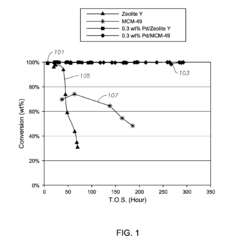
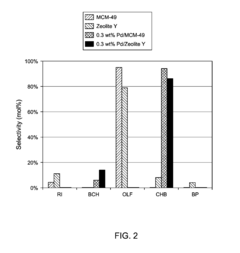
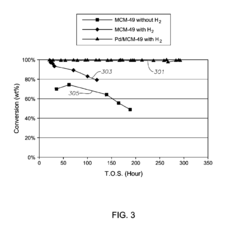
The Impact of Carbolic Acid on Chemical Manufacturing Efficiency
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbolic Acid Evolution
Carbolic acid, also known as phenol, has undergone significant evolution since its discovery in the early 19th century. Initially isolated from coal tar in 1834 by German chemist Friedlieb Ferdinand Runge, carbolic acid quickly gained prominence due to its antiseptic properties. This discovery marked the beginning of a transformative journey for this compound in chemical manufacturing.
The evolution of carbolic acid can be traced through several key stages. In the mid-19th century, it found widespread use as a disinfectant, particularly in medical settings. Joseph Lister's pioneering work in antiseptic surgery using carbolic acid solutions revolutionized medical practices and significantly reduced post-operative infections.
As industrial processes advanced, the production methods for carbolic acid evolved. The early 20th century saw the development of the cumene process, which became the dominant method for large-scale phenol production. This process, invented by Heinrich Hock and Sergei Lang in 1944, utilized benzene and propylene as starting materials, significantly improving manufacturing efficiency and yield.
The 1950s and 1960s witnessed further refinements in carbolic acid production. The introduction of zeolite catalysts in the cumene process enhanced selectivity and reduced energy consumption. This period also saw the emergence of alternative production routes, such as the Raschig process, which used chlorobenzene as a starting material.
In recent decades, the focus has shifted towards more sustainable and environmentally friendly production methods. Green chemistry initiatives have led to the exploration of bio-based routes for phenol production, utilizing renewable resources such as lignin from biomass. These developments aim to reduce the carbon footprint of carbolic acid manufacturing while maintaining or improving efficiency.
The digital era has brought about significant advancements in process control and optimization for carbolic acid production. The integration of artificial intelligence and machine learning algorithms has enabled real-time monitoring and adjustment of manufacturing parameters, leading to improved yield and quality consistency.
Today, carbolic acid continues to be a crucial component in various industries, including pharmaceuticals, plastics, and agrochemicals. Its evolution has been marked by continuous improvements in production efficiency, safety, and environmental sustainability. As we look to the future, ongoing research focuses on developing even more efficient and eco-friendly production methods, ensuring that carbolic acid remains a vital player in chemical manufacturing for years to come.
The evolution of carbolic acid can be traced through several key stages. In the mid-19th century, it found widespread use as a disinfectant, particularly in medical settings. Joseph Lister's pioneering work in antiseptic surgery using carbolic acid solutions revolutionized medical practices and significantly reduced post-operative infections.
As industrial processes advanced, the production methods for carbolic acid evolved. The early 20th century saw the development of the cumene process, which became the dominant method for large-scale phenol production. This process, invented by Heinrich Hock and Sergei Lang in 1944, utilized benzene and propylene as starting materials, significantly improving manufacturing efficiency and yield.
The 1950s and 1960s witnessed further refinements in carbolic acid production. The introduction of zeolite catalysts in the cumene process enhanced selectivity and reduced energy consumption. This period also saw the emergence of alternative production routes, such as the Raschig process, which used chlorobenzene as a starting material.
In recent decades, the focus has shifted towards more sustainable and environmentally friendly production methods. Green chemistry initiatives have led to the exploration of bio-based routes for phenol production, utilizing renewable resources such as lignin from biomass. These developments aim to reduce the carbon footprint of carbolic acid manufacturing while maintaining or improving efficiency.
The digital era has brought about significant advancements in process control and optimization for carbolic acid production. The integration of artificial intelligence and machine learning algorithms has enabled real-time monitoring and adjustment of manufacturing parameters, leading to improved yield and quality consistency.
Today, carbolic acid continues to be a crucial component in various industries, including pharmaceuticals, plastics, and agrochemicals. Its evolution has been marked by continuous improvements in production efficiency, safety, and environmental sustainability. As we look to the future, ongoing research focuses on developing even more efficient and eco-friendly production methods, ensuring that carbolic acid remains a vital player in chemical manufacturing for years to come.
