Role of Boron in Borosilicate Glass Properties
JUL 3, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Borosilicate Glass Evolution and Objectives
Borosilicate glass has a rich history dating back to the late 19th century when German glassmaker Otto Schott first developed this innovative material. The evolution of borosilicate glass has been driven by the need for materials with superior thermal, chemical, and mechanical properties. Initially, the primary focus was on creating glass with improved thermal shock resistance for laboratory glassware and industrial applications.
As the technology progressed, the role of boron in enhancing glass properties became increasingly apparent. Boron oxide (B2O3) was found to be a crucial component in reducing the thermal expansion coefficient of glass, thereby improving its resistance to thermal shock. This discovery led to the widespread adoption of borosilicate glass in various industries, including laboratory equipment, cookware, and lighting.
The objectives of borosilicate glass development have evolved over time to meet the changing demands of various sectors. In the mid-20th century, the focus shifted towards improving the chemical durability of the glass, making it suitable for use in the pharmaceutical and chemical industries. This led to the development of specialized borosilicate glass compositions with enhanced resistance to acids, alkalis, and other corrosive substances.
In recent decades, the objectives have expanded to include the development of borosilicate glass with improved optical properties for use in high-performance optics and photonics applications. The ability to fine-tune the refractive index and dispersion characteristics of borosilicate glass by adjusting the boron content has opened up new possibilities in fields such as astronomy, laser technology, and fiber optics.
Another important objective in the evolution of borosilicate glass has been to enhance its mechanical strength and durability. This has led to the development of chemically strengthened borosilicate glass, which finds applications in aerospace, automotive, and consumer electronics industries. The ongoing research in this area aims to further improve the impact resistance and scratch resistance of borosilicate glass without compromising its other desirable properties.
Looking ahead, the objectives for borosilicate glass development are likely to focus on sustainability and energy efficiency. This includes developing manufacturing processes that require less energy and produce fewer emissions, as well as exploring ways to incorporate recycled materials into borosilicate glass production. Additionally, there is growing interest in developing borosilicate glass compositions with enhanced functionality, such as self-cleaning surfaces, antimicrobial properties, or smart glass capabilities.
As the technology progressed, the role of boron in enhancing glass properties became increasingly apparent. Boron oxide (B2O3) was found to be a crucial component in reducing the thermal expansion coefficient of glass, thereby improving its resistance to thermal shock. This discovery led to the widespread adoption of borosilicate glass in various industries, including laboratory equipment, cookware, and lighting.
The objectives of borosilicate glass development have evolved over time to meet the changing demands of various sectors. In the mid-20th century, the focus shifted towards improving the chemical durability of the glass, making it suitable for use in the pharmaceutical and chemical industries. This led to the development of specialized borosilicate glass compositions with enhanced resistance to acids, alkalis, and other corrosive substances.
In recent decades, the objectives have expanded to include the development of borosilicate glass with improved optical properties for use in high-performance optics and photonics applications. The ability to fine-tune the refractive index and dispersion characteristics of borosilicate glass by adjusting the boron content has opened up new possibilities in fields such as astronomy, laser technology, and fiber optics.
Another important objective in the evolution of borosilicate glass has been to enhance its mechanical strength and durability. This has led to the development of chemically strengthened borosilicate glass, which finds applications in aerospace, automotive, and consumer electronics industries. The ongoing research in this area aims to further improve the impact resistance and scratch resistance of borosilicate glass without compromising its other desirable properties.
Looking ahead, the objectives for borosilicate glass development are likely to focus on sustainability and energy efficiency. This includes developing manufacturing processes that require less energy and produce fewer emissions, as well as exploring ways to incorporate recycled materials into borosilicate glass production. Additionally, there is growing interest in developing borosilicate glass compositions with enhanced functionality, such as self-cleaning surfaces, antimicrobial properties, or smart glass capabilities.
Market Demand Analysis for Borosilicate Glass
The global market for borosilicate glass has been experiencing steady growth, driven by increasing demand across various industries. The unique properties of borosilicate glass, particularly its high thermal resistance and chemical durability, have made it a preferred material in laboratory equipment, pharmaceutical packaging, and high-end cookware.
In the laboratory and scientific research sector, borosilicate glass remains the gold standard for glassware due to its resistance to thermal shock and chemical corrosion. The expansion of research and development activities in pharmaceuticals, biotechnology, and materials science has fueled the demand for high-quality laboratory equipment, thereby boosting the market for borosilicate glass.
