The Function of Carbolic Acid in Electrochemical Sensor Technologies
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbolic Acid in Sensors: Background and Objectives
Carbolic acid, also known as phenol, has emerged as a crucial component in the development of electrochemical sensor technologies. The evolution of this field can be traced back to the early 20th century when phenol's unique properties were first recognized in analytical chemistry. Over the decades, researchers have continuously explored and expanded the applications of carbolic acid in sensing mechanisms, leading to significant advancements in various industries.
The primary objective of incorporating carbolic acid in electrochemical sensors is to enhance sensitivity, selectivity, and stability. These sensors play a vital role in detecting and quantifying specific analytes in complex matrices, making them indispensable in environmental monitoring, healthcare diagnostics, food safety, and industrial process control. The integration of carbolic acid has revolutionized the way we approach these challenges, offering improved performance and reliability.
As the field progresses, researchers aim to overcome existing limitations and push the boundaries of sensor capabilities. Key goals include developing sensors with lower detection limits, broader dynamic ranges, and enhanced resistance to interfering substances. Additionally, there is a growing focus on creating miniaturized, portable, and cost-effective sensor platforms that can provide rapid and accurate results in real-time.
The technological trajectory of carbolic acid in electrochemical sensors is closely aligned with broader trends in materials science, nanotechnology, and data analytics. Innovations in these areas are expected to drive further advancements in sensor design and functionality. For instance, the integration of nanomaterials and smart polymers with carbolic acid-based sensing elements shows promise for creating next-generation sensors with unprecedented performance characteristics.
Looking ahead, the field is poised for significant growth and innovation. Researchers are exploring novel approaches to harness the unique properties of carbolic acid, such as its ability to form self-assembled monolayers and its electrochemical behavior under various conditions. These efforts are expected to yield sensors with improved specificity, faster response times, and greater durability, addressing the evolving needs of diverse applications across multiple sectors.
In conclusion, the background and objectives of carbolic acid in electrochemical sensor technologies reflect a dynamic and rapidly evolving field. From its historical roots to its current applications and future potential, carbolic acid continues to play a pivotal role in shaping the landscape of sensor technology. As research progresses, we can anticipate groundbreaking developments that will further expand the capabilities and applications of these sensors, ultimately contributing to advancements in various scientific and industrial domains.
The primary objective of incorporating carbolic acid in electrochemical sensors is to enhance sensitivity, selectivity, and stability. These sensors play a vital role in detecting and quantifying specific analytes in complex matrices, making them indispensable in environmental monitoring, healthcare diagnostics, food safety, and industrial process control. The integration of carbolic acid has revolutionized the way we approach these challenges, offering improved performance and reliability.
As the field progresses, researchers aim to overcome existing limitations and push the boundaries of sensor capabilities. Key goals include developing sensors with lower detection limits, broader dynamic ranges, and enhanced resistance to interfering substances. Additionally, there is a growing focus on creating miniaturized, portable, and cost-effective sensor platforms that can provide rapid and accurate results in real-time.
The technological trajectory of carbolic acid in electrochemical sensors is closely aligned with broader trends in materials science, nanotechnology, and data analytics. Innovations in these areas are expected to drive further advancements in sensor design and functionality. For instance, the integration of nanomaterials and smart polymers with carbolic acid-based sensing elements shows promise for creating next-generation sensors with unprecedented performance characteristics.
Looking ahead, the field is poised for significant growth and innovation. Researchers are exploring novel approaches to harness the unique properties of carbolic acid, such as its ability to form self-assembled monolayers and its electrochemical behavior under various conditions. These efforts are expected to yield sensors with improved specificity, faster response times, and greater durability, addressing the evolving needs of diverse applications across multiple sectors.