Market Demand Analysis
The market demand for carbolic acid, also known as phenol, has been steadily increasing due to its widespread applications in various industries. The chemical manufacturing sector, in particular, has witnessed a surge in demand for carbolic acid as a key raw material. This compound plays a crucial role in the production of numerous downstream products, including plastics, pharmaceuticals, and agrochemicals.
In the plastics industry, carbolic acid is a vital component in the manufacture of bisphenol A (BPA), which is used to produce polycarbonate plastics and epoxy resins. The growing demand for lightweight and durable materials in automotive and construction sectors has significantly boosted the consumption of carbolic acid. Additionally, the increasing use of polycarbonate in electronic devices and packaging materials has further fueled market growth.
The pharmaceutical industry represents another major consumer of carbolic acid. It serves as a precursor for various drugs, including aspirin, and is used in the synthesis of numerous pharmaceutical intermediates. With the global healthcare sector expanding and the rising prevalence of chronic diseases, the demand for carbolic acid in pharmaceutical manufacturing is expected to continue its upward trajectory.
In the agrochemical sector, carbolic acid is utilized in the production of herbicides and pesticides. As the agricultural industry faces challenges in meeting the food demands of a growing global population, the use of these chemical products has intensified, thereby driving the demand for carbolic acid.
The market for carbolic acid is also influenced by environmental regulations and sustainability concerns. Manufacturers are increasingly focusing on developing eco-friendly production processes and exploring bio-based alternatives. This trend has led to research and development efforts aimed at improving the efficiency of carbolic acid production while minimizing environmental impact.
Geographically, Asia-Pacific has emerged as the largest consumer of carbolic acid, primarily due to the rapid industrialization and economic growth in countries like China and India. The region's expanding manufacturing sector, coupled with increasing investments in infrastructure development, has significantly contributed to the rising demand for carbolic acid and its derivatives.
The global carbolic acid market is projected to experience substantial growth in the coming years, driven by the expanding end-use industries and technological advancements in production processes. However, market dynamics are subject to fluctuations in raw material prices, particularly benzene, which is the primary feedstock for carbolic acid production. These price variations can impact the overall manufacturing efficiency and profitability of chemical producers.
In the plastics industry, carbolic acid is a vital component in the manufacture of bisphenol A (BPA), which is used to produce polycarbonate plastics and epoxy resins. The growing demand for lightweight and durable materials in automotive and construction sectors has significantly boosted the consumption of carbolic acid. Additionally, the increasing use of polycarbonate in electronic devices and packaging materials has further fueled market growth.
The pharmaceutical industry represents another major consumer of carbolic acid. It serves as a precursor for various drugs, including aspirin, and is used in the synthesis of numerous pharmaceutical intermediates. With the global healthcare sector expanding and the rising prevalence of chronic diseases, the demand for carbolic acid in pharmaceutical manufacturing is expected to continue its upward trajectory.
In the agrochemical sector, carbolic acid is utilized in the production of herbicides and pesticides. As the agricultural industry faces challenges in meeting the food demands of a growing global population, the use of these chemical products has intensified, thereby driving the demand for carbolic acid.
The market for carbolic acid is also influenced by environmental regulations and sustainability concerns. Manufacturers are increasingly focusing on developing eco-friendly production processes and exploring bio-based alternatives. This trend has led to research and development efforts aimed at improving the efficiency of carbolic acid production while minimizing environmental impact.
Geographically, Asia-Pacific has emerged as the largest consumer of carbolic acid, primarily due to the rapid industrialization and economic growth in countries like China and India. The region's expanding manufacturing sector, coupled with increasing investments in infrastructure development, has significantly contributed to the rising demand for carbolic acid and its derivatives.
The global carbolic acid market is projected to experience substantial growth in the coming years, driven by the expanding end-use industries and technological advancements in production processes. However, market dynamics are subject to fluctuations in raw material prices, particularly benzene, which is the primary feedstock for carbolic acid production. These price variations can impact the overall manufacturing efficiency and profitability of chemical producers.
Technical Challenges
The use of carbolic acid in chemical manufacturing processes presents several significant technical challenges that impact efficiency and overall production. One of the primary issues is the corrosive nature of carbolic acid, which can lead to accelerated wear and degradation of equipment and infrastructure. This necessitates the use of specialized materials and coatings, increasing both initial investment and maintenance costs for manufacturing facilities.
