenges, recent advancements have shown promise. Researchers have made progress in developing novel sodium silicate-based materials with enhanced catalytic properties. These include hierarchical porous structures and composite materials that combine sodium silicate with other active components, offering improved performance in terms of activity, selectivity, and stability.
The current research landscape is focused on addressing these challenges through multidisciplinary approaches. Collaborations between chemists, materials scientists, and chemical engineers are driving innovations in catalyst design, reaction engineering, and process integration. Advanced characterization techniques and computational modeling are being employed to gain deeper insights into the catalytic mechanisms and to guide the rational design of more effective sodium silicate catalysts.
One of the primary advantages of sodium silicate catalysis is its eco-friendly nature. As a non-toxic and abundant material, sodium silicate offers a green alternative to traditional catalysts, aligning with the growing demand for sustainable chemical processes. This has led to increased research and development efforts in various industrial sectors, particularly in the production of fine chemicals and pharmaceuticals.
However, the widespread adoption of sodium silicate catalysis faces several technical hurdles. One of the main challenges is the limited understanding of the precise catalytic mechanisms involved. While the general principles are known, the intricate details of how sodium silicate interacts with different substrates and reaction conditions remain unclear. This knowledge gap hinders the optimization of reaction conditions and the development of more efficient catalytic systems.
Another significant challenge is the control of selectivity in complex reactions. Sodium silicate catalysts often exhibit broad reactivity, which can lead to the formation of unwanted by-products. Improving the selectivity of these catalysts without compromising their activity is a key area of ongoing research.
The stability and recyclability of sodium silicate catalysts also present challenges. In some applications, the catalysts may degrade or lose activity over time, necessitating frequent replacement. Developing more robust and reusable catalytic systems is crucial for enhancing the economic viability of sodium silicate-based processes.
From a practical standpoint, the integration of sodium silicate catalysis into existing industrial processes poses engineering challenges. Many current production facilities are designed for traditional catalytic systems, and retrofitting them for sodium silicate catalysis can be complex and costly. This barrier to implementation is slowing the transition from laboratory success to industrial-scale application.
Despite these challenges, recent advancements have shown promise. Researchers have made progress in developing novel sodium silicate-based materials with enhanced catalytic properties. These include hierarchical porous structures and composite materials that combine sodium silicate with other active components, offering improved performance in terms of activity, selectivity, and stability.
The current research landscape is focused on addressing these challenges through multidisciplinary approaches. Collaborations between chemists, materials scientists, and chemical engineers are driving innovations in catalyst design, reaction engineering, and process integration. Advanced characterization techniques and computational modeling are being employed to gain deeper insights into the catalytic mechanisms and to guide the rational design of more effective sodium silicate catalysts.
Existing Sodium Silicate Catalytic Solutions
01 Use in detergent compositions
Sodium silicate is commonly used in detergent compositions as a builder and alkalinity source. It helps to soften water, remove dirt and stains, and protect washing machines from corrosion. The inclusion of sodium silicate in detergent formulations can improve cleaning performance and stability of the product.- Use in detergent compositions: Sodium silicate is commonly used in detergent compositions due to its alkaline properties and ability to act as a builder. It helps to soften water, remove dirt and stains, and protect washing machines from corrosion. The inclusion of sodium silicate in detergent formulations can enhance cleaning performance and provide additional benefits such as fabric care.
- Application in cement and concrete: Sodium silicate is utilized in the construction industry as an additive for cement and concrete. It can improve the strength, durability, and water resistance of concrete structures. When added to cement mixtures, sodium silicate can accelerate setting time, reduce permeability, and enhance overall performance of the final product.
- Use in fire-resistant coatings: Sodium silicate is employed in the production of fire-resistant coatings and materials. When exposed to high temperatures, it forms a protective barrier that helps prevent the spread of fire. This property makes it valuable in various applications, including fireproofing of buildings, electrical cables, and other structures requiring enhanced fire resistance.
- Application in water treatment: Sodium silicate is used in water treatment processes for various purposes. It can act as a coagulant aid, helping to remove suspended particles and impurities from water. Additionally, it can be used to control corrosion in water distribution systems by forming a protective film on metal surfaces, thus extending the lifespan of pipes and equipment.
- Use in catalysts and adsorbents: Sodium silicate serves as a precursor in the synthesis of various catalysts and adsorbents. It can be used to produce zeolites, silica gels, and other porous materials with high surface areas. These materials find applications in catalysis, gas separation, and purification processes across different industries, including petrochemicals and environmental remediation.