In conclusion, the background and objectives of carbolic acid in electrochemical sensor technologies reflect a dynamic and rapidly evolving field. From its historical roots to its current applications and future potential, carbolic acid continues to play a pivotal role in shaping the landscape of sensor technology. As research progresses, we can anticipate groundbreaking developments that will further expand the capabilities and applications of these sensors, ultimately contributing to advancements in various scientific and industrial domains.
Market Analysis for Carbolic Acid-Based Sensors
The market for carbolic acid-based electrochemical sensors is experiencing significant growth, driven by increasing demand across various industries. These sensors, which utilize carbolic acid (phenol) as a key component, offer high sensitivity and selectivity in detecting a wide range of analytes. The healthcare sector represents a major market segment, with applications in diagnostic devices, point-of-care testing, and continuous health monitoring systems. The environmental monitoring industry also shows strong demand, as these sensors are effective in detecting pollutants and toxic substances in water and air.
In the industrial sector, carbolic acid-based sensors find applications in quality control processes, particularly in the food and beverage industry for detecting contaminants and ensuring product safety. The pharmaceutical industry utilizes these sensors in drug discovery and development processes, contributing to market expansion. Additionally, the agriculture sector is adopting these sensors for soil analysis and crop health monitoring, further diversifying the market.
The global market for electrochemical sensors, including those based on carbolic acid, is projected to grow steadily over the next five years. This growth is attributed to technological advancements, increasing awareness of environmental and health issues, and stringent regulations across industries. Emerging economies in Asia-Pacific and Latin America are expected to present significant growth opportunities due to rapid industrialization and increasing investments in healthcare infrastructure.
However, the market faces challenges such as the high cost of advanced sensor technologies and the need for skilled personnel to operate and interpret sensor data. Competition from alternative sensing technologies, such as optical and piezoelectric sensors, also impacts market dynamics. Despite these challenges, ongoing research and development efforts are focused on improving sensor performance, reducing costs, and expanding application areas.
Key market trends include the miniaturization of sensors for portable and wearable devices, integration with IoT and AI technologies for real-time data analysis, and the development of multi-analyte sensing platforms. The shift towards personalized medicine and the growing emphasis on preventive healthcare are expected to drive demand for carbolic acid-based sensors in the medical diagnostics sector.
In conclusion, the market for carbolic acid-based electrochemical sensors shows promising growth potential across multiple industries. The combination of technological advancements, expanding application areas, and increasing regulatory requirements is likely to sustain market growth in the coming years. Companies investing in research and development to address current limitations and explore new applications are well-positioned to capitalize on this growing market opportunity.
In the industrial sector, carbolic acid-based sensors find applications in quality control processes, particularly in the food and beverage industry for detecting contaminants and ensuring product safety. The pharmaceutical industry utilizes these sensors in drug discovery and development processes, contributing to market expansion. Additionally, the agriculture sector is adopting these sensors for soil analysis and crop health monitoring, further diversifying the market.
The global market for electrochemical sensors, including those based on carbolic acid, is projected to grow steadily over the next five years. This growth is attributed to technological advancements, increasing awareness of environmental and health issues, and stringent regulations across industries. Emerging economies in Asia-Pacific and Latin America are expected to present significant growth opportunities due to rapid industrialization and increasing investments in healthcare infrastructure.
However, the market faces challenges such as the high cost of advanced sensor technologies and the need for skilled personnel to operate and interpret sensor data. Competition from alternative sensing technologies, such as optical and piezoelectric sensors, also impacts market dynamics. Despite these challenges, ongoing research and development efforts are focused on improving sensor performance, reducing costs, and expanding application areas.
Key market trends include the miniaturization of sensors for portable and wearable devices, integration with IoT and AI technologies for real-time data analysis, and the development of multi-analyte sensing platforms. The shift towards personalized medicine and the growing emphasis on preventive healthcare are expected to drive demand for carbolic acid-based sensors in the medical diagnostics sector.
