Hydroxyethylcellulose Correlation with Tissue Engineering Scaffolds
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HEC in TE Scaffolds: Background and Objectives
Hydroxyethylcellulose (HEC) has emerged as a promising material in the field of tissue engineering scaffolds, offering unique properties that align with the requirements of biocompatible and biodegradable structures. The evolution of tissue engineering as a discipline has been driven by the need to develop functional replacements for damaged or diseased tissues and organs. In this context, HEC, a cellulose derivative, has gained attention due to its versatility and biocompatibility.
The primary objective of researching the correlation between HEC and tissue engineering scaffolds is to explore and optimize the potential of this material in creating effective support structures for cell growth and tissue regeneration. This research aims to address the critical challenges in tissue engineering, such as achieving appropriate mechanical properties, controlled degradation rates, and favorable cell-material interactions.
HEC's journey in tissue engineering began with its recognition as a biocompatible polymer with excellent film-forming properties. Its non-toxic nature and ability to form hydrogels have made it an attractive candidate for various biomedical applications. The evolution of HEC usage in tissue engineering scaffolds has been marked by continuous efforts to enhance its properties and expand its applicability across different tissue types.
The technological progression in this field has seen a shift from simple HEC-based hydrogels to more complex, composite scaffolds that combine HEC with other materials to achieve specific mechanical and biological properties. This evolution reflects the growing understanding of the importance of mimicking the natural extracellular matrix in tissue engineering applications.
Current research trends focus on several key areas, including the optimization of HEC-based scaffold fabrication techniques, such as 3D printing and electrospinning, to create structures with precise architectures. Additionally, there is significant interest in modifying HEC to improve its cell adhesion properties and to incorporate bioactive molecules for enhanced tissue regeneration.
The objectives of ongoing research in this field are multifaceted. They include developing HEC-based scaffolds with tunable mechanical properties to match those of target tissues, improving the scaffold's ability to support cell proliferation and differentiation, and enhancing the material's degradation profile to synchronize with the rate of new tissue formation.
Furthermore, researchers aim to explore the potential of HEC in creating smart, responsive scaffolds that can adapt to the changing microenvironment during tissue regeneration. This includes investigating the incorporation of growth factors and other bioactive molecules into HEC-based scaffolds to guide and accelerate tissue formation.
As the field progresses, there is also a growing emphasis on translational research, focusing on bridging the gap between laboratory findings and clinical applications. This involves addressing scalability issues, ensuring reproducibility of scaffold properties, and conducting comprehensive in vivo studies to validate the efficacy and safety of HEC-based tissue engineering scaffolds.
The primary objective of researching the correlation between HEC and tissue engineering scaffolds is to explore and optimize the potential of this material in creating effective support structures for cell growth and tissue regeneration. This research aims to address the critical challenges in tissue engineering, such as achieving appropriate mechanical properties, controlled degradation rates, and favorable cell-material interactions.
HEC's journey in tissue engineering began with its recognition as a biocompatible polymer with excellent film-forming properties. Its non-toxic nature and ability to form hydrogels have made it an attractive candidate for various biomedical applications. The evolution of HEC usage in tissue engineering scaffolds has been marked by continuous efforts to enhance its properties and expand its applicability across different tissue types.
The technological progression in this field has seen a shift from simple HEC-based hydrogels to more complex, composite scaffolds that combine HEC with other materials to achieve specific mechanical and biological properties. This evolution reflects the growing understanding of the importance of mimicking the natural extracellular matrix in tissue engineering applications.
Current research trends focus on several key areas, including the optimization of HEC-based scaffold fabrication techniques, such as 3D printing and electrospinning, to create structures with precise architectures. Additionally, there is significant interest in modifying HEC to improve its cell adhesion properties and to incorporate bioactive molecules for enhanced tissue regeneration.
The objectives of ongoing research in this field are multifaceted. They include developing HEC-based scaffolds with tunable mechanical properties to match those of target tissues, improving the scaffold's ability to support cell proliferation and differentiation, and enhancing the material's degradation profile to synchronize with the rate of new tissue formation.
Furthermore, researchers aim to explore the potential of HEC in creating smart, responsive scaffolds that can adapt to the changing microenvironment during tissue regeneration. This includes investigating the incorporation of growth factors and other bioactive molecules into HEC-based scaffolds to guide and accelerate tissue formation.
As the field progresses, there is also a growing emphasis on translational research, focusing on bridging the gap between laboratory findings and clinical applications. This involves addressing scalability issues, ensuring reproducibility of scaffold properties, and conducting comprehensive in vivo studies to validate the efficacy and safety of HEC-based tissue engineering scaffolds.
Market Analysis for HEC-based Scaffolds
The market for Hydroxyethylcellulose (HEC)-based tissue engineering scaffolds is experiencing significant growth, driven by the increasing demand for advanced biomaterials in regenerative medicine and tissue engineering applications. HEC, a cellulose derivative, has gained attention in the scaffold market due to its biocompatibility, biodegradability, and versatile mechanical properties.