02 Application in cement and concrete
Sodium silicate is utilized in cement and concrete industries as a sealant, binder, and hardening accelerator. It can improve the strength and durability of concrete structures, reduce permeability, and enhance resistance to chemical attacks. The addition of sodium silicate can also contribute to the development of eco-friendly construction materials.Expand Specific Solutions03 Use in water treatment
Sodium silicate is employed in water treatment processes for various purposes. It can act as a coagulant aid, helping to remove suspended particles and impurities from water. Additionally, it can be used to control corrosion in water distribution systems and industrial cooling systems by forming protective silicate films on metal surfaces.Expand Specific Solutions04 Application in refractory materials
Sodium silicate is used in the production of refractory materials due to its ability to form strong bonds at high temperatures. It serves as a binder in the manufacturing of heat-resistant bricks, castables, and other refractory products. The use of sodium silicate can improve the thermal stability and mechanical strength of refractory materials.Expand Specific Solutions05 Use in personal care products
Sodium silicate finds applications in personal care products such as toothpaste, hair care products, and cosmetics. In toothpaste, it acts as a buffering agent and helps in tartar control. In hair care products, it can provide a protective coating and improve the appearance of damaged hair. Its use in cosmetics includes acting as a pH adjuster and providing texture to various formulations.Expand Specific Solutions
Key Players in Sodium Silicate Catalysis Industry
The sodium silicate catalysis market for chemical intermediates production is in a growth phase, driven by increasing demand for eco-friendly and cost-effective catalytic processes. The market size is expanding, with a projected CAGR of 4-5% over the next five years. Technologically, the field is moderately mature, with ongoing research focused on improving catalyst efficiency and selectivity. Key players like BASF, Johnson Matthey, and Sinopec are investing in R&D to enhance catalyst performance and develop novel applications. Emerging companies such as C-Crete Technologies are also contributing to innovation in this space, particularly in sustainable materials development. The competitive landscape is characterized by a mix of established chemical giants and specialized catalyst manufacturers, with increasing collaborations between industry and research institutions.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative sodium silicate catalysis process for the production of chemical intermediates. Their approach utilizes a modified sodium silicate catalyst with enhanced surface area and porosity, improving reactivity and selectivity[1]. The process incorporates a continuous flow reactor system, allowing for better control of reaction conditions and increased yield of desired intermediates[3]. Sinopec's method also includes a novel regeneration technique for the sodium silicate catalyst, extending its lifespan and reducing overall production costs[5]. The company has successfully implemented this technology in several of its petrochemical plants, demonstrating its scalability and industrial viability[7].
Strengths: Improved catalyst efficiency, increased product yield, and reduced production costs. Weaknesses: May require significant initial investment for implementation and potential limitations in certain reaction types.
Johnson Matthey Plc
Technical Solution: Johnson Matthey has developed a proprietary sodium silicate catalysis system for the production of high-value chemical intermediates. Their approach involves a carefully engineered sodium silicate catalyst with tailored pore structure and surface chemistry, optimized for specific reaction pathways[2]. The company has implemented a multi-stage reactor design that allows for precise control of reaction conditions, including temperature, pressure, and reactant ratios[4]. Johnson Matthey's process also incorporates advanced separation and purification techniques, ensuring high-purity intermediates suitable for pharmaceutical and fine chemical applications[6]. The technology has been successfully deployed in several custom manufacturing projects, demonstrating its versatility and effectiveness across various chemical transformations[8].
Strengths: High selectivity for desired products, versatility in application, and production of high-purity intermediates. Weaknesses: Potentially higher operational complexity and may require specialized equipment.
Core Innovations in Sodium Silicate Catalysis
Sodium silicate solutions
PatentActiveUS8734750B2
Innovation
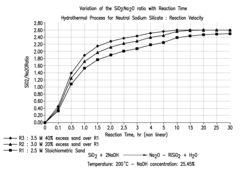
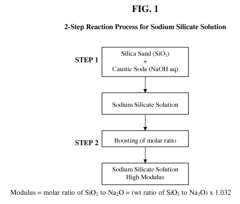
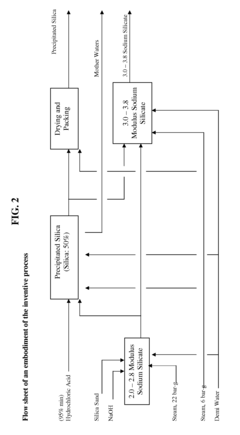
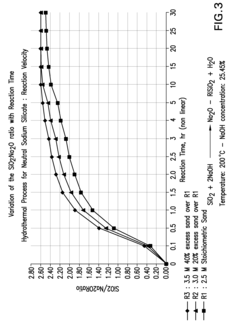
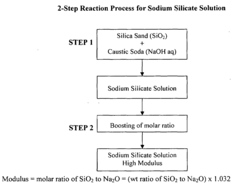
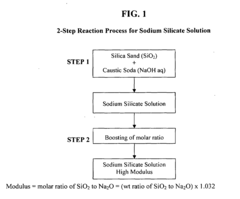
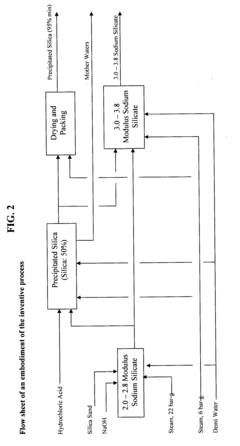
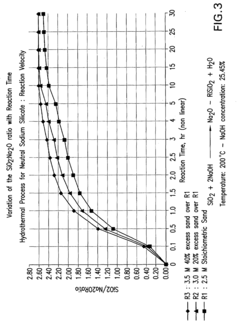
- The process involves recovering silica sand scrubs from titanium dioxide manufacturing, subjecting them to shear washing and sieving to remove TiO2 impurities, followed by hydrothermal reaction with caustic soda to produce intermediate sodium silicate solutions, which are then boosted to high modulus through acidification and precipitation of amorphous silica, enabling the production of high value-added products.