In conclusion, the market for carbolic acid-based electrochemical sensors shows promising growth potential across multiple industries. The combination of technological advancements, expanding application areas, and increasing regulatory requirements is likely to sustain market growth in the coming years. Companies investing in research and development to address current limitations and explore new applications are well-positioned to capitalize on this growing market opportunity.
Current Challenges in Electrochemical Sensor Technology
Electrochemical sensor technology has made significant strides in recent years, yet several challenges persist that hinder its widespread adoption and optimal performance. One of the primary obstacles is the issue of selectivity. Many sensors struggle to differentiate between similar analytes, leading to false positives or inaccurate readings. This is particularly problematic in complex biological samples where multiple interfering substances may be present.
Sensitivity is another area of concern, especially for detecting trace amounts of analytes in environmental or clinical samples. While some sensors have achieved impressive detection limits, there is a constant push for even lower thresholds to meet the demands of emerging applications in fields such as early disease diagnosis and environmental monitoring.
Stability and longevity of electrochemical sensors remain significant challenges. Many sensors suffer from drift over time, requiring frequent recalibration or replacement. This is particularly problematic for implantable or long-term monitoring devices. The degradation of electrode materials and fouling of sensor surfaces by sample components can lead to decreased sensitivity and altered response characteristics.
Miniaturization and integration pose additional hurdles. As the demand for portable, wearable, and implantable sensors grows, researchers face the challenge of maintaining sensor performance while reducing size and power consumption. This often involves complex trade-offs between sensitivity, selectivity, and device dimensions.
The development of reliable, reproducible manufacturing processes for electrochemical sensors is another significant challenge. Batch-to-batch variations can lead to inconsistent sensor performance, making quality control and standardization difficult. This is particularly crucial for mass-produced sensors intended for point-of-care diagnostics or widespread environmental monitoring.
Biocompatibility is a critical concern for sensors designed for in vivo applications. Ensuring that sensor materials do not provoke adverse biological responses while maintaining functionality in the complex in vivo environment is an ongoing challenge. This includes addressing issues such as protein adsorption, which can interfere with sensor performance.
Finally, the integration of electrochemical sensors with data processing and communication systems presents both technical and regulatory challenges. Ensuring data security, developing robust algorithms for signal processing, and meeting regulatory requirements for medical devices are all areas that require ongoing research and development efforts.
Sensitivity is another area of concern, especially for detecting trace amounts of analytes in environmental or clinical samples. While some sensors have achieved impressive detection limits, there is a constant push for even lower thresholds to meet the demands of emerging applications in fields such as early disease diagnosis and environmental monitoring.
Stability and longevity of electrochemical sensors remain significant challenges. Many sensors suffer from drift over time, requiring frequent recalibration or replacement. This is particularly problematic for implantable or long-term monitoring devices. The degradation of electrode materials and fouling of sensor surfaces by sample components can lead to decreased sensitivity and altered response characteristics.
Miniaturization and integration pose additional hurdles. As the demand for portable, wearable, and implantable sensors grows, researchers face the challenge of maintaining sensor performance while reducing size and power consumption. This often involves complex trade-offs between sensitivity, selectivity, and device dimensions.
The development of reliable, reproducible manufacturing processes for electrochemical sensors is another significant challenge. Batch-to-batch variations can lead to inconsistent sensor performance, making quality control and standardization difficult. This is particularly crucial for mass-produced sensors intended for point-of-care diagnostics or widespread environmental monitoring.
Biocompatibility is a critical concern for sensors designed for in vivo applications. Ensuring that sensor materials do not provoke adverse biological responses while maintaining functionality in the complex in vivo environment is an ongoing challenge. This includes addressing issues such as protein adsorption, which can interfere with sensor performance.
Finally, the integration of electrochemical sensors with data processing and communication systems presents both technical and regulatory challenges. Ensuring data security, developing robust algorithms for signal processing, and meeting regulatory requirements for medical devices are all areas that require ongoing research and development efforts.