The global tissue engineering market, which encompasses HEC-based scaffolds, is projected to expand rapidly in the coming years. This growth is fueled by factors such as the rising prevalence of chronic diseases, an aging population, and advancements in regenerative medicine technologies. HEC-based scaffolds are particularly well-positioned to capitalize on this market expansion due to their unique properties and potential applications.
One of the key drivers for HEC-based scaffolds is their ability to mimic the extracellular matrix, providing an ideal environment for cell growth and tissue regeneration. This characteristic makes them suitable for a wide range of applications, including bone tissue engineering, cartilage repair, and wound healing. The versatility of HEC-based scaffolds allows them to address multiple market segments within the broader tissue engineering field.
The pharmaceutical and biotechnology industries are major consumers of HEC-based scaffolds, utilizing them in drug discovery and development processes. These scaffolds provide a more physiologically relevant platform for testing new therapeutics, potentially reducing the time and cost associated with bringing new drugs to market. This application is expected to drive significant demand for HEC-based scaffolds in the coming years.
Geographically, North America and Europe currently dominate the market for HEC-based scaffolds, owing to their advanced healthcare infrastructure and substantial investment in research and development. However, the Asia-Pacific region is anticipated to witness the fastest growth, driven by increasing healthcare expenditure, growing awareness of regenerative medicine, and improving regulatory frameworks in countries like China and India.
Despite the promising outlook, the market for HEC-based scaffolds faces challenges such as high production costs and regulatory hurdles. Manufacturers are investing in research to develop more cost-effective production methods and to improve the scalability of HEC-based scaffold technologies. Additionally, ongoing efforts to standardize regulatory processes for tissue-engineered products are expected to facilitate market growth in the long term.
In conclusion, the market for HEC-based tissue engineering scaffolds shows strong growth potential, driven by technological advancements, increasing applications in regenerative medicine, and growing demand for personalized healthcare solutions. As research continues to uncover new applications and improve existing technologies, HEC-based scaffolds are poised to play a crucial role in shaping the future of tissue engineering and regenerative medicine.
The global tissue engineering market, which encompasses HEC-based scaffolds, is projected to expand rapidly in the coming years. This growth is fueled by factors such as the rising prevalence of chronic diseases, an aging population, and advancements in regenerative medicine technologies. HEC-based scaffolds are particularly well-positioned to capitalize on this market expansion due to their unique properties and potential applications.
One of the key drivers for HEC-based scaffolds is their ability to mimic the extracellular matrix, providing an ideal environment for cell growth and tissue regeneration. This characteristic makes them suitable for a wide range of applications, including bone tissue engineering, cartilage repair, and wound healing. The versatility of HEC-based scaffolds allows them to address multiple market segments within the broader tissue engineering field.
The pharmaceutical and biotechnology industries are major consumers of HEC-based scaffolds, utilizing them in drug discovery and development processes. These scaffolds provide a more physiologically relevant platform for testing new therapeutics, potentially reducing the time and cost associated with bringing new drugs to market. This application is expected to drive significant demand for HEC-based scaffolds in the coming years.
Geographically, North America and Europe currently dominate the market for HEC-based scaffolds, owing to their advanced healthcare infrastructure and substantial investment in research and development. However, the Asia-Pacific region is anticipated to witness the fastest growth, driven by increasing healthcare expenditure, growing awareness of regenerative medicine, and improving regulatory frameworks in countries like China and India.
Despite the promising outlook, the market for HEC-based scaffolds faces challenges such as high production costs and regulatory hurdles. Manufacturers are investing in research to develop more cost-effective production methods and to improve the scalability of HEC-based scaffold technologies. Additionally, ongoing efforts to standardize regulatory processes for tissue-engineered products are expected to facilitate market growth in the long term.
In conclusion, the market for HEC-based tissue engineering scaffolds shows strong growth potential, driven by technological advancements, increasing applications in regenerative medicine, and growing demand for personalized healthcare solutions. As research continues to uncover new applications and improve existing technologies, HEC-based scaffolds are poised to play a crucial role in shaping the future of tissue engineering and regenerative medicine.
HEC Scaffold Technology: Current Status and Challenges
Hydroxyethylcellulose (HEC) has emerged as a promising material in the field of tissue engineering scaffolds, offering unique properties that align well with the requirements of biocompatible and biodegradable structures. The current status of HEC scaffold technology reflects significant advancements, yet it also faces several challenges that researchers are actively addressing.
One of the primary advantages of HEC in scaffold development is its excellent biocompatibility. Studies have shown that HEC-based scaffolds support cell adhesion, proliferation, and differentiation, making them suitable for various tissue engineering applications. The material's ability to form hydrogels with tunable mechanical properties has been particularly beneficial in mimicking the extracellular matrix of different tissues.