Another challenge lies in the handling and storage of carbolic acid. Its toxicity and potential for environmental contamination require stringent safety protocols and containment measures. This not only adds complexity to the manufacturing process but also increases the risk of workplace accidents and potential regulatory non-compliance.
The volatility of carbolic acid poses difficulties in maintaining consistent concentration levels during various stages of production. Temperature fluctuations can lead to changes in the acid's properties, affecting reaction rates and product quality. This variability necessitates precise control systems and monitoring equipment, adding another layer of complexity to the manufacturing process.
Furthermore, the purification of carbolic acid to meet high-grade industrial standards is a technically demanding process. Impurities can significantly impact the efficiency of downstream processes and the quality of final products. Advanced separation and purification techniques are required, which can be energy-intensive and time-consuming, potentially creating bottlenecks in production.
The disposal of carbolic acid waste and by-products presents another significant challenge. Environmental regulations mandate strict treatment and disposal procedures, which can be costly and technologically complex. Developing efficient recycling or neutralization methods for these waste streams is an ongoing area of research and development in the industry.
Lastly, the optimization of reaction conditions involving carbolic acid to maximize yield and minimize side reactions remains a persistent challenge. This requires a deep understanding of reaction kinetics and the ability to fine-tune process parameters. The development of novel catalysts and reaction pathways to improve selectivity and reduce energy consumption is an active area of investigation, with potential to significantly enhance manufacturing efficiency.
Another challenge lies in the handling and storage of carbolic acid. Its toxicity and potential for environmental contamination require stringent safety protocols and containment measures. This not only adds complexity to the manufacturing process but also increases the risk of workplace accidents and potential regulatory non-compliance.
The volatility of carbolic acid poses difficulties in maintaining consistent concentration levels during various stages of production. Temperature fluctuations can lead to changes in the acid's properties, affecting reaction rates and product quality. This variability necessitates precise control systems and monitoring equipment, adding another layer of complexity to the manufacturing process.
Furthermore, the purification of carbolic acid to meet high-grade industrial standards is a technically demanding process. Impurities can significantly impact the efficiency of downstream processes and the quality of final products. Advanced separation and purification techniques are required, which can be energy-intensive and time-consuming, potentially creating bottlenecks in production.
The disposal of carbolic acid waste and by-products presents another significant challenge. Environmental regulations mandate strict treatment and disposal procedures, which can be costly and technologically complex. Developing efficient recycling or neutralization methods for these waste streams is an ongoing area of research and development in the industry.
Lastly, the optimization of reaction conditions involving carbolic acid to maximize yield and minimize side reactions remains a persistent challenge. This requires a deep understanding of reaction kinetics and the ability to fine-tune process parameters. The development of novel catalysts and reaction pathways to improve selectivity and reduce energy consumption is an active area of investigation, with potential to significantly enhance manufacturing efficiency.
Current Manufacturing
01 Carbolic acid in medical applications
Carbolic acid, also known as phenol, has been used in various medical applications due to its antiseptic properties. It has been utilized in disinfectants, surgical procedures, and wound treatments to prevent infections and promote healing. The efficiency of carbolic acid in these applications has been studied and improved over time.- Carbolic acid in disinfectant compositions: Carbolic acid, also known as phenol, is utilized in various disinfectant compositions to enhance their efficiency. These compositions are designed for use in medical, industrial, and household settings to effectively eliminate harmful microorganisms. The inclusion of carbolic acid in these formulations contributes to their broad-spectrum antimicrobial activity.
- Carbolic acid in water treatment systems: Water treatment systems incorporate carbolic acid to improve their efficiency in purifying water. The addition of carbolic acid helps in the removal of contaminants and pathogens from water sources. These systems are employed in various applications, including industrial water treatment, wastewater management, and drinking water purification.
- Carbolic acid in medical devices and equipment: Medical devices and equipment utilize carbolic acid to enhance their sterilization and disinfection efficiency. The incorporation of carbolic acid in these applications helps maintain a sterile environment in healthcare settings, reducing the risk of infections and improving patient safety. This approach is particularly useful in surgical instruments and medical diagnostic equipment.
- Carbolic acid in industrial cleaning processes: Industrial cleaning processes benefit from the use of carbolic acid to increase their efficiency. The addition of carbolic acid to cleaning formulations enhances their ability to remove tough stains, grease, and other contaminants from various surfaces. This application is particularly useful in manufacturing facilities, food processing plants, and other industrial settings where thorough cleaning is essential.