Existing Carbolic Acid Sensor Solutions
01 Historical use of carbolic acid in medical applications
Carbolic acid, also known as phenol, has a long history of use in medical and pharmaceutical applications. It was widely used as an antiseptic and disinfectant in the late 19th and early 20th centuries. Its properties made it valuable for wound treatment and surgical procedures, although its use has since been largely replaced by safer alternatives.- Historical use of carbolic acid in medical applications: Carbolic acid, also known as phenol, has been historically used in various medical applications. It was one of the earliest antiseptics used in surgery and wound care due to its ability to kill bacteria and other microorganisms. This compound played a significant role in the development of modern antiseptic techniques in the late 19th and early 20th centuries.
- Carbolic acid in industrial and chemical processes: Carbolic acid is widely used in industrial and chemical processes. It serves as a precursor for many organic compounds and is utilized in the production of plastics, pharmaceuticals, and other chemical products. Its versatile properties make it an important raw material in various manufacturing sectors.
- Environmental and safety considerations of carbolic acid: Due to its toxic nature, the use and handling of carbolic acid require strict safety measures. Environmental concerns have led to the development of treatment and disposal methods for carbolic acid waste. Modern applications focus on minimizing exposure risks and environmental impact while harnessing its beneficial properties.
- Carbolic acid derivatives and their applications: Various derivatives of carbolic acid have been developed for specific applications. These compounds often retain some of the beneficial properties of carbolic acid while mitigating its toxicity or enhancing certain characteristics. Such derivatives find use in fields like pharmaceuticals, cosmetics, and materials science.
- Modern alternatives and substitutes for carbolic acid: Given the health and environmental concerns associated with carbolic acid, research has focused on developing safer alternatives and substitutes. These modern compounds aim to provide similar antimicrobial or chemical properties while offering improved safety profiles and reduced environmental impact.
02 Carbolic acid in modern industrial applications
In contemporary industrial settings, carbolic acid finds use in various applications. It serves as a precursor in the production of plastics, resins, and other synthetic materials. Its disinfectant properties are still utilized in certain industrial cleaning processes, albeit with strict safety measures due to its corrosive nature.Expand Specific Solutions03 Safety measures and handling of carbolic acid
Due to the hazardous nature of carbolic acid, specialized equipment and safety measures are necessary for its handling and storage. This includes the use of protective gear, proper ventilation systems, and specific containment methods to prevent exposure and environmental contamination.Expand Specific Solutions04 Carbolic acid in water treatment and purification
Carbolic acid and its derivatives have applications in water treatment and purification processes. They can be used in small quantities as part of disinfection systems, particularly in industrial wastewater treatment. However, their use is carefully regulated due to potential environmental impacts.Expand Specific Solutions05 Research and development of carbolic acid derivatives
Ongoing research focuses on developing new derivatives and applications of carbolic acid. This includes exploring its potential in advanced materials, pharmaceuticals, and chemical synthesis. Scientists are working on mitigating its toxic effects while harnessing its beneficial properties for various industrial and medical applications.Expand Specific Solutions
Key Players in Electrochemical Sensor Industry
The electrochemical sensor technology for carbolic acid detection is in a growth phase, with increasing market demand driven by applications in environmental monitoring, healthcare, and industrial processes. The global market size for electrochemical sensors is projected to expand significantly in the coming years. While the technology is relatively mature, ongoing research focuses on improving sensitivity, selectivity, and miniaturization. Key players like Abbott Point of Care, Drägerwerk, and 3M Innovative Properties are advancing the field through innovative sensor designs and materials. Universities such as Cambridge, Bath, and South China University of Technology are contributing to fundamental research, while companies like Avantium and Dioxide Materials are developing novel electrode materials and sensing platforms to enhance performance and expand applications.
Abbott Point of Care, Inc.