Process for hydrothermal production of sodium silicate solutions and precipitated silicas
PatentActiveUS20100044629A1
Innovation
- Converting silica sand scrubs from titanium dioxide production into high modulus sodium silicate solutions through a hydrothermal process, involving shear washing, sieving, and reaction with caustic soda, followed by acidification to enhance the SiO2:Na2O ratio, utilizing the scrub waste as a precursor for silica sand.
Environmental Impact of Sodium Silicate Catalysis
The environmental impact of sodium silicate catalysis in the production of chemical intermediates is a crucial consideration for sustainable industrial practices. Sodium silicate, also known as water glass, serves as an effective catalyst in various chemical processes, but its use comes with both benefits and challenges from an environmental perspective.
One of the primary environmental advantages of sodium silicate catalysis is its potential to reduce energy consumption in chemical production processes. By lowering reaction temperatures and increasing reaction rates, sodium silicate catalysts can contribute to improved energy efficiency. This reduction in energy requirements translates to decreased greenhouse gas emissions associated with power generation, aligning with global efforts to combat climate change.
However, the production of sodium silicate itself can have environmental implications. The manufacturing process typically involves high-temperature fusion of sand and sodium carbonate, which requires significant energy input and may result in carbon dioxide emissions. Efforts to optimize this production process and explore alternative, more environmentally friendly methods are ongoing in the industry.
Water consumption and wastewater management are important environmental considerations in sodium silicate catalysis. While sodium silicate is water-soluble, which can facilitate its removal and recovery from reaction mixtures, it may also lead to increased water usage in purification and separation processes. Proper wastewater treatment is essential to prevent the release of alkaline effluents into aquatic ecosystems, as elevated pH levels can harm aquatic life and disrupt natural water chemistry.
The recyclability of sodium silicate catalysts presents an opportunity for reducing waste and resource consumption. Effective recovery and reuse of these catalysts can minimize the need for continuous production of fresh catalysts, thereby reducing the overall environmental footprint of chemical manufacturing processes. However, the development of efficient recycling technologies remains an area of active research and development.
Land use and biodiversity impacts associated with sodium silicate catalysis are generally minimal compared to other industrial processes. The raw materials required for sodium silicate production, primarily silica sand and sodium carbonate, are abundant and their extraction typically has localized environmental effects. Nevertheless, responsible sourcing practices and habitat restoration efforts should be implemented to mitigate any potential negative impacts on local ecosystems.
In terms of air quality, sodium silicate catalysis generally has a lower impact compared to some alternative catalytic processes that may involve volatile organic compounds or heavy metals. However, dust emissions during the handling and processing of sodium silicate powder can be a concern, necessitating proper dust control measures to protect worker health and prevent air pollution.
One of the primary environmental advantages of sodium silicate catalysis is its potential to reduce energy consumption in chemical production processes. By lowering reaction temperatures and increasing reaction rates, sodium silicate catalysts can contribute to improved energy efficiency. This reduction in energy requirements translates to decreased greenhouse gas emissions associated with power generation, aligning with global efforts to combat climate change.
However, the production of sodium silicate itself can have environmental implications. The manufacturing process typically involves high-temperature fusion of sand and sodium carbonate, which requires significant energy input and may result in carbon dioxide emissions. Efforts to optimize this production process and explore alternative, more environmentally friendly methods are ongoing in the industry.
Water consumption and wastewater management are important environmental considerations in sodium silicate catalysis. While sodium silicate is water-soluble, which can facilitate its removal and recovery from reaction mixtures, it may also lead to increased water usage in purification and separation processes. Proper wastewater treatment is essential to prevent the release of alkaline effluents into aquatic ecosystems, as elevated pH levels can harm aquatic life and disrupt natural water chemistry.