- Carbolic acid in personal care and hygiene products: Personal care and hygiene products incorporate carbolic acid to improve their antimicrobial efficiency. These products include soaps, hand sanitizers, and other personal hygiene items. The addition of carbolic acid enhances their ability to eliminate harmful bacteria and other microorganisms, providing better protection against infections and improving overall hygiene.
02 Carbolic acid in industrial processes
The efficiency of carbolic acid has been explored in various industrial processes, including chemical synthesis, polymer production, and as a precursor for other compounds. Its reactivity and versatility make it a valuable component in manufacturing processes, with ongoing research to optimize its use and improve efficiency.Expand Specific Solutions03 Carbolic acid in water treatment
Carbolic acid has been investigated for its potential use in water treatment processes. Its disinfectant properties make it effective in removing harmful microorganisms from water. Research has focused on improving the efficiency of carbolic acid in water purification systems and developing methods to minimize its environmental impact.Expand Specific Solutions04 Carbolic acid in agricultural applications
The efficiency of carbolic acid has been studied in agricultural contexts, including pest control and soil treatment. Its antimicrobial properties make it useful in managing plant diseases and improving crop yields. Ongoing research aims to optimize its application methods and minimize potential environmental risks.Expand Specific Solutions05 Carbolic acid in personal care products
Carbolic acid has been utilized in various personal care products due to its antiseptic properties. Its efficiency in soaps, shampoos, and other hygiene products has been studied to improve formulations and ensure safety. Research continues to explore new applications and enhance the effectiveness of carbolic acid in personal care.Expand Specific Solutions
Key Industry Players
The impact of carbolic acid on chemical manufacturing efficiency is a complex issue in a mature industry with significant market size. The competitive landscape is characterized by established players like BASF, Sumitomo Chemical, and Evonik Operations, alongside innovative companies such as Genomatica and Dioxide Materials. These firms are at various stages of technological development, from traditional petrochemical processes to more sustainable bio-based approaches. The market is driven by increasing demand for efficient and environmentally friendly manufacturing processes, with a focus on cost reduction and improved product quality. As the industry evolves, collaborations between large corporations and specialized technology providers are becoming more common, fostering innovation and addressing challenges in chemical manufacturing efficiency.
Bayer Intellectual Property GmbH
Technical Solution: Bayer has developed a novel approach to carbolic acid production focusing on sustainability and efficiency. Their process utilizes a proprietary catalyst system that enables the direct oxidation of benzene to phenol, bypassing the traditional cumene intermediate[3]. This one-step process significantly reduces energy consumption and waste generation. Additionally, Bayer has implemented advanced process control systems and AI-driven optimization algorithms to fine-tune reaction conditions, resulting in improved yield and product quality[4]. The company is also exploring the integration of renewable hydrogen sources in the production process to further reduce its environmental impact.
Strengths: Direct benzene-to-phenol process, advanced process control, and AI optimization. Weaknesses: Potential high initial investment costs, reliance on benzene as a feedstock.
BASF Corp.
Technical Solution: BASF has developed an innovative process for manufacturing carbolic acid (phenol) using cumene as a starting material. This process, known as the cumene process, involves the oxidation of cumene to form cumene hydroperoxide, which is then cleaved to produce phenol and acetone[1]. BASF has further optimized this process by implementing advanced catalysts and reactor designs, resulting in improved yield and energy efficiency. The company has also invested in developing a bio-based route for phenol production, utilizing renewable feedstocks to reduce the carbon footprint of the manufacturing process[2].
Strengths: High-efficiency cumene process, advanced catalysts, and bio-based alternatives. Weaknesses: Dependence on petroleum-derived feedstocks for the traditional process, potential environmental concerns.
Innovative Techniques
Process for the liquid phase selective hydroxylation of benzene
PatentInactiveUS20080234524A1
Innovation
- A process involving the liquid phase selective hydroxylation of benzene using hydrogen peroxide as the oxidant and vanadyl pyrophosphate as the catalyst in acetonitrile solvent under mild conditions, with specific molar ratios and temperature control, to achieve high conversion and selectivity of benzene to phenol.
Process for Producing Phenol and/or Cyclohexanone from Cyclohexylbenzene
PatentInactiveUS20150203429A1
Innovation
- A process that involves the oxidation of cyclohexylbenzene to cyclohexylbenzene hydroperoxide, followed by cleavage to produce phenol and cyclohexanone, with the additional steps of dehydration and hydrogenation of by-products phenylcyclohexanols and phenylcyclohexanones to recycle back to cyclohexylbenzene, using a bifunctional catalyst with a solid acid and hydrogenating metal component.
