Sodium silicate in microemulsion templating of silica spheres
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Silica Sphere Synthesis Background and Objectives
Silica spheres have been a subject of intense research and development in materials science for several decades. The synthesis of these spheres has evolved significantly, with the microemulsion templating method using sodium silicate emerging as a promising approach. This technique offers unique advantages in controlling the size, shape, and properties of silica spheres, making it highly relevant for various applications in industries such as electronics, catalysis, and biomedicine.
The historical context of silica sphere synthesis dates back to the 1960s with the pioneering work of Stöber and colleagues. Their method, known as the Stöber process, involved the hydrolysis and condensation of tetraethyl orthosilicate (TEOS) in an alcohol-water mixture with ammonia as a catalyst. This breakthrough laid the foundation for subsequent advancements in silica sphere synthesis techniques.
As research progressed, scientists sought to overcome limitations in size control and uniformity inherent in the Stöber method. This led to the exploration of alternative approaches, including the use of microemulsions as templates. The introduction of sodium silicate as a silica precursor in microemulsion systems marked a significant milestone in this field, offering a more cost-effective and environmentally friendly alternative to TEOS.
The primary objectives of utilizing sodium silicate in microemulsion templating for silica sphere synthesis are multifaceted. Firstly, researchers aim to achieve precise control over particle size distribution, targeting monodisperse spheres with diameters ranging from nanometers to micrometers. Secondly, there is a focus on enhancing the structural integrity and surface properties of the spheres, which are crucial for their performance in various applications.
Another key goal is to develop a scalable and economically viable production process. The use of sodium silicate, an abundant and inexpensive precursor, aligns well with this objective. Additionally, researchers are working towards improving the sustainability of the synthesis process by minimizing the use of harmful solvents and reducing energy consumption.
The evolution of this technology is driven by the growing demand for advanced materials in cutting-edge applications. For instance, in the field of drug delivery, precisely engineered silica spheres can serve as carriers for controlled release of therapeutic agents. In catalysis, these spheres can act as supports for active catalytic species, enhancing reaction efficiency and selectivity.
Looking ahead, the field of silica sphere synthesis using sodium silicate in microemulsion templating is poised for further advancements. Researchers are exploring ways to functionalize the surface of these spheres, incorporate additional components to create multifunctional materials, and develop novel templating strategies to achieve even greater control over particle morphology and internal structure.
The historical context of silica sphere synthesis dates back to the 1960s with the pioneering work of Stöber and colleagues. Their method, known as the Stöber process, involved the hydrolysis and condensation of tetraethyl orthosilicate (TEOS) in an alcohol-water mixture with ammonia as a catalyst. This breakthrough laid the foundation for subsequent advancements in silica sphere synthesis techniques.
As research progressed, scientists sought to overcome limitations in size control and uniformity inherent in the Stöber method. This led to the exploration of alternative approaches, including the use of microemulsions as templates. The introduction of sodium silicate as a silica precursor in microemulsion systems marked a significant milestone in this field, offering a more cost-effective and environmentally friendly alternative to TEOS.
The primary objectives of utilizing sodium silicate in microemulsion templating for silica sphere synthesis are multifaceted. Firstly, researchers aim to achieve precise control over particle size distribution, targeting monodisperse spheres with diameters ranging from nanometers to micrometers. Secondly, there is a focus on enhancing the structural integrity and surface properties of the spheres, which are crucial for their performance in various applications.
Another key goal is to develop a scalable and economically viable production process. The use of sodium silicate, an abundant and inexpensive precursor, aligns well with this objective. Additionally, researchers are working towards improving the sustainability of the synthesis process by minimizing the use of harmful solvents and reducing energy consumption.
The evolution of this technology is driven by the growing demand for advanced materials in cutting-edge applications. For instance, in the field of drug delivery, precisely engineered silica spheres can serve as carriers for controlled release of therapeutic agents. In catalysis, these spheres can act as supports for active catalytic species, enhancing reaction efficiency and selectivity.