Recent research has focused on improving the mechanical strength of HEC scaffolds, as this has been a limiting factor in certain applications. Crosslinking techniques, such as chemical crosslinking with agents like glutaraldehyde or physical crosslinking through freeze-thawing cycles, have shown promising results in enhancing the structural integrity of HEC scaffolds. These advancements have expanded the potential use of HEC in load-bearing tissue engineering applications.
Another area of progress is the development of composite scaffolds incorporating HEC with other materials. For instance, HEC-collagen composites have demonstrated improved cell attachment and proliferation compared to pure HEC scaffolds. Similarly, HEC-nanocellulose composites have shown enhanced mechanical properties and bioactivity.
Despite these advancements, several challenges remain in the field of HEC scaffold technology. One significant issue is the control of degradation rates. While HEC is biodegradable, fine-tuning its degradation to match the rate of tissue regeneration remains a complex task. Researchers are exploring various strategies, including chemical modifications and blending with other polymers, to achieve optimal degradation profiles.
Another challenge lies in the scalability and reproducibility of HEC scaffold production. Current methods often yield scaffolds with heterogeneous structures, which can lead to inconsistent performance in tissue engineering applications. Developing standardized manufacturing processes that ensure uniform scaffold properties is crucial for the clinical translation of HEC-based tissue engineering solutions.
Furthermore, while HEC scaffolds have shown promise in vitro, their performance in vivo requires further investigation. The complex biological environment of the human body presents additional challenges, including immune responses and vascularization, which need to be addressed for successful clinical applications.
One of the primary advantages of HEC in scaffold development is its excellent biocompatibility. Studies have shown that HEC-based scaffolds support cell adhesion, proliferation, and differentiation, making them suitable for various tissue engineering applications. The material's ability to form hydrogels with tunable mechanical properties has been particularly beneficial in mimicking the extracellular matrix of different tissues.
Recent research has focused on improving the mechanical strength of HEC scaffolds, as this has been a limiting factor in certain applications. Crosslinking techniques, such as chemical crosslinking with agents like glutaraldehyde or physical crosslinking through freeze-thawing cycles, have shown promising results in enhancing the structural integrity of HEC scaffolds. These advancements have expanded the potential use of HEC in load-bearing tissue engineering applications.
Another area of progress is the development of composite scaffolds incorporating HEC with other materials. For instance, HEC-collagen composites have demonstrated improved cell attachment and proliferation compared to pure HEC scaffolds. Similarly, HEC-nanocellulose composites have shown enhanced mechanical properties and bioactivity.
Despite these advancements, several challenges remain in the field of HEC scaffold technology. One significant issue is the control of degradation rates. While HEC is biodegradable, fine-tuning its degradation to match the rate of tissue regeneration remains a complex task. Researchers are exploring various strategies, including chemical modifications and blending with other polymers, to achieve optimal degradation profiles.
Another challenge lies in the scalability and reproducibility of HEC scaffold production. Current methods often yield scaffolds with heterogeneous structures, which can lead to inconsistent performance in tissue engineering applications. Developing standardized manufacturing processes that ensure uniform scaffold properties is crucial for the clinical translation of HEC-based tissue engineering solutions.
Furthermore, while HEC scaffolds have shown promise in vitro, their performance in vivo requires further investigation. The complex biological environment of the human body presents additional challenges, including immune responses and vascularization, which need to be addressed for successful clinical applications.
Current HEC Scaffold Fabrication Methods
01 Use as a thickening agent in various industries
Hydroxyethylcellulose is widely used as a thickening agent in various industries, including cosmetics, pharmaceuticals, and oil drilling. It helps to increase the viscosity of solutions and provides stability to formulations.- Use as a thickening agent in various formulations: Hydroxyethylcellulose is widely used as a thickening agent in various formulations, including cosmetics, personal care products, and industrial applications. It helps to improve the viscosity and stability of liquid and semi-solid products, enhancing their texture and performance.
- Application in oil and gas industry: Hydroxyethylcellulose is utilized in the oil and gas industry as a component in drilling fluids and fracturing fluids. It helps control fluid loss, improve viscosity, and enhance the overall performance of these fluids in well operations.
- Use in pharmaceutical formulations: Hydroxyethylcellulose is employed in pharmaceutical formulations as a binder, film-forming agent, and controlled-release matrix. It helps in the development of various drug delivery systems, improving the stability and release characteristics of active ingredients.
- Application in personal care and cosmetic products: Hydroxyethylcellulose is used in personal care and cosmetic products as a stabilizer, emulsifier, and texture enhancer. It helps improve the feel and consistency of products such as shampoos, lotions, and creams, while also providing moisturizing properties.
- Use in adhesive and coating formulations: Hydroxyethylcellulose is utilized in adhesive and coating formulations to improve their rheological properties, water retention, and film-forming characteristics. It enhances the performance and durability of various adhesives and coatings used in industrial and consumer applications.