Technical Solution: Abbott Point of Care has developed advanced electrochemical sensor technologies incorporating carbolic acid for point-of-care diagnostics. Their approach utilizes carbolic acid as a key component in the electrode modification process, enhancing the sensor's selectivity and sensitivity. The company's proprietary method involves the electropolymerization of carbolic acid derivatives on electrode surfaces, creating a stable and conductive film that facilitates electron transfer and improves analyte detection[1]. This technique has been particularly effective in developing glucose sensors with extended lifetimes and reduced interference from other blood components[2]. Abbott's sensors also incorporate nanomaterials functionalized with carbolic acid groups, further improving the electrochemical performance and expanding the range of detectable biomarkers[3].
Strengths: High sensitivity and selectivity, extended sensor lifetime, reduced interference. Weaknesses: Potentially higher production costs, complexity in large-scale manufacturing.
Cambridge Enterprise Ltd.
Technical Solution: Cambridge Enterprise, representing the University of Cambridge's research commercialization efforts, has pioneered innovative uses of carbolic acid in electrochemical sensors. Their approach focuses on the development of novel electrode materials incorporating carbolic acid derivatives for enhanced sensing capabilities. The research team has successfully synthesized graphene-carbolic acid composites that exhibit exceptional electrochemical properties, including increased electron transfer rates and improved analyte adsorption[4]. These materials have been applied in the detection of various environmental pollutants and biomarkers with remarkable sensitivity. Additionally, Cambridge Enterprise has developed a unique surface modification technique using carbolic acid to create highly stable and reproducible sensor platforms, addressing common issues in long-term sensor performance[5].
Strengths: Cutting-edge materials science, high sensitivity for diverse analytes. Weaknesses: Early-stage technology, potential scalability challenges.
Core Innovations in Carbolic Acid Sensing
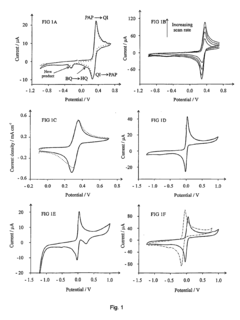
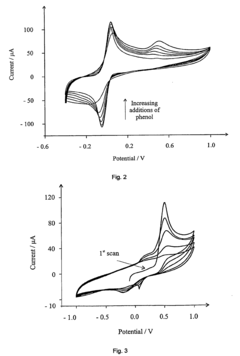
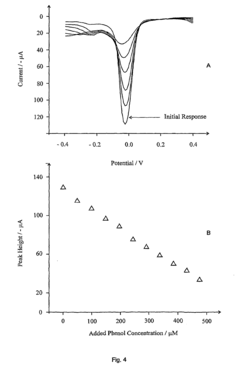
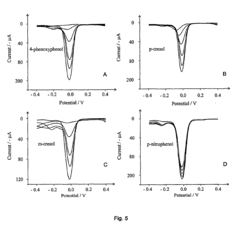
Detection of phenols
PatentActiveEP1891423B1
Innovation
- The method involves electrochemically oxidizing p-aminophenol to form a benzoquinone monoamine, which reacts with phenols indirectly, avoiding direct oxidation and electrode passivation, using a redox reaction with a detectable redox couple to determine phenol presence and concentration.
Detection of phenols
PatentWO2006134386A1
Innovation
- The method involves electrochemically oxidizing p-aminophenol to form a benzoquinone monoamine, which reacts with phenols, allowing for indirect detection through monitoring the reduction of the oxidized p-aminophenol, thereby avoiding direct oxidation of phenols and reducing electrode passivation.
Environmental Impact of Carbolic Acid in Sensors
The use of carbolic acid, also known as phenol, in electrochemical sensor technologies has raised significant environmental concerns. As these sensors become more prevalent in various applications, the potential environmental impact of carbolic acid requires careful consideration.
Carbolic acid, when released into the environment, can have detrimental effects on ecosystems and human health. In aquatic environments, even low concentrations of phenol can be toxic to fish and other aquatic organisms. It can disrupt the natural balance of ecosystems by affecting the growth and reproduction of various species. The bioaccumulation of phenol in the food chain poses risks to higher-level organisms, including humans.