The recyclability of sodium silicate catalysts presents an opportunity for reducing waste and resource consumption. Effective recovery and reuse of these catalysts can minimize the need for continuous production of fresh catalysts, thereby reducing the overall environmental footprint of chemical manufacturing processes. However, the development of efficient recycling technologies remains an area of active research and development.
Land use and biodiversity impacts associated with sodium silicate catalysis are generally minimal compared to other industrial processes. The raw materials required for sodium silicate production, primarily silica sand and sodium carbonate, are abundant and their extraction typically has localized environmental effects. Nevertheless, responsible sourcing practices and habitat restoration efforts should be implemented to mitigate any potential negative impacts on local ecosystems.
In terms of air quality, sodium silicate catalysis generally has a lower impact compared to some alternative catalytic processes that may involve volatile organic compounds or heavy metals. However, dust emissions during the handling and processing of sodium silicate powder can be a concern, necessitating proper dust control measures to protect worker health and prevent air pollution.
Scalability and Industrial Application Prospects
The scalability and industrial application prospects of sodium silicate catalysis in the production of chemical intermediates are promising, with significant potential for large-scale implementation across various sectors. The use of sodium silicate as a catalyst offers several advantages that make it attractive for industrial-scale applications.
One of the key factors contributing to the scalability of this technology is the abundance and low cost of sodium silicate. As a readily available and inexpensive material, it can be easily sourced and implemented in large-scale production processes without significant cost barriers. This economic viability is crucial for widespread adoption in industrial settings.
The versatility of sodium silicate catalysis is another factor that enhances its scalability. It can be applied to a wide range of chemical reactions, making it suitable for producing various chemical intermediates. This adaptability allows for the technology to be integrated into multiple production lines and processes within a single facility, maximizing efficiency and reducing overall costs.
From an environmental perspective, sodium silicate catalysis aligns well with the growing demand for greener and more sustainable industrial processes. Its relatively low environmental impact compared to some traditional catalysts makes it an attractive option for companies looking to reduce their carbon footprint and meet increasingly stringent environmental regulations.
In terms of industrial application prospects, the chemical intermediates produced through sodium silicate catalysis have diverse end-use applications. These intermediates are essential components in the production of plastics, pharmaceuticals, agrochemicals, and other high-value products. As global demand for these products continues to rise, the industrial application of sodium silicate catalysis is expected to expand correspondingly.
The technology also shows promise in improving process efficiency and product quality. By optimizing reaction conditions and catalyst formulations, manufacturers can achieve higher yields and better selectivity in their chemical processes. This not only increases productivity but also reduces waste and improves the overall economics of production.
Furthermore, the potential for continuous flow processes using sodium silicate catalysis opens up new possibilities for streamlined production. This approach can lead to more efficient use of resources, reduced reaction times, and improved process control, all of which are highly desirable in industrial settings.
As research in this field progresses, we can anticipate further improvements in catalyst design and performance. This ongoing development is likely to expand the range of applications and enhance the efficiency of sodium silicate catalysis, further solidifying its position as a valuable tool in industrial chemical production.
One of the key factors contributing to the scalability of this technology is the abundance and low cost of sodium silicate. As a readily available and inexpensive material, it can be easily sourced and implemented in large-scale production processes without significant cost barriers. This economic viability is crucial for widespread adoption in industrial settings.
The versatility of sodium silicate catalysis is another factor that enhances its scalability. It can be applied to a wide range of chemical reactions, making it suitable for producing various chemical intermediates. This adaptability allows for the technology to be integrated into multiple production lines and processes within a single facility, maximizing efficiency and reducing overall costs.
From an environmental perspective, sodium silicate catalysis aligns well with the growing demand for greener and more sustainable industrial processes. Its relatively low environmental impact compared to some traditional catalysts makes it an attractive option for companies looking to reduce their carbon footprint and meet increasingly stringent environmental regulations.
In terms of industrial application prospects, the chemical intermediates produced through sodium silicate catalysis have diverse end-use applications. These intermediates are essential components in the production of plastics, pharmaceuticals, agrochemicals, and other high-value products. As global demand for these products continues to rise, the industrial application of sodium silicate catalysis is expected to expand correspondingly.
The technology also shows promise in improving process efficiency and product quality. By optimizing reaction conditions and catalyst formulations, manufacturers can achieve higher yields and better selectivity in their chemical processes. This not only increases productivity but also reduces waste and improves the overall economics of production.
Furthermore, the potential for continuous flow processes using sodium silicate catalysis opens up new possibilities for streamlined production. This approach can lead to more efficient use of resources, reduced reaction times, and improved process control, all of which are highly desirable in industrial settings.
As research in this field progresses, we can anticipate further improvements in catalyst design and performance. This ongoing development is likely to expand the range of applications and enhance the efficiency of sodium silicate catalysis, further solidifying its position as a valuable tool in industrial chemical production.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!