02 Application in personal care products
Hydroxyethylcellulose is commonly used in personal care products such as shampoos, lotions, and creams. It acts as a thickener, emulsifier, and stabilizer, improving the texture and consistency of these products.Expand Specific Solutions03 Use in pharmaceutical formulations
Hydroxyethylcellulose is utilized in pharmaceutical formulations as a binder, film-former, and controlled-release agent. It helps in the production of tablets, capsules, and topical preparations, improving drug delivery and stability.Expand Specific Solutions04 Application in oil and gas industry
Hydroxyethylcellulose is used in the oil and gas industry as a component of drilling fluids and fracturing fluids. It helps control fluid loss, improve viscosity, and enhance the efficiency of drilling and extraction processes.Expand Specific Solutions05 Modification and derivatization for specific applications
Hydroxyethylcellulose can be chemically modified or derivatized to enhance its properties for specific applications. These modifications can improve its solubility, stability, or compatibility with other ingredients in various formulations.Expand Specific Solutions
Key Players in HEC Scaffold Development
The research on the correlation between Hydroxyethylcellulose and Tissue Engineering Scaffolds is in a developing stage, with growing market potential and increasing technological maturity. The field is attracting attention from both academic institutions and industry players, indicating a competitive landscape. Key players like Sichuan University, Drexel University, and