Looking ahead, the field of silica sphere synthesis using sodium silicate in microemulsion templating is poised for further advancements. Researchers are exploring ways to functionalize the surface of these spheres, incorporate additional components to create multifunctional materials, and develop novel templating strategies to achieve even greater control over particle morphology and internal structure.
Market Analysis for Silica Spheres
The global market for silica spheres has been experiencing significant growth, driven by their diverse applications across various industries. These microspheres, often produced through microemulsion templating methods using sodium silicate, have found extensive use in sectors such as healthcare, cosmetics, electronics, and advanced materials.
In the healthcare industry, silica spheres are increasingly utilized in drug delivery systems, diagnostic imaging, and biosensors. The pharmaceutical market, in particular, has shown a strong demand for these particles due to their biocompatibility and ability to encapsulate and release drugs in a controlled manner. This application has been further bolstered by the growing emphasis on targeted therapies and personalized medicine.
The cosmetics sector represents another substantial market for silica spheres. These particles are widely used in skincare products, sunscreens, and color cosmetics due to their light-diffusing properties and ability to improve texture and stability. The rising consumer focus on high-performance personal care products has been a key driver in this segment.
In the electronics industry, silica spheres play a crucial role in the production of high-precision optical coatings, display technologies, and semiconductor materials. The ongoing miniaturization trend in electronics and the development of advanced display technologies have contributed to the increased demand for silica spheres with specific size distributions and surface properties.
The advanced materials sector, including the production of composites, ceramics, and specialty coatings, has also shown a growing interest in silica spheres. These particles are used to enhance mechanical properties, thermal stability, and chemical resistance in various materials, opening up new possibilities for product innovation across multiple industries.
Geographically, North America and Europe have been leading markets for silica spheres, primarily due to their well-established pharmaceutical and cosmetics industries. However, the Asia-Pacific region is emerging as a rapidly growing market, driven by the expansion of electronics manufacturing and increasing investments in healthcare infrastructure.
The market for silica spheres is characterized by a high degree of customization, with manufacturers offering products tailored to specific application requirements. This trend has led to increased collaboration between silica sphere producers and end-users, fostering innovation and market growth.
In the healthcare industry, silica spheres are increasingly utilized in drug delivery systems, diagnostic imaging, and biosensors. The pharmaceutical market, in particular, has shown a strong demand for these particles due to their biocompatibility and ability to encapsulate and release drugs in a controlled manner. This application has been further bolstered by the growing emphasis on targeted therapies and personalized medicine.
The cosmetics sector represents another substantial market for silica spheres. These particles are widely used in skincare products, sunscreens, and color cosmetics due to their light-diffusing properties and ability to improve texture and stability. The rising consumer focus on high-performance personal care products has been a key driver in this segment.
In the electronics industry, silica spheres play a crucial role in the production of high-precision optical coatings, display technologies, and semiconductor materials. The ongoing miniaturization trend in electronics and the development of advanced display technologies have contributed to the increased demand for silica spheres with specific size distributions and surface properties.
The advanced materials sector, including the production of composites, ceramics, and specialty coatings, has also shown a growing interest in silica spheres. These particles are used to enhance mechanical properties, thermal stability, and chemical resistance in various materials, opening up new possibilities for product innovation across multiple industries.
Geographically, North America and Europe have been leading markets for silica spheres, primarily due to their well-established pharmaceutical and cosmetics industries. However, the Asia-Pacific region is emerging as a rapidly growing market, driven by the expansion of electronics manufacturing and increasing investments in healthcare infrastructure.
The market for silica spheres is characterized by a high degree of customization, with manufacturers offering products tailored to specific application requirements. This trend has led to increased collaboration between silica sphere producers and end-users, fostering innovation and market growth.
Microemulsion Templating Challenges
Microemulsion templating for the synthesis of silica spheres using sodium silicate faces several significant challenges that researchers and industry professionals must address. One of the primary obstacles is achieving precise control over the size and uniformity of the silica spheres. The delicate balance of surfactants, co-surfactants, and oil phases in the microemulsion system can be easily disrupted, leading to polydispersity in the final product. This lack of consistency can significantly impact the performance of the silica spheres in various applications, such as catalysis, drug delivery, and optical materials.