Environmental Impact
The use of carbolic acid in chemical manufacturing processes has significant environmental implications that warrant careful consideration. While it enhances production efficiency, its potential environmental impact raises concerns across various ecological domains.
Carbolic acid, also known as phenol, can be toxic to aquatic life even at low concentrations. When released into water bodies, it can disrupt ecosystems by affecting the growth and reproduction of fish, algae, and other aquatic organisms. The bioaccumulation of phenol in the food chain poses risks to higher-level predators and potentially to human health through contaminated seafood consumption.
Air pollution is another critical environmental concern associated with carbolic acid usage. Volatile organic compounds (VOCs) released during manufacturing processes contribute to smog formation and can lead to respiratory issues in nearby communities. Long-term exposure to these emissions may have broader public health implications, particularly in densely populated areas near chemical manufacturing facilities.
Soil contamination is a persistent issue when carbolic acid is improperly handled or disposed of. It can leach into groundwater, affecting soil microorganisms and plant life. This contamination can persist for extended periods, potentially impacting agricultural productivity and biodiversity in affected areas.
The production and disposal of carbolic acid also contribute to the overall carbon footprint of chemical manufacturing. Energy-intensive processes required for its synthesis and the treatment of waste products increase greenhouse gas emissions, contributing to global climate change concerns.
To mitigate these environmental risks, chemical manufacturers are increasingly adopting cleaner production technologies and implementing stringent waste management protocols. Advanced filtration systems and closed-loop manufacturing processes help reduce emissions and minimize the release of carbolic acid into the environment. Additionally, research into green chemistry alternatives aims to develop less harmful substitutes that maintain manufacturing efficiency while reducing ecological impact.
Regulatory bodies worldwide have implemented strict guidelines for the handling, storage, and disposal of carbolic acid. Compliance with these regulations is crucial for chemical manufacturers to minimize environmental risks and maintain sustainable operations. Regular environmental impact assessments and monitoring programs are essential to track and mitigate the long-term effects of carbolic acid usage on surrounding ecosystems.
As the chemical industry continues to evolve, balancing manufacturing efficiency with environmental stewardship remains a critical challenge. The development of innovative, eco-friendly technologies and the adoption of circular economy principles in chemical manufacturing processes will be key to addressing the environmental concerns associated with carbolic acid and similar compounds.
Carbolic acid, also known as phenol, can be toxic to aquatic life even at low concentrations. When released into water bodies, it can disrupt ecosystems by affecting the growth and reproduction of fish, algae, and other aquatic organisms. The bioaccumulation of phenol in the food chain poses risks to higher-level predators and potentially to human health through contaminated seafood consumption.
Air pollution is another critical environmental concern associated with carbolic acid usage. Volatile organic compounds (VOCs) released during manufacturing processes contribute to smog formation and can lead to respiratory issues in nearby communities. Long-term exposure to these emissions may have broader public health implications, particularly in densely populated areas near chemical manufacturing facilities.
Soil contamination is a persistent issue when carbolic acid is improperly handled or disposed of. It can leach into groundwater, affecting soil microorganisms and plant life. This contamination can persist for extended periods, potentially impacting agricultural productivity and biodiversity in affected areas.
The production and disposal of carbolic acid also contribute to the overall carbon footprint of chemical manufacturing. Energy-intensive processes required for its synthesis and the treatment of waste products increase greenhouse gas emissions, contributing to global climate change concerns.
To mitigate these environmental risks, chemical manufacturers are increasingly adopting cleaner production technologies and implementing stringent waste management protocols. Advanced filtration systems and closed-loop manufacturing processes help reduce emissions and minimize the release of carbolic acid into the environment. Additionally, research into green chemistry alternatives aims to develop less harmful substitutes that maintain manufacturing efficiency while reducing ecological impact.
Regulatory bodies worldwide have implemented strict guidelines for the handling, storage, and disposal of carbolic acid. Compliance with these regulations is crucial for chemical manufacturers to minimize environmental risks and maintain sustainable operations. Regular environmental impact assessments and monitoring programs are essential to track and mitigate the long-term effects of carbolic acid usage on surrounding ecosystems.
As the chemical industry continues to evolve, balancing manufacturing efficiency with environmental stewardship remains a critical challenge. The development of innovative, eco-friendly technologies and the adoption of circular economy principles in chemical manufacturing processes will be key to addressing the environmental concerns associated with carbolic acid and similar compounds.