Tongji University are contributing to academic research, while companies such as Ethicon, Inc. and Hercules Corp. are exploring commercial applications. The market size is expanding as tissue engineering gains traction in regenerative medicine. As the technology matures, we can expect more collaborations between academia and industry, potentially leading to innovative products and therapies in the coming years.
Agency for Science, Technology & Research
Technical Solution: The Agency for Science, Technology & Research (A*STAR) has developed innovative tissue engineering scaffolds using hydroxyethylcellulose (HEC) as a key component. Their approach involves creating a composite scaffold by combining HEC with other biocompatible polymers to enhance mechanical properties and cell adhesion. The scaffolds are fabricated using advanced 3D printing techniques, allowing for precise control over pore size and architecture[1]. A*STAR researchers have also incorporated growth factors and bioactive molecules into the HEC-based scaffolds to promote tissue regeneration and vascularization[3]. The scaffolds have shown promising results in supporting the growth and differentiation of various cell types, including mesenchymal stem cells and osteoblasts[5].
Strengths: Advanced 3D printing capabilities, integration of bioactive molecules, and customizable scaffold properties. Weaknesses: Potential limitations in mechanical strength for load-bearing applications and scalability for large-scale production.
Sichuan University
Technical Solution: Sichuan University has made significant advancements in utilizing hydroxyethylcellulose for tissue engineering scaffolds. Their research team has developed a novel approach that combines HEC with natural polymers like chitosan and gelatin to create hybrid scaffolds with enhanced biocompatibility and mechanical properties[2]. The university has also pioneered the use of electrospinning techniques to fabricate HEC-based nanofiber scaffolds, which mimic the extracellular matrix structure and promote cell attachment and proliferation[4]. Additionally, they have explored the incorporation of nanoparticles into HEC scaffolds to improve their antimicrobial properties and drug delivery capabilities[6]. These scaffolds have shown promising results in various tissue engineering applications, including skin regeneration and cartilage repair.
Strengths: Innovative hybrid scaffold designs, expertise in electrospinning techniques, and multifunctional scaffold properties. Weaknesses: Potential challenges in scaling up production and ensuring consistent quality across large batches.
Innovations in HEC-based Scaffold Design
Tissue engineering scaffolds promoting matrix protein production
PatentInactiveUS7635592B2
Innovation
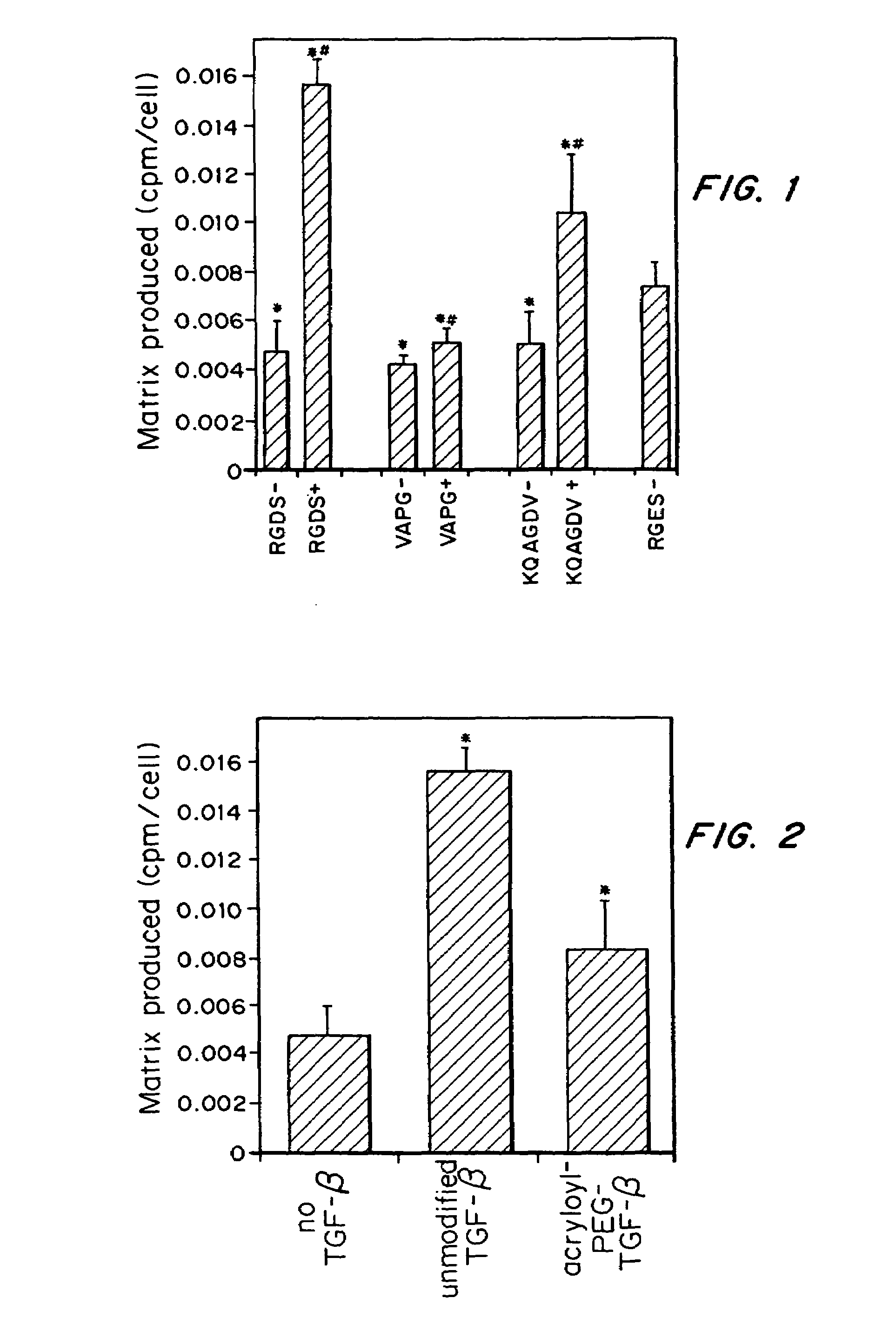
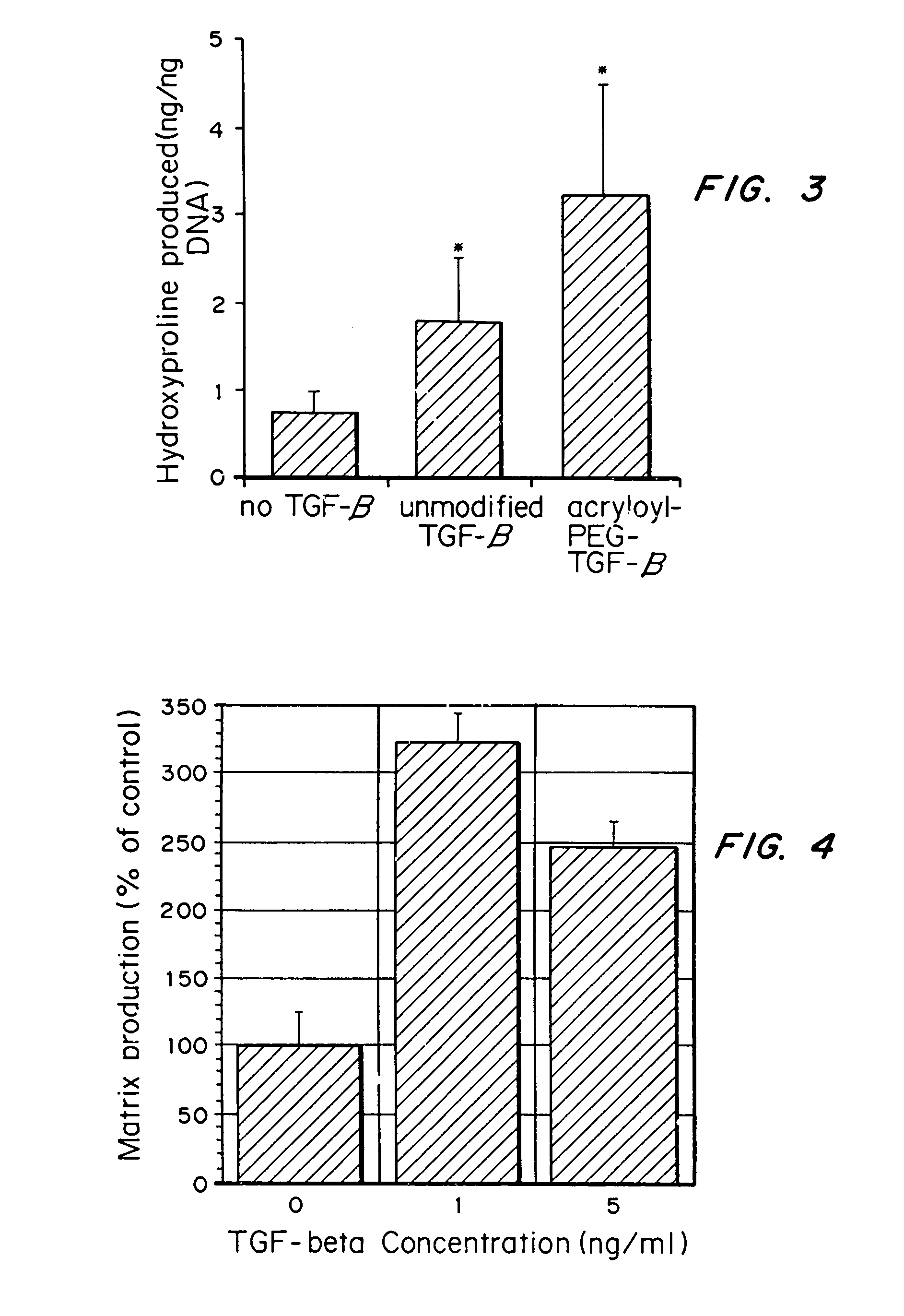
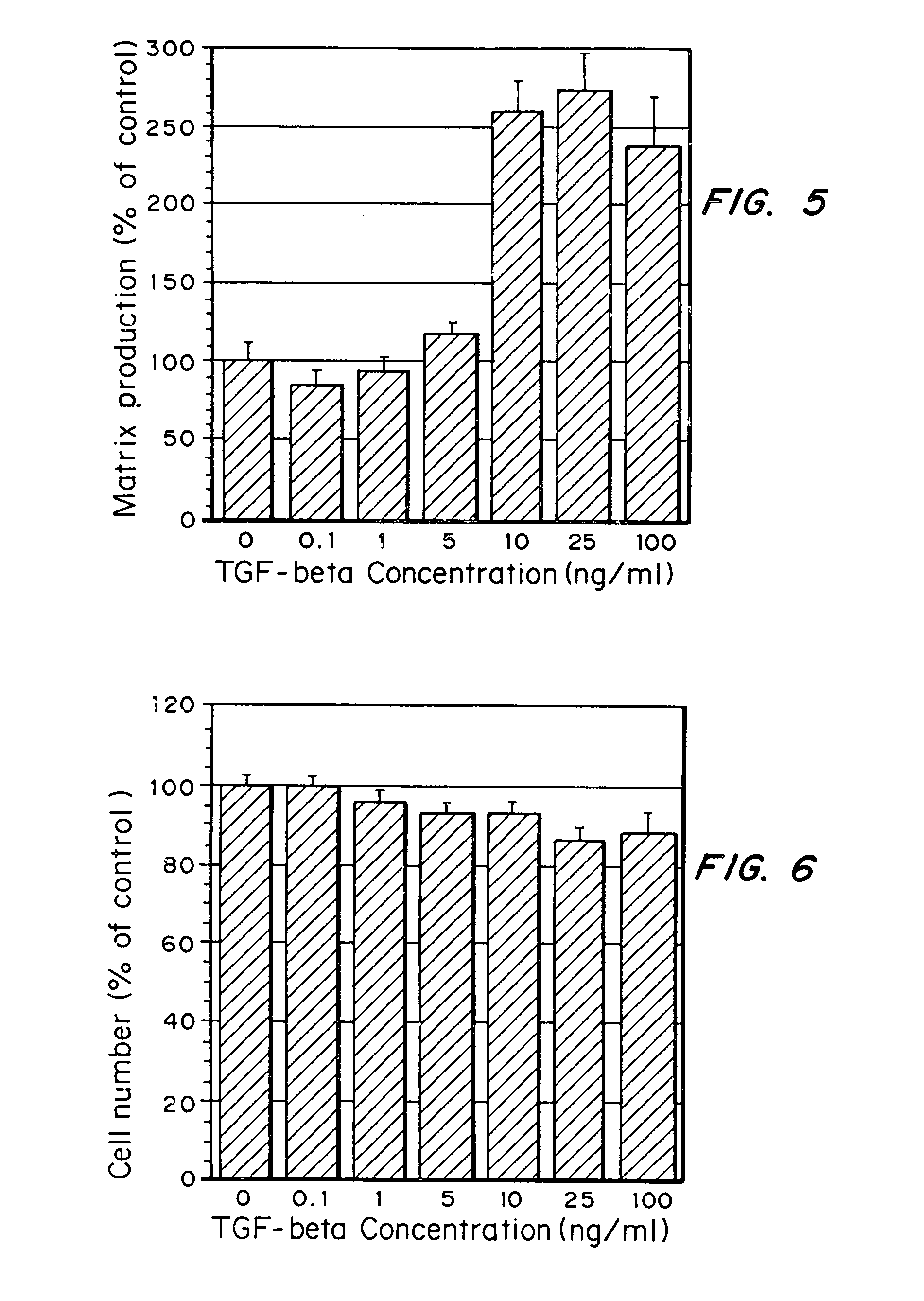
- Conjugating or immobilizing matrix-enhancing molecules like transforming growth factor-beta (TGF-β) to scaffolds using a polymer such as polyethylene glycol monoacrylate, allowing for increased ECM production without promoting cell proliferation, by tethering TGF-β to the scaffold material.