Another critical challenge lies in the stability of the microemulsion system during the templating process. The introduction of sodium silicate can alter the interfacial properties of the microemulsion, potentially causing phase separation or premature gelation. This instability can result in incomplete formation of silica spheres or the creation of irregular structures, compromising the quality and functionality of the final product. Researchers must carefully optimize the composition and reaction conditions to maintain a stable microemulsion throughout the entire synthesis process.
The reaction kinetics of silica formation from sodium silicate within the microemulsion droplets present additional complexities. The rate of hydrolysis and condensation reactions can vary significantly depending on factors such as pH, temperature, and the concentration of reactants. Controlling these parameters to achieve a uniform growth rate across all droplets is challenging but essential for producing monodisperse silica spheres. Furthermore, the diffusion of reactants and products across the oil-water interface can influence the growth mechanism, adding another layer of complexity to the process.
Scalability and reproducibility of microemulsion templating for large-scale production of silica spheres remain significant hurdles. While laboratory-scale synthesis may yield promising results, translating these processes to industrial scales often encounters difficulties in maintaining consistent quality and properties. The sensitive nature of microemulsions makes them susceptible to minor variations in processing conditions, which can be more pronounced in larger batch sizes. Developing robust and scalable protocols that can accommodate industrial demands without compromising the quality of the silica spheres is a ongoing challenge for researchers and engineers in this field.
Environmental and economic considerations also pose challenges to the widespread adoption of microemulsion templating using sodium silicate. The use of large volumes of organic solvents and surfactants raises concerns about waste generation and disposal. Additionally, the cost-effectiveness of the process compared to alternative methods of silica sphere synthesis must be carefully evaluated, taking into account factors such as raw material costs, energy consumption, and purification requirements. Addressing these challenges is crucial for the sustainable development and commercial viability of microemulsion-templated silica spheres.
Another critical challenge lies in the stability of the microemulsion system during the templating process. The introduction of sodium silicate can alter the interfacial properties of the microemulsion, potentially causing phase separation or premature gelation. This instability can result in incomplete formation of silica spheres or the creation of irregular structures, compromising the quality and functionality of the final product. Researchers must carefully optimize the composition and reaction conditions to maintain a stable microemulsion throughout the entire synthesis process.
The reaction kinetics of silica formation from sodium silicate within the microemulsion droplets present additional complexities. The rate of hydrolysis and condensation reactions can vary significantly depending on factors such as pH, temperature, and the concentration of reactants. Controlling these parameters to achieve a uniform growth rate across all droplets is challenging but essential for producing monodisperse silica spheres. Furthermore, the diffusion of reactants and products across the oil-water interface can influence the growth mechanism, adding another layer of complexity to the process.
Scalability and reproducibility of microemulsion templating for large-scale production of silica spheres remain significant hurdles. While laboratory-scale synthesis may yield promising results, translating these processes to industrial scales often encounters difficulties in maintaining consistent quality and properties. The sensitive nature of microemulsions makes them susceptible to minor variations in processing conditions, which can be more pronounced in larger batch sizes. Developing robust and scalable protocols that can accommodate industrial demands without compromising the quality of the silica spheres is a ongoing challenge for researchers and engineers in this field.
Environmental and economic considerations also pose challenges to the widespread adoption of microemulsion templating using sodium silicate. The use of large volumes of organic solvents and surfactants raises concerns about waste generation and disposal. Additionally, the cost-effectiveness of the process compared to alternative methods of silica sphere synthesis must be carefully evaluated, taking into account factors such as raw material costs, energy consumption, and purification requirements. Addressing these challenges is crucial for the sustainable development and commercial viability of microemulsion-templated silica spheres.