The pharmaceutical industry represents another significant market for borosilicate glass, particularly in packaging applications. The material's inert nature and ability to maintain the integrity of sensitive drugs have led to its widespread use in vials, ampoules, and syringes. With the global pharmaceutical market projected to grow, the demand for borosilicate glass in this sector is expected to increase correspondingly.
Consumer goods, especially kitchenware and home appliances, have also contributed to the rising demand for borosilicate glass. The material's heat resistance and durability make it ideal for bakeware, cookware, and storage containers. As consumers become more health-conscious and seek alternatives to plastic, the market for borosilicate glass products in this segment has expanded.
The automotive and aerospace industries have shown growing interest in borosilicate glass for specialized applications. Its use in high-performance lighting, sensors, and display systems has opened new avenues for market growth. The increasing adoption of advanced technologies in vehicles and aircraft is likely to drive further demand in these sectors.
Emerging applications in solar energy and fiber optics have also contributed to the market's expansion. Borosilicate glass's low coefficient of thermal expansion and excellent optical properties make it suitable for solar collectors and optical fibers, aligning with the global push towards renewable energy and improved telecommunications infrastructure.
Geographically, North America and Europe have traditionally been the largest markets for borosilicate glass, owing to their well-established pharmaceutical and research sectors. However, the Asia-Pacific region is emerging as a significant growth driver, fueled by rapid industrialization, increasing research activities, and growing healthcare infrastructure in countries like China and India.
In the laboratory and scientific research sector, borosilicate glass remains the gold standard for glassware due to its resistance to thermal shock and chemical corrosion. The expansion of research and development activities in pharmaceuticals, biotechnology, and materials science has fueled the demand for high-quality laboratory equipment, thereby boosting the market for borosilicate glass.
The pharmaceutical industry represents another significant market for borosilicate glass, particularly in packaging applications. The material's inert nature and ability to maintain the integrity of sensitive drugs have led to its widespread use in vials, ampoules, and syringes. With the global pharmaceutical market projected to grow, the demand for borosilicate glass in this sector is expected to increase correspondingly.
Consumer goods, especially kitchenware and home appliances, have also contributed to the rising demand for borosilicate glass. The material's heat resistance and durability make it ideal for bakeware, cookware, and storage containers. As consumers become more health-conscious and seek alternatives to plastic, the market for borosilicate glass products in this segment has expanded.
The automotive and aerospace industries have shown growing interest in borosilicate glass for specialized applications. Its use in high-performance lighting, sensors, and display systems has opened new avenues for market growth. The increasing adoption of advanced technologies in vehicles and aircraft is likely to drive further demand in these sectors.
Emerging applications in solar energy and fiber optics have also contributed to the market's expansion. Borosilicate glass's low coefficient of thermal expansion and excellent optical properties make it suitable for solar collectors and optical fibers, aligning with the global push towards renewable energy and improved telecommunications infrastructure.
Geographically, North America and Europe have traditionally been the largest markets for borosilicate glass, owing to their well-established pharmaceutical and research sectors. However, the Asia-Pacific region is emerging as a significant growth driver, fueled by rapid industrialization, increasing research activities, and growing healthcare infrastructure in countries like China and India.
Current Boron Integration Challenges
The integration of boron into borosilicate glass presents several significant challenges that researchers and manufacturers must address to optimize glass properties and production processes. One of the primary difficulties lies in controlling the volatilization of boron during the glass melting process. Boron oxide (B2O3) has a relatively low boiling point compared to other glass components, which can lead to substantial losses during high-temperature melting. This volatilization not only affects the final composition of the glass but also creates environmental concerns due to the release of boron-containing vapors.
Another challenge is maintaining the homogeneity of boron distribution within the glass matrix. Boron tends to form distinct structural units, such as BO3 triangles and BO4 tetrahedra, which can segregate or cluster depending on the overall glass composition and processing conditions. Achieving a uniform distribution of these structural units is crucial for ensuring consistent glass properties throughout the material.
The incorporation of boron also impacts the glass transition temperature and thermal expansion coefficient of borosilicate glasses. While boron generally helps to lower the thermal expansion, precise control over these properties requires careful balancing of the boron content with other glass components. This balancing act becomes particularly challenging when trying to meet specific thermal and mechanical property requirements for various applications.