Scaffolds for use in tissue engineering and method for preparing scaffolds
PatentPendingUS20220184276A1
Innovation
- A scaffold design featuring a gradient and staggered distribution of volume-building components, allowing for precise control of pore size and shape, combined with a novel copolymer of ε-caprolactone and p-dioxanone for additive manufacturing, ensuring degradability and improved mechanical properties.
Biocompatibility and Safety Considerations
Biocompatibility and safety considerations are paramount in the development and application of hydroxyethylcellulose (HEC) for tissue engineering scaffolds. The interaction between HEC-based scaffolds and biological systems must be thoroughly evaluated to ensure the safety and efficacy of these materials in regenerative medicine applications.
HEC, a cellulose derivative, has shown promising potential as a scaffold material due to its biocompatibility, biodegradability, and versatile physicochemical properties. However, comprehensive assessments of its long-term effects on cellular behavior and tissue regeneration are essential. In vitro studies have demonstrated that HEC scaffolds generally support cell adhesion, proliferation, and differentiation of various cell types, including mesenchymal stem cells and fibroblasts. These findings suggest a favorable cellular response to HEC-based materials.
The biodegradation profile of HEC scaffolds is a critical factor in their biocompatibility. The rate of degradation should ideally match the rate of tissue regeneration to ensure proper structural support throughout the healing process. Studies have shown that HEC undergoes enzymatic degradation in physiological conditions, with the degradation rate controllable through chemical modifications or crosslinking strategies. This tunability allows for the optimization of scaffold properties to suit specific tissue engineering applications.
Immunogenicity is another crucial aspect of biocompatibility that requires careful consideration. Initial studies indicate that HEC scaffolds exhibit low immunogenicity, with minimal inflammatory responses observed in both in vitro and in vivo models. However, more extensive research is needed to fully characterize the immune response to HEC-based materials across different tissue types and implantation sites.
Safety considerations extend beyond biocompatibility to include potential toxicity of degradation products and the risk of unintended biological interactions. While HEC is generally regarded as safe for various biomedical applications, the specific formulations and modifications used in tissue engineering scaffolds warrant thorough toxicological evaluations. This includes assessing the potential for systemic toxicity, genotoxicity, and carcinogenicity of both the scaffold material and its degradation products.
The mechanical properties of HEC scaffolds also play a role in their safety profile. The scaffold should provide adequate support to the regenerating tissue without causing stress shielding or mechanical mismatch, which could lead to impaired tissue function or implant failure. Tailoring the mechanical properties of HEC scaffolds through various fabrication techniques and composite formulations is an active area of research aimed at optimizing their performance and safety in diverse tissue engineering applications.
In conclusion, while initial studies on HEC-based tissue engineering scaffolds show promising biocompatibility and safety profiles, continued research is essential to fully elucidate their long-term effects and optimize their performance in clinical applications. Rigorous preclinical testing and regulatory compliance will be crucial in translating HEC-based scaffold technologies from the laboratory to clinical practice.
HEC, a cellulose derivative, has shown promising potential as a scaffold material due to its biocompatibility, biodegradability, and versatile physicochemical properties. However, comprehensive assessments of its long-term effects on cellular behavior and tissue regeneration are essential. In vitro studies have demonstrated that HEC scaffolds generally support cell adhesion, proliferation, and differentiation of various cell types, including mesenchymal stem cells and fibroblasts. These findings suggest a favorable cellular response to HEC-based materials.
The biodegradation profile of HEC scaffolds is a critical factor in their biocompatibility. The rate of degradation should ideally match the rate of tissue regeneration to ensure proper structural support throughout the healing process. Studies have shown that HEC undergoes enzymatic degradation in physiological conditions, with the degradation rate controllable through chemical modifications or crosslinking strategies. This tunability allows for the optimization of scaffold properties to suit specific tissue engineering applications.
Immunogenicity is another crucial aspect of biocompatibility that requires careful consideration. Initial studies indicate that HEC scaffolds exhibit low immunogenicity, with minimal inflammatory responses observed in both in vitro and in vivo models. However, more extensive research is needed to fully characterize the immune response to HEC-based materials across different tissue types and implantation sites.
Safety considerations extend beyond biocompatibility to include potential toxicity of degradation products and the risk of unintended biological interactions. While HEC is generally regarded as safe for various biomedical applications, the specific formulations and modifications used in tissue engineering scaffolds warrant thorough toxicological evaluations. This includes assessing the potential for systemic toxicity, genotoxicity, and carcinogenicity of both the scaffold material and its degradation products.