Current Sodium Silicate Microemulsion Techniques
01 Synthesis methods for controlling silica sphere size
Various synthesis methods can be employed to control the size of silica spheres. These include sol-gel processes, microemulsion techniques, and hydrothermal methods. By adjusting parameters such as pH, temperature, and reactant concentrations, researchers can fine-tune the size of the resulting silica spheres, ranging from nanometers to micrometers in diameter.- Synthesis methods for controlling silica sphere size: Various synthesis methods can be employed to control the size of silica spheres. These include sol-gel processes, microemulsion techniques, and hydrothermal methods. By adjusting parameters such as pH, temperature, and reactant concentrations, researchers can fine-tune the size of the resulting silica spheres, ranging from nanometers to micrometers in diameter.
- Morphology control of silica spheres: The morphology of silica spheres can be controlled through various techniques. These include templating methods, surface modification, and the use of structure-directing agents. By manipulating these factors, researchers can create silica spheres with different shapes, such as hollow spheres, core-shell structures, or porous spheres with specific surface characteristics.
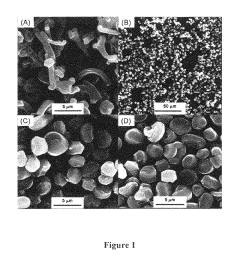
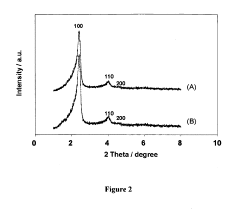
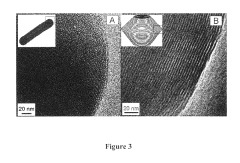
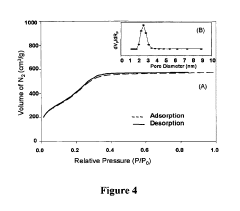
- Characterization techniques for silica sphere size and morphology: Various analytical techniques are used to characterize the size and morphology of silica spheres. These include electron microscopy (SEM, TEM), dynamic light scattering (DLS), and X-ray diffraction (XRD). These methods provide detailed information about particle size distribution, surface features, and internal structures of the silica spheres.
- Applications of size-controlled silica spheres: Size-controlled silica spheres find applications in various fields. They are used in chromatography as stationary phases, in drug delivery systems, as fillers in polymer composites, and in optical coatings. The ability to precisely control the size of silica spheres allows for tailored properties in these applications, such as improved separation efficiency or controlled drug release rates.
- Influence of size and morphology on silica sphere properties: The size and morphology of silica spheres significantly influence their properties and performance in various applications. Factors such as surface area, pore structure, and particle uniformity are directly affected by size and shape. These characteristics, in turn, impact properties like adsorption capacity, optical properties, and mechanical strength, which are crucial in applications ranging from catalysis to photonics.
02 Morphology control of silica spheres
The morphology of silica spheres can be tailored through various techniques. These include the use of structure-directing agents, templating methods, and surface modification approaches. By controlling the synthesis conditions and additives, it is possible to create silica spheres with different shapes, such as hollow spheres, core-shell structures, or porous spheres with specific surface features.Expand Specific Solutions03 Characterization techniques for silica sphere size and morphology
Various analytical techniques are employed to characterize the size and morphology of silica spheres. These include electron microscopy (SEM, TEM), dynamic light scattering (DLS), and atomic force microscopy (AFM). These methods provide detailed information about particle size distribution, surface features, and internal structures of silica spheres.Expand Specific Solutions04 Applications of size-controlled silica spheres
Size-controlled silica spheres find applications in various fields. They are used in chromatography as stationary phases, in drug delivery systems, as templates for creating porous materials, and in optical coatings. The ability to precisely control the size of silica spheres allows for tailoring their properties for specific applications in industries such as electronics, healthcare, and materials science.Expand Specific Solutions05 Surface modification of silica spheres
Surface modification techniques are used to alter the properties of silica spheres. These include grafting of organic functional groups, coating with metals or polymers, and creating core-shell structures. Such modifications can enhance the dispersibility, reactivity, or compatibility of silica spheres with various matrices, expanding their potential applications in areas such as catalysis, sensing, and composite materials.Expand Specific Solutions
Key Players in Silica Nanomaterials Industry