Furthermore, the presence of boron affects the chemical durability of the glass. Although borosilicate glasses are known for their excellent chemical resistance, excessive boron content can lead to decreased durability in certain environments, particularly under alkaline conditions. This necessitates careful optimization of the boron content to maintain the desired chemical stability while preserving other beneficial properties.
The interaction between boron and other network-forming elements, such as silicon and aluminum, adds another layer of complexity to glass formulation. These interactions can lead to unexpected changes in glass structure and properties, making it challenging to predict and control the final characteristics of the glass based solely on composition.
Lastly, the measurement and analysis of boron content in glass pose technical difficulties. Traditional analytical methods may not always provide accurate results for boron concentration, especially at low levels. This complicates quality control processes and makes it challenging to ensure consistent boron incorporation across different production batches.
Another challenge is maintaining the homogeneity of boron distribution within the glass matrix. Boron tends to form distinct structural units, such as BO3 triangles and BO4 tetrahedra, which can segregate or cluster depending on the overall glass composition and processing conditions. Achieving a uniform distribution of these structural units is crucial for ensuring consistent glass properties throughout the material.
The incorporation of boron also impacts the glass transition temperature and thermal expansion coefficient of borosilicate glasses. While boron generally helps to lower the thermal expansion, precise control over these properties requires careful balancing of the boron content with other glass components. This balancing act becomes particularly challenging when trying to meet specific thermal and mechanical property requirements for various applications.
Furthermore, the presence of boron affects the chemical durability of the glass. Although borosilicate glasses are known for their excellent chemical resistance, excessive boron content can lead to decreased durability in certain environments, particularly under alkaline conditions. This necessitates careful optimization of the boron content to maintain the desired chemical stability while preserving other beneficial properties.
The interaction between boron and other network-forming elements, such as silicon and aluminum, adds another layer of complexity to glass formulation. These interactions can lead to unexpected changes in glass structure and properties, making it challenging to predict and control the final characteristics of the glass based solely on composition.
Lastly, the measurement and analysis of boron content in glass pose technical difficulties. Traditional analytical methods may not always provide accurate results for boron concentration, especially at low levels. This complicates quality control processes and makes it challenging to ensure consistent boron incorporation across different production batches.
Boron Incorporation Techniques
01 Chemical composition and thermal properties
Borosilicate glass is characterized by its unique chemical composition, primarily consisting of silica and boron oxide. This composition gives it excellent thermal properties, including low thermal expansion and high resistance to thermal shock. These characteristics make it suitable for applications requiring temperature stability and resistance to sudden temperature changes.- Chemical composition and thermal properties: Borosilicate glass is characterized by its unique chemical composition, primarily consisting of silica and boron oxide. This composition gives it excellent thermal properties, including low thermal expansion and high resistance to thermal shock. These characteristics make it suitable for applications requiring temperature stability and resistance to sudden temperature changes.
- Optical properties and transparency: Borosilicate glass exhibits superior optical properties, including high transparency and clarity. It has a low refractive index and low dispersion, making it ideal for use in optical instruments and laboratory glassware. The glass can be engineered to have specific optical characteristics for various applications in optics and photonics.
- Chemical resistance and durability: One of the key properties of borosilicate glass is its exceptional chemical resistance. It is highly resistant to corrosion from acids, alkalis, and other chemicals, making it suitable for laboratory equipment and chemical processing. The glass also demonstrates high durability and scratch resistance, contributing to its longevity in various applications.
- Electrical insulation properties: Borosilicate glass possesses excellent electrical insulation properties, making it suitable for use in electrical and electronic applications. It has a low dielectric constant and low electrical conductivity, which contribute to its effectiveness as an insulator in various electronic components and devices.
- Manufacturing processes and formability: Borosilicate glass can be manufactured using various processes, including melting, forming, and annealing. It exhibits good formability, allowing for the creation of complex shapes and structures. The glass can be molded, blown, or drawn into various forms, making it versatile for different applications in industry and consumer products.