The mechanical properties of HEC scaffolds also play a role in their safety profile. The scaffold should provide adequate support to the regenerating tissue without causing stress shielding or mechanical mismatch, which could lead to impaired tissue function or implant failure. Tailoring the mechanical properties of HEC scaffolds through various fabrication techniques and composite formulations is an active area of research aimed at optimizing their performance and safety in diverse tissue engineering applications.
In conclusion, while initial studies on HEC-based tissue engineering scaffolds show promising biocompatibility and safety profiles, continued research is essential to fully elucidate their long-term effects and optimize their performance in clinical applications. Rigorous preclinical testing and regulatory compliance will be crucial in translating HEC-based scaffold technologies from the laboratory to clinical practice.
Regulatory Landscape for HEC-based Medical Devices
The regulatory landscape for Hydroxyethylcellulose (HEC)-based medical devices, particularly in the context of tissue engineering scaffolds, is complex and evolving. Regulatory bodies worldwide, such as the FDA in the United States and the EMA in Europe, have established frameworks to ensure the safety and efficacy of these innovative medical products.
In the United States, HEC-based tissue engineering scaffolds are typically classified as combination products, falling under the jurisdiction of the FDA's Center for Biologics Evaluation and Research (CBER) or the Center for Devices and Radiological Health (CDRH), depending on their primary mode of action. The regulatory pathway often involves premarket approval (PMA) or the 510(k) clearance process, with the latter being more common for devices similar to existing approved products.
European regulations for HEC-based medical devices are governed by the Medical Device Regulation (MDR) and the In Vitro Diagnostic Regulation (IVDR). These regulations emphasize a risk-based approach, with higher-risk devices subject to more stringent controls. Manufacturers must demonstrate compliance with essential requirements, including biocompatibility, sterilization, and performance characteristics.
In both regions, manufacturers are required to implement quality management systems, such as ISO 13485, to ensure consistent product quality and safety. Clinical evidence is crucial for regulatory approval, with requirements varying based on the device's classification and intended use. Post-market surveillance is also a key component of the regulatory framework, ensuring ongoing safety and performance monitoring.
Regulatory considerations specific to HEC-based scaffolds include biocompatibility testing, degradation studies, and assessment of potential immunological responses. The use of HEC in tissue engineering applications may require additional scrutiny due to its interaction with biological systems and potential long-term effects on tissue regeneration.
Globally, harmonization efforts, such as the International Medical Device Regulators Forum (IMDRF), aim to streamline regulatory processes across different regions. However, manufacturers must still navigate country-specific requirements, particularly in emerging markets where regulations for advanced medical technologies may be less developed.
As the field of tissue engineering continues to advance, regulatory frameworks are expected to evolve to address novel technologies and applications. This may include the development of specific guidance documents for HEC-based scaffolds and other biomaterials used in regenerative medicine. Manufacturers and researchers must stay informed of these regulatory developments to ensure compliance and facilitate the translation of innovative HEC-based technologies into clinical practice.
In the United States, HEC-based tissue engineering scaffolds are typically classified as combination products, falling under the jurisdiction of the FDA's Center for Biologics Evaluation and Research (CBER) or the Center for Devices and Radiological Health (CDRH), depending on their primary mode of action. The regulatory pathway often involves premarket approval (PMA) or the 510(k) clearance process, with the latter being more common for devices similar to existing approved products.
European regulations for HEC-based medical devices are governed by the Medical Device Regulation (MDR) and the In Vitro Diagnostic Regulation (IVDR). These regulations emphasize a risk-based approach, with higher-risk devices subject to more stringent controls. Manufacturers must demonstrate compliance with essential requirements, including biocompatibility, sterilization, and performance characteristics.
In both regions, manufacturers are required to implement quality management systems, such as ISO 13485, to ensure consistent product quality and safety. Clinical evidence is crucial for regulatory approval, with requirements varying based on the device's classification and intended use. Post-market surveillance is also a key component of the regulatory framework, ensuring ongoing safety and performance monitoring.
Regulatory considerations specific to HEC-based scaffolds include biocompatibility testing, degradation studies, and assessment of potential immunological responses. The use of HEC in tissue engineering applications may require additional scrutiny due to its interaction with biological systems and potential long-term effects on tissue regeneration.
Globally, harmonization efforts, such as the International Medical Device Regulators Forum (IMDRF), aim to streamline regulatory processes across different regions. However, manufacturers must still navigate country-specific requirements, particularly in emerging markets where regulations for advanced medical technologies may be less developed.
As the field of tissue engineering continues to advance, regulatory frameworks are expected to evolve to address novel technologies and applications. This may include the development of specific guidance documents for HEC-based scaffolds and other biomaterials used in regenerative medicine. Manufacturers and researchers must stay informed of these regulatory developments to ensure compliance and facilitate the translation of innovative HEC-based technologies into clinical practice.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!