The field of sodium silicate microemulsion templating for silica sphere synthesis is in a growth phase, with increasing market potential due to applications in various industries. The global market for advanced materials, including engineered silica, is projected to expand significantly in the coming years. Technologically, the process is moderately mature, with ongoing research to optimize particle size control and uniformity. Key players like Tronox LLC and China Petroleum & Chemical Corp. are leveraging their chemical expertise to advance this technology. Academic institutions such as Zhejiang University and University of Vermont are contributing to fundamental research, while companies like Henkel AG & Co. KGaA are exploring industrial applications. The collaboration between industry and academia is driving innovation and commercialization efforts in this field.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative approach to synthesizing silica spheres using sodium silicate in microemulsion templating for applications in the petroleum and chemical industries. Their method involves creating a water-in-oil microemulsion system where sodium silicate is confined within nanoscale water droplets stabilized by surfactants. By controlling the microemulsion composition and reaction conditions, they can produce silica spheres with tailored properties for specific applications[1]. Sinopec's researchers have focused on developing silica spheres with high thermal stability and mechanical strength for use as catalyst supports in petroleum refining processes. They have also explored the incorporation of metal nanoparticles into the silica spheres to create multifunctional catalysts with enhanced activity and selectivity[2][3]. Additionally, Sinopec has developed a continuous flow process for the large-scale production of these silica spheres, demonstrating the potential for industrial implementation[4].
Strengths: Tailored properties for specific industrial applications, potential for large-scale production, integration with existing petroleum and chemical processes. Weaknesses: May be limited to applications within the petroleum and chemical industries, potential challenges in adapting the technology for other sectors.
Zhejiang University
Technical Solution: Zhejiang University has developed a novel approach to synthesizing silica spheres using sodium silicate in microemulsion templating. Their method involves creating a reverse microemulsion system where sodium silicate is confined within nanoscale water droplets surrounded by a continuous oil phase. By carefully controlling the water-to-surfactant ratio and reaction conditions, they can produce monodisperse silica spheres with tunable sizes ranging from 20 to 200 nm[1]. The researchers have also explored the incorporation of various functional groups and nanoparticles into the silica spheres during the synthesis process, enabling the creation of multifunctional materials for applications in catalysis, drug delivery, and sensing[2][3]. Additionally, they have developed a continuous flow microreactor system for the large-scale production of these silica spheres, demonstrating the potential for industrial-scale synthesis[4].
Strengths: Precise control over particle size and functionality, potential for large-scale production, versatility in creating multifunctional materials. Weaknesses: Complexity of the microemulsion system may require careful optimization for different applications.
Innovations in Microemulsion Templating
Synthesis of mesoporous silica shapes using sodium silicate as a silica source
PatentInactiveUS10457560B1
Innovation
- A novel method for synthesizing micrometer-sized mesoporous silica particles in various shapes, such as hard spheres, discoids, and origami structures, using sodium silicate as an inorganic silica precursor, with ionic or copolymer surfactants as structure directing agents and strong acids as condensation catalysts, allowing for control over particle morphology and internal architecture.
Environmental Impact of Silica Sphere Production
The production of silica spheres through microemulsion templating using sodium silicate has significant environmental implications that warrant careful consideration. This process, while innovative and efficient, raises concerns about resource consumption, energy usage, and potential ecological impacts.
Water usage is a primary environmental concern in silica sphere production. The microemulsion process requires substantial amounts of water, both as a reaction medium and for washing the final product. This high water demand can strain local water resources, particularly in water-scarce regions. Additionally, the wastewater generated during the production process may contain residual chemicals and nanoparticles, necessitating proper treatment before discharge to prevent water pollution.