02 Optical properties and transparency
Borosilicate glass exhibits superior optical properties, including high transparency and clarity. It has a low refractive index and low dispersion, making it ideal for use in optical instruments and laboratory glassware. The glass can be engineered to have specific optical characteristics for various applications in the fields of optics and photonics.Expand Specific Solutions03 Chemical resistance and durability
One of the key properties of borosilicate glass is its exceptional chemical resistance. It is highly resistant to corrosion from acids, alkalis, and other chemical substances. This durability makes it an ideal material for laboratory equipment, pharmaceutical packaging, and industrial applications where chemical inertness is crucial.Expand Specific Solutions04 Mechanical strength and impact resistance
Borosilicate glass possesses high mechanical strength and improved impact resistance compared to conventional soda-lime glass. These properties make it suitable for use in cookware, laboratory equipment, and other applications requiring durability. The glass can be further strengthened through various treatments to enhance its mechanical properties.Expand Specific Solutions05 Electrical insulation and dielectric properties
Borosilicate glass exhibits excellent electrical insulation properties and low dielectric loss. These characteristics make it suitable for use in electrical and electronic applications, such as insulators, capacitors, and substrate materials for electronic components. The glass can be tailored to have specific dielectric properties for various electronic applications.Expand Specific Solutions
Key Borosilicate Glass Manufacturers
The borosilicate glass industry is in a mature stage, with established players and steady market growth. The global market size for borosilicate glass is projected to reach $5.7 billion by 2027, driven by increasing demand in pharmaceuticals, laboratories, and electronics. Technologically, borosilicate glass production is well-developed, with companies like SCHOTT AG, Corning, Inc., and AGC, Inc. leading innovation. These firms, along with others such as Nippon Electric Glass and Hunan Kibing, are continuously improving properties like thermal resistance, chemical durability, and optical clarity. The competitive landscape is characterized by a mix of large multinational corporations and specialized regional manufacturers, with ongoing research focused on enhancing performance and expanding applications in emerging sectors.
SCHOTT AG
Technical Solution: SCHOTT AG has developed advanced borosilicate glass compositions with optimized boron content to enhance specific properties. Their BOROFLOAT® glass, for instance, contains approximately 10-13% boron oxide, which contributes to its excellent thermal shock resistance and low coefficient of thermal expansion[1]. SCHOTT's research has shown that increasing boron content can improve chemical durability and reduce alkali ion release, making their glasses suitable for pharmaceutical packaging[2]. They have also explored the role of boron in radiation shielding glasses, where boron's neutron absorption properties are utilized[3].
Strengths: Excellent thermal and chemical properties, versatile applications from laboratory to pharmaceutical use. Weaknesses: Higher production costs compared to soda-lime glass, potential for boron volatilization during melting process.
Corning, Inc.
Technical Solution: Corning has pioneered the development of borosilicate glasses, with their Pyrex® brand being synonymous with borosilicate glassware. Their research focuses on tailoring boron content to achieve specific material properties. For instance, they have developed low-expansion borosilicate glasses with boron oxide content ranging from 7-13% for applications in astronomy and space exploration[4]. Corning's scientists have also investigated the role of boron in improving the glass network structure, which enhances mechanical strength and chemical durability[5]. Their recent innovations include borosilicate glasses with improved infrared transmission for optical applications[6].
Strengths: Long-standing expertise in borosilicate glass, wide range of specialized compositions for various industries. Weaknesses: High-end products may be more expensive, some compositions may require specialized manufacturing processes.
Boron-Silicon Interaction Mechanisms
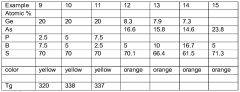
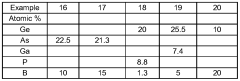
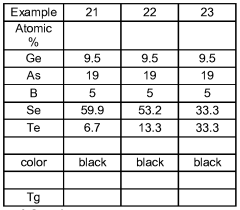
Chalcogenide glass
PatentWO2013006392A1
Innovation
- The glassforming region is expanded in Ge-B-S, Se; Ge-P-B-S, Se; As-B-S, Se; Ge-As-B-S, Se; and Ge-Ga-B-S, Se systems by using a method involving a precursor glass or crystalline material combined with elemental boron, melted in a carbon vessel to minimize contamination and enhance boron coordination, resulting in improved thermal stability and chemical durability.