Safety Regulations
Safety regulations play a crucial role in the use of carbolic acid in chemical manufacturing processes. The handling and application of this potent compound require strict adherence to established guidelines to ensure worker safety and environmental protection.
Regulatory bodies such as the Occupational Safety and Health Administration (OSHA) in the United States and the European Chemicals Agency (ECHA) have set forth comprehensive safety standards for carbolic acid usage. These regulations encompass various aspects, including proper storage, handling procedures, personal protective equipment (PPE), and emergency response protocols.
Storage requirements for carbolic acid are particularly stringent due to its corrosive and toxic nature. Facilities must maintain dedicated storage areas with proper ventilation, temperature control, and containment measures to prevent accidental spills or leaks. Regular inspections and maintenance of storage facilities are mandated to ensure compliance with safety standards.
Personal protective equipment is a critical component of safety regulations for carbolic acid handling. Workers must be equipped with appropriate PPE, including chemical-resistant gloves, goggles, face shields, and protective clothing. Respiratory protection may also be necessary in certain situations, particularly when dealing with high concentrations or in poorly ventilated areas.
Training and education form an integral part of safety regulations. Employees involved in carbolic acid handling must undergo comprehensive training programs that cover proper handling techniques, emergency procedures, and the use of safety equipment. Regular refresher courses and safety drills are often required to maintain a high level of preparedness.
Exposure limits for carbolic acid are strictly regulated to minimize health risks. Workplace air quality monitoring and regular health assessments for exposed workers are typically mandated. These measures help in early detection of potential health issues and ensure that exposure levels remain within safe limits.
Emergency response protocols are another critical aspect of safety regulations. Facilities must have well-defined procedures for handling spills, leaks, or accidental exposures. This includes the availability of emergency showers, eyewash stations, and first aid equipment in areas where carbolic acid is used or stored.
Environmental regulations also play a significant role in the use of carbolic acid in chemical manufacturing. Proper disposal methods for waste products and contaminated materials are strictly enforced to prevent environmental contamination. Wastewater treatment and air emission controls are often required to mitigate the environmental impact of carbolic acid usage.
Compliance with these safety regulations is not only a legal requirement but also a crucial factor in maintaining operational efficiency. By ensuring a safe working environment, companies can minimize the risk of accidents, reduce downtime, and improve overall productivity in chemical manufacturing processes involving carbolic acid.
Regulatory bodies such as the Occupational Safety and Health Administration (OSHA) in the United States and the European Chemicals Agency (ECHA) have set forth comprehensive safety standards for carbolic acid usage. These regulations encompass various aspects, including proper storage, handling procedures, personal protective equipment (PPE), and emergency response protocols.
Storage requirements for carbolic acid are particularly stringent due to its corrosive and toxic nature. Facilities must maintain dedicated storage areas with proper ventilation, temperature control, and containment measures to prevent accidental spills or leaks. Regular inspections and maintenance of storage facilities are mandated to ensure compliance with safety standards.
Personal protective equipment is a critical component of safety regulations for carbolic acid handling. Workers must be equipped with appropriate PPE, including chemical-resistant gloves, goggles, face shields, and protective clothing. Respiratory protection may also be necessary in certain situations, particularly when dealing with high concentrations or in poorly ventilated areas.
Training and education form an integral part of safety regulations. Employees involved in carbolic acid handling must undergo comprehensive training programs that cover proper handling techniques, emergency procedures, and the use of safety equipment. Regular refresher courses and safety drills are often required to maintain a high level of preparedness.
Exposure limits for carbolic acid are strictly regulated to minimize health risks. Workplace air quality monitoring and regular health assessments for exposed workers are typically mandated. These measures help in early detection of potential health issues and ensure that exposure levels remain within safe limits.
Emergency response protocols are another critical aspect of safety regulations. Facilities must have well-defined procedures for handling spills, leaks, or accidental exposures. This includes the availability of emergency showers, eyewash stations, and first aid equipment in areas where carbolic acid is used or stored.
Environmental regulations also play a significant role in the use of carbolic acid in chemical manufacturing. Proper disposal methods for waste products and contaminated materials are strictly enforced to prevent environmental contamination. Wastewater treatment and air emission controls are often required to mitigate the environmental impact of carbolic acid usage.
Compliance with these safety regulations is not only a legal requirement but also a crucial factor in maintaining operational efficiency. By ensuring a safe working environment, companies can minimize the risk of accidents, reduce downtime, and improve overall productivity in chemical manufacturing processes involving carbolic acid.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!