Energy consumption is another critical factor. The synthesis of silica spheres often involves heating and stirring processes, which can be energy-intensive. The environmental footprint of this energy usage depends largely on the source of electricity used in production facilities. Reliance on fossil fuels for energy generation can contribute to greenhouse gas emissions and air pollution, while renewable energy sources can mitigate these impacts.
Chemical usage in the production process also poses environmental risks. Sodium silicate, the primary precursor, is generally considered less harmful than some alternative silica sources. However, other chemicals used in the microemulsion system, such as surfactants and co-solvents, may have varying degrees of toxicity and environmental persistence. The potential for these chemicals to enter ecosystems through accidental spills or improper disposal must be carefully managed.
The production of silica spheres generates waste materials, including unreacted precursors, byproducts, and rejected batches. Proper disposal or recycling of these wastes is crucial to minimize environmental impact. Some waste streams may contain nanomaterials, raising concerns about their potential effects on ecosystems if released into the environment.
The environmental impact of transportation associated with raw material sourcing and product distribution should also be considered. Long-distance transportation contributes to carbon emissions and air pollution, emphasizing the importance of optimizing supply chains and considering local sourcing options where possible.
On a positive note, the precise control over particle size and morphology offered by microemulsion templating can lead to more efficient use of materials in various applications. This efficiency could potentially reduce overall resource consumption and waste generation in downstream industries that utilize silica spheres.
In conclusion, while the microemulsion templating of silica spheres using sodium silicate offers technological advantages, its environmental impact is multifaceted. Addressing these environmental concerns through sustainable practices, such as water recycling, energy efficiency improvements, and responsible chemical management, is essential for the long-term viability and acceptability of this production method.
Water usage is a primary environmental concern in silica sphere production. The microemulsion process requires substantial amounts of water, both as a reaction medium and for washing the final product. This high water demand can strain local water resources, particularly in water-scarce regions. Additionally, the wastewater generated during the production process may contain residual chemicals and nanoparticles, necessitating proper treatment before discharge to prevent water pollution.
Energy consumption is another critical factor. The synthesis of silica spheres often involves heating and stirring processes, which can be energy-intensive. The environmental footprint of this energy usage depends largely on the source of electricity used in production facilities. Reliance on fossil fuels for energy generation can contribute to greenhouse gas emissions and air pollution, while renewable energy sources can mitigate these impacts.
Chemical usage in the production process also poses environmental risks. Sodium silicate, the primary precursor, is generally considered less harmful than some alternative silica sources. However, other chemicals used in the microemulsion system, such as surfactants and co-solvents, may have varying degrees of toxicity and environmental persistence. The potential for these chemicals to enter ecosystems through accidental spills or improper disposal must be carefully managed.
The production of silica spheres generates waste materials, including unreacted precursors, byproducts, and rejected batches. Proper disposal or recycling of these wastes is crucial to minimize environmental impact. Some waste streams may contain nanomaterials, raising concerns about their potential effects on ecosystems if released into the environment.
The environmental impact of transportation associated with raw material sourcing and product distribution should also be considered. Long-distance transportation contributes to carbon emissions and air pollution, emphasizing the importance of optimizing supply chains and considering local sourcing options where possible.
On a positive note, the precise control over particle size and morphology offered by microemulsion templating can lead to more efficient use of materials in various applications. This efficiency could potentially reduce overall resource consumption and waste generation in downstream industries that utilize silica spheres.
In conclusion, while the microemulsion templating of silica spheres using sodium silicate offers technological advantages, its environmental impact is multifaceted. Addressing these environmental concerns through sustainable practices, such as water recycling, energy efficiency improvements, and responsible chemical management, is essential for the long-term viability and acceptability of this production method.
Scale-up Considerations for Industrial Applications
Scaling up the production of silica spheres using sodium silicate in microemulsion templating presents several challenges and considerations for industrial applications. The transition from laboratory-scale synthesis to large-scale manufacturing requires careful optimization of process parameters and equipment design.