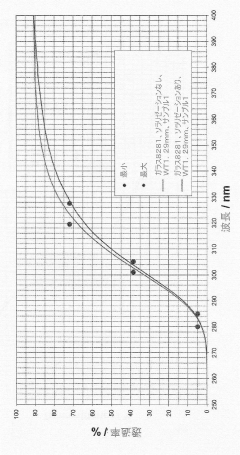
Solarization-resistant borosilicate glass, use thereof in production of glass and tube, and use thereof in irradiation unit
PatentActiveJP2014114204A
Innovation
- A borosilicate glass composition with specific proportions of SiO2, B2O3, Al2O3, Na2O, K2O, TiO2, MoO3, and V2O5, along with controlled alkali and alkaline earth metal oxides, achieves a balanced UV edge and high visible/near-infrared transmission, enhancing solarization resistance.
Environmental Impact of Borosilicate Production
The production of borosilicate glass, while offering numerous benefits in terms of durability and heat resistance, does have environmental implications that warrant careful consideration. The manufacturing process involves high-temperature melting of raw materials, including silica, boron oxide, and other additives, which requires significant energy consumption. This energy-intensive production contributes to greenhouse gas emissions, particularly when fossil fuels are used as the primary energy source.
The extraction of boron, a key component in borosilicate glass, also poses environmental challenges. Boron mining operations can lead to soil erosion, habitat disruption, and potential contamination of groundwater resources. Additionally, the processing of boron ore generates waste materials that require proper management to prevent environmental pollution.
Water usage in borosilicate glass production is another area of environmental concern. The manufacturing process requires substantial amounts of water for cooling and cleaning purposes, potentially straining local water resources in areas where production facilities are located. Proper water management and recycling systems are crucial to mitigate this impact.
The use of chemicals in the production process, including various fluxes and fining agents, may result in the generation of hazardous waste. Proper handling, treatment, and disposal of these waste materials are essential to prevent soil and water contamination. Implementing advanced waste management techniques and exploring opportunities for waste reduction and recycling can help minimize the environmental footprint of borosilicate production.
On a positive note, the durability and recyclability of borosilicate glass contribute to its overall environmental sustainability. The long lifespan of borosilicate products reduces the need for frequent replacements, thereby conserving resources in the long term. Furthermore, borosilicate glass can be recycled, although the process may require specialized facilities due to its unique composition.
Efforts to improve the environmental impact of borosilicate production are ongoing. These include the development of more energy-efficient melting technologies, the use of renewable energy sources in manufacturing, and the implementation of closed-loop systems for water and material recycling. Additionally, research into alternative raw materials and production methods that reduce the reliance on boron mining could further enhance the environmental profile of borosilicate glass production.
The extraction of boron, a key component in borosilicate glass, also poses environmental challenges. Boron mining operations can lead to soil erosion, habitat disruption, and potential contamination of groundwater resources. Additionally, the processing of boron ore generates waste materials that require proper management to prevent environmental pollution.
Water usage in borosilicate glass production is another area of environmental concern. The manufacturing process requires substantial amounts of water for cooling and cleaning purposes, potentially straining local water resources in areas where production facilities are located. Proper water management and recycling systems are crucial to mitigate this impact.
The use of chemicals in the production process, including various fluxes and fining agents, may result in the generation of hazardous waste. Proper handling, treatment, and disposal of these waste materials are essential to prevent soil and water contamination. Implementing advanced waste management techniques and exploring opportunities for waste reduction and recycling can help minimize the environmental footprint of borosilicate production.
On a positive note, the durability and recyclability of borosilicate glass contribute to its overall environmental sustainability. The long lifespan of borosilicate products reduces the need for frequent replacements, thereby conserving resources in the long term. Furthermore, borosilicate glass can be recycled, although the process may require specialized facilities due to its unique composition.
Efforts to improve the environmental impact of borosilicate production are ongoing. These include the development of more energy-efficient melting technologies, the use of renewable energy sources in manufacturing, and the implementation of closed-loop systems for water and material recycling. Additionally, research into alternative raw materials and production methods that reduce the reliance on boron mining could further enhance the environmental profile of borosilicate glass production.
Borosilicate Glass Safety Standards
Borosilicate glass safety standards have evolved significantly over the years to ensure the protection of consumers and workers in various industries. These standards encompass a wide range of requirements, including chemical composition, thermal shock resistance, mechanical strength, and durability. The American Society for Testing and Materials (ASTM) and the International Organization for Standardization (ISO) have developed comprehensive guidelines for borosilicate glass safety.