Soil contamination is another critical issue associated with the use of carbolic acid in sensors. When sensors are improperly disposed of or when leakage occurs, phenol can seep into the soil, affecting soil microorganisms and plant life. This contamination can persist for extended periods, leading to long-term environmental degradation.
Air pollution is also a concern, particularly during the manufacturing process of sensors containing carbolic acid. Volatile organic compounds (VOCs) released during production can contribute to smog formation and have adverse effects on air quality. Workers in manufacturing facilities may be at risk of exposure to these harmful emissions.
The disposal of sensors containing carbolic acid presents additional environmental challenges. Improper disposal can lead to the release of phenol into landfills or water systems, potentially contaminating groundwater sources. This highlights the need for proper waste management protocols and recycling initiatives specific to electrochemical sensors.
To mitigate these environmental risks, several approaches are being explored. Research is ongoing to develop alternative, more environmentally friendly compounds that can replace carbolic acid in sensor technologies without compromising performance. Additionally, improved manufacturing processes and stricter regulations are being implemented to reduce emissions and prevent environmental contamination during production and disposal.
Efforts are also being made to enhance the durability and lifespan of sensors, reducing the frequency of replacement and, consequently, the overall environmental impact. Furthermore, the development of biodegradable sensor components is an area of active research, aiming to minimize the long-term environmental footprint of these devices.
In conclusion, while carbolic acid plays a crucial role in electrochemical sensor technologies, its environmental impact cannot be overlooked. Balancing technological advancement with environmental stewardship remains a key challenge in this field, necessitating ongoing research and innovation to develop more sustainable sensor solutions.
Carbolic acid, when released into the environment, can have detrimental effects on ecosystems and human health. In aquatic environments, even low concentrations of phenol can be toxic to fish and other aquatic organisms. It can disrupt the natural balance of ecosystems by affecting the growth and reproduction of various species. The bioaccumulation of phenol in the food chain poses risks to higher-level organisms, including humans.
Soil contamination is another critical issue associated with the use of carbolic acid in sensors. When sensors are improperly disposed of or when leakage occurs, phenol can seep into the soil, affecting soil microorganisms and plant life. This contamination can persist for extended periods, leading to long-term environmental degradation.
Air pollution is also a concern, particularly during the manufacturing process of sensors containing carbolic acid. Volatile organic compounds (VOCs) released during production can contribute to smog formation and have adverse effects on air quality. Workers in manufacturing facilities may be at risk of exposure to these harmful emissions.
The disposal of sensors containing carbolic acid presents additional environmental challenges. Improper disposal can lead to the release of phenol into landfills or water systems, potentially contaminating groundwater sources. This highlights the need for proper waste management protocols and recycling initiatives specific to electrochemical sensors.
To mitigate these environmental risks, several approaches are being explored. Research is ongoing to develop alternative, more environmentally friendly compounds that can replace carbolic acid in sensor technologies without compromising performance. Additionally, improved manufacturing processes and stricter regulations are being implemented to reduce emissions and prevent environmental contamination during production and disposal.
Efforts are also being made to enhance the durability and lifespan of sensors, reducing the frequency of replacement and, consequently, the overall environmental impact. Furthermore, the development of biodegradable sensor components is an area of active research, aiming to minimize the long-term environmental footprint of these devices.
In conclusion, while carbolic acid plays a crucial role in electrochemical sensor technologies, its environmental impact cannot be overlooked. Balancing technological advancement with environmental stewardship remains a key challenge in this field, necessitating ongoing research and innovation to develop more sustainable sensor solutions.
Regulatory Framework for Chemical Sensors
The regulatory framework for chemical sensors, particularly those utilizing carbolic acid in electrochemical sensor technologies, is a complex and evolving landscape. Governments and international organizations have established various guidelines and standards to ensure the safety, reliability, and ethical use of these sensors across different industries.