One of the primary considerations is the maintenance of uniform droplet size distribution in the microemulsion system. As the batch size increases, achieving consistent droplet formation becomes more challenging due to variations in shear forces and mixing dynamics. Industrial-scale reactors must be designed with advanced mixing technologies, such as high-shear mixers or rotor-stator systems, to ensure homogeneous dispersion of the aqueous sodium silicate phase within the oil continuous phase.
Temperature control is another critical factor in scale-up. The kinetics of silica formation and the stability of the microemulsion are highly temperature-dependent. Large-scale reactors must incorporate efficient heat transfer systems to maintain precise temperature control throughout the reaction volume. This may involve the use of jacketed vessels, internal cooling coils, or external heat exchangers.
The choice of surfactants and co-surfactants for stabilizing the microemulsion becomes more crucial in industrial settings. Factors such as cost-effectiveness, environmental impact, and compatibility with large-scale processing equipment must be considered. Additionally, the recovery and recycling of these components should be integrated into the process design to improve economic viability and reduce waste.
Reaction time and aging processes may need to be adjusted for larger batch sizes. The diffusion of reactants and products within the microemulsion droplets can be affected by the increased volume, potentially altering the kinetics of silica formation. Careful optimization of reaction conditions and aging protocols is necessary to maintain product quality and consistency.
Separation and purification of the silica spheres from the reaction mixture present significant challenges in industrial-scale production. Centrifugation, which is commonly used in laboratory settings, may not be practical for large volumes. Alternative separation techniques such as membrane filtration or continuous flow centrifugation may need to be explored and optimized for efficient product recovery.
Quality control and characterization methods must be adapted for high-throughput industrial production. In-line monitoring techniques, such as dynamic light scattering or automated microscopy, should be implemented to ensure consistent particle size distribution and morphology throughout the production process.
Environmental and safety considerations become more prominent at industrial scales. The handling and disposal of large quantities of organic solvents and surfactants require robust containment systems and waste treatment facilities. Additionally, the potential for aerosol formation during processing necessitates appropriate ventilation and personal protective equipment for workers.
One of the primary considerations is the maintenance of uniform droplet size distribution in the microemulsion system. As the batch size increases, achieving consistent droplet formation becomes more challenging due to variations in shear forces and mixing dynamics. Industrial-scale reactors must be designed with advanced mixing technologies, such as high-shear mixers or rotor-stator systems, to ensure homogeneous dispersion of the aqueous sodium silicate phase within the oil continuous phase.
Temperature control is another critical factor in scale-up. The kinetics of silica formation and the stability of the microemulsion are highly temperature-dependent. Large-scale reactors must incorporate efficient heat transfer systems to maintain precise temperature control throughout the reaction volume. This may involve the use of jacketed vessels, internal cooling coils, or external heat exchangers.
The choice of surfactants and co-surfactants for stabilizing the microemulsion becomes more crucial in industrial settings. Factors such as cost-effectiveness, environmental impact, and compatibility with large-scale processing equipment must be considered. Additionally, the recovery and recycling of these components should be integrated into the process design to improve economic viability and reduce waste.
Reaction time and aging processes may need to be adjusted for larger batch sizes. The diffusion of reactants and products within the microemulsion droplets can be affected by the increased volume, potentially altering the kinetics of silica formation. Careful optimization of reaction conditions and aging protocols is necessary to maintain product quality and consistency.
Separation and purification of the silica spheres from the reaction mixture present significant challenges in industrial-scale production. Centrifugation, which is commonly used in laboratory settings, may not be practical for large volumes. Alternative separation techniques such as membrane filtration or continuous flow centrifugation may need to be explored and optimized for efficient product recovery.
Quality control and characterization methods must be adapted for high-throughput industrial production. In-line monitoring techniques, such as dynamic light scattering or automated microscopy, should be implemented to ensure consistent particle size distribution and morphology throughout the production process.
Environmental and safety considerations become more prominent at industrial scales. The handling and disposal of large quantities of organic solvents and surfactants require robust containment systems and waste treatment facilities. Additionally, the potential for aerosol formation during processing necessitates appropriate ventilation and personal protective equipment for workers.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!