One of the primary safety standards for borosilicate glass is ASTM C1036, which specifies the quality requirements for flat glass used in buildings. This standard outlines the acceptable levels of imperfections, such as bubbles, stones, and scratches, as well as the minimum thickness requirements for different applications. Additionally, ASTM C1048 provides specifications for heat-strengthened and fully tempered flat glass, which are crucial for enhancing the safety of borosilicate glass products.
The ISO 3585 standard specifically addresses the properties of borosilicate glass, including its chemical composition, physical characteristics, and thermal properties. This standard ensures that borosilicate glass meets the necessary requirements for laboratory and industrial applications, where resistance to thermal shock and chemical corrosion is essential.
For laboratory glassware, ASTM E438 sets the standards for glass and glass products used in laboratory apparatus. This standard covers the chemical durability, thermal shock resistance, and dimensional tolerances of borosilicate glass used in scientific research and testing environments. Similarly, ISO 1773 provides specifications for laboratory glassware made from borosilicate glass, ensuring consistency and reliability in scientific experiments.
In the pharmaceutical industry, borosilicate glass safety standards are particularly stringent. The United States Pharmacopeia (USP) and the European Pharmacopoeia (Ph. Eur.) have established guidelines for glass containers used in drug packaging. These standards, such as USP <660> and Ph. Eur. 3.2.1, outline the requirements for chemical resistance, hydrolytic resistance, and thermal shock resistance of borosilicate glass containers.
The food and beverage industry also relies on specific safety standards for borosilicate glass. The U.S. Food and Drug Administration (FDA) has established regulations under 21 CFR 177.1520 for glass and ceramic food contact surfaces, which include borosilicate glass products. These regulations ensure that the glass does not leach harmful substances into food or beverages during storage or preparation.
Occupational safety standards for workers handling borosilicate glass are outlined in various regulations, such as those set by the Occupational Safety and Health Administration (OSHA) in the United States. These standards address proper handling techniques, personal protective equipment requirements, and safe disposal methods for broken glass.
As technology and manufacturing processes continue to advance, borosilicate glass safety standards are regularly reviewed and updated to address new challenges and applications. This ongoing process ensures that borosilicate glass products maintain their reputation for safety and reliability across diverse industries and applications.
One of the primary safety standards for borosilicate glass is ASTM C1036, which specifies the quality requirements for flat glass used in buildings. This standard outlines the acceptable levels of imperfections, such as bubbles, stones, and scratches, as well as the minimum thickness requirements for different applications. Additionally, ASTM C1048 provides specifications for heat-strengthened and fully tempered flat glass, which are crucial for enhancing the safety of borosilicate glass products.
The ISO 3585 standard specifically addresses the properties of borosilicate glass, including its chemical composition, physical characteristics, and thermal properties. This standard ensures that borosilicate glass meets the necessary requirements for laboratory and industrial applications, where resistance to thermal shock and chemical corrosion is essential.
For laboratory glassware, ASTM E438 sets the standards for glass and glass products used in laboratory apparatus. This standard covers the chemical durability, thermal shock resistance, and dimensional tolerances of borosilicate glass used in scientific research and testing environments. Similarly, ISO 1773 provides specifications for laboratory glassware made from borosilicate glass, ensuring consistency and reliability in scientific experiments.
In the pharmaceutical industry, borosilicate glass safety standards are particularly stringent. The United States Pharmacopeia (USP) and the European Pharmacopoeia (Ph. Eur.) have established guidelines for glass containers used in drug packaging. These standards, such as USP <660> and Ph. Eur. 3.2.1, outline the requirements for chemical resistance, hydrolytic resistance, and thermal shock resistance of borosilicate glass containers.
The food and beverage industry also relies on specific safety standards for borosilicate glass. The U.S. Food and Drug Administration (FDA) has established regulations under 21 CFR 177.1520 for glass and ceramic food contact surfaces, which include borosilicate glass products. These regulations ensure that the glass does not leach harmful substances into food or beverages during storage or preparation.
Occupational safety standards for workers handling borosilicate glass are outlined in various regulations, such as those set by the Occupational Safety and Health Administration (OSHA) in the United States. These standards address proper handling techniques, personal protective equipment requirements, and safe disposal methods for broken glass.
As technology and manufacturing processes continue to advance, borosilicate glass safety standards are regularly reviewed and updated to address new challenges and applications. This ongoing process ensures that borosilicate glass products maintain their reputation for safety and reliability across diverse industries and applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!