In the United States, the Environmental Protection Agency (EPA) plays a crucial role in regulating chemical sensors used for environmental monitoring. The EPA's regulations focus on the accuracy and precision of sensors detecting hazardous substances, including those that may utilize carbolic acid as a component. The agency has set forth specific performance criteria and calibration requirements for sensors used in air and water quality monitoring.
The Food and Drug Administration (FDA) oversees the regulation of chemical sensors in medical applications. For electrochemical sensors incorporating carbolic acid that are used in medical devices or diagnostic tools, manufacturers must comply with the FDA's premarket approval process. This includes demonstrating the safety and efficacy of the sensor technology through rigorous clinical trials and risk assessments.
On the international stage, the International Organization for Standardization (ISO) has developed several standards relevant to chemical sensors. ISO 17025, for instance, provides general requirements for the competence of testing and calibration laboratories, which applies to facilities developing and testing electrochemical sensors. Additionally, ISO 15197 sets specific requirements for blood glucose monitoring systems, which may employ similar electrochemical sensing principles.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which impacts the use of carbolic acid and other chemicals in sensor technologies. Manufacturers must ensure that their sensors comply with REACH requirements, particularly if the sensors are intended for use in environmental monitoring or consumer products within the EU market.
Occupational safety regulations also play a significant role in the deployment of chemical sensors in workplace environments. In the United States, the Occupational Safety and Health Administration (OSHA) has established permissible exposure limits for various chemicals, including phenol (carbolic acid). Sensors used to monitor workplace air quality must meet OSHA's standards for accuracy and reliability.
As the field of electrochemical sensor technologies continues to advance, regulatory bodies are adapting their frameworks to address emerging challenges. This includes considerations for nanotechnology-based sensors and the integration of artificial intelligence in sensor data analysis. Future regulations are likely to focus on data privacy and security aspects of networked sensor systems, as well as the environmental impact of sensor production and disposal.
In the United States, the Environmental Protection Agency (EPA) plays a crucial role in regulating chemical sensors used for environmental monitoring. The EPA's regulations focus on the accuracy and precision of sensors detecting hazardous substances, including those that may utilize carbolic acid as a component. The agency has set forth specific performance criteria and calibration requirements for sensors used in air and water quality monitoring.
The Food and Drug Administration (FDA) oversees the regulation of chemical sensors in medical applications. For electrochemical sensors incorporating carbolic acid that are used in medical devices or diagnostic tools, manufacturers must comply with the FDA's premarket approval process. This includes demonstrating the safety and efficacy of the sensor technology through rigorous clinical trials and risk assessments.
On the international stage, the International Organization for Standardization (ISO) has developed several standards relevant to chemical sensors. ISO 17025, for instance, provides general requirements for the competence of testing and calibration laboratories, which applies to facilities developing and testing electrochemical sensors. Additionally, ISO 15197 sets specific requirements for blood glucose monitoring systems, which may employ similar electrochemical sensing principles.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which impacts the use of carbolic acid and other chemicals in sensor technologies. Manufacturers must ensure that their sensors comply with REACH requirements, particularly if the sensors are intended for use in environmental monitoring or consumer products within the EU market.
Occupational safety regulations also play a significant role in the deployment of chemical sensors in workplace environments. In the United States, the Occupational Safety and Health Administration (OSHA) has established permissible exposure limits for various chemicals, including phenol (carbolic acid). Sensors used to monitor workplace air quality must meet OSHA's standards for accuracy and reliability.
As the field of electrochemical sensor technologies continues to advance, regulatory bodies are adapting their frameworks to address emerging challenges. This includes considerations for nanotechnology-based sensors and the integration of artificial intelligence in sensor data analysis. Future regulations are likely to focus on data privacy and security aspects of networked sensor systems, as well as the environmental impact of sensor production and disposal.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!