What are the aging mechanisms in Nylon 6 exposed to UV radiation
OCT 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
UV-Induced Nylon 6 Degradation Background and Objectives
Nylon 6, a widely used engineering thermoplastic, has been extensively employed in various industrial applications since its development in the 1930s. This semi-crystalline polyamide possesses excellent mechanical properties, chemical resistance, and thermal stability, making it suitable for automotive components, electrical insulation, and outdoor applications. However, when exposed to ultraviolet (UV) radiation, Nylon 6 undergoes significant degradation that compromises its structural integrity and functional properties over time.
The evolution of UV-induced degradation research in Nylon 6 has progressed through several key phases. Initial studies in the 1950s and 1960s primarily focused on identifying visible changes in physical properties. The 1970s and 1980s saw advancements in analytical techniques that enabled researchers to investigate chemical modifications at the molecular level. Recent decades have witnessed sophisticated approaches combining spectroscopic methods, mechanical testing, and computational modeling to comprehensively understand the complex degradation mechanisms.
Current technological trends in this field are moving toward developing more accurate prediction models for service life estimation, creating advanced UV stabilization systems, and establishing standardized testing protocols that better simulate real-world exposure conditions. The integration of nanotechnology and smart materials into Nylon 6 formulations represents another significant trend aimed at enhancing UV resistance while maintaining or improving other material properties.
The primary objective of investigating UV-induced aging mechanisms in Nylon 6 is to establish a fundamental understanding of the photochemical and photophysical processes that lead to material deterioration. This knowledge is essential for developing effective stabilization strategies and extending the service life of Nylon 6 products exposed to outdoor environments.
Additionally, this research aims to correlate molecular-level changes with macroscopic property alterations, enabling the development of accelerated testing methodologies that can reliably predict long-term performance. Such predictive capabilities would significantly reduce product development cycles and enhance material selection processes for specific applications.
Another critical goal is to identify key indicators or markers of early-stage degradation that could serve as warning signs before catastrophic failure occurs. This would facilitate the implementation of preventive maintenance strategies and improve safety in critical applications where Nylon 6 components are exposed to UV radiation.
Furthermore, understanding these aging mechanisms will contribute to the broader field of polymer science by elucidating fundamental photochemical reaction pathways that may be applicable to other polyamide materials. The insights gained will also support sustainability efforts by potentially reducing material waste through extended product lifespans and enabling more efficient recycling processes for aged Nylon 6 materials.
The evolution of UV-induced degradation research in Nylon 6 has progressed through several key phases. Initial studies in the 1950s and 1960s primarily focused on identifying visible changes in physical properties. The 1970s and 1980s saw advancements in analytical techniques that enabled researchers to investigate chemical modifications at the molecular level. Recent decades have witnessed sophisticated approaches combining spectroscopic methods, mechanical testing, and computational modeling to comprehensively understand the complex degradation mechanisms.
Current technological trends in this field are moving toward developing more accurate prediction models for service life estimation, creating advanced UV stabilization systems, and establishing standardized testing protocols that better simulate real-world exposure conditions. The integration of nanotechnology and smart materials into Nylon 6 formulations represents another significant trend aimed at enhancing UV resistance while maintaining or improving other material properties.
The primary objective of investigating UV-induced aging mechanisms in Nylon 6 is to establish a fundamental understanding of the photochemical and photophysical processes that lead to material deterioration. This knowledge is essential for developing effective stabilization strategies and extending the service life of Nylon 6 products exposed to outdoor environments.
Additionally, this research aims to correlate molecular-level changes with macroscopic property alterations, enabling the development of accelerated testing methodologies that can reliably predict long-term performance. Such predictive capabilities would significantly reduce product development cycles and enhance material selection processes for specific applications.
Another critical goal is to identify key indicators or markers of early-stage degradation that could serve as warning signs before catastrophic failure occurs. This would facilitate the implementation of preventive maintenance strategies and improve safety in critical applications where Nylon 6 components are exposed to UV radiation.
Furthermore, understanding these aging mechanisms will contribute to the broader field of polymer science by elucidating fundamental photochemical reaction pathways that may be applicable to other polyamide materials. The insights gained will also support sustainability efforts by potentially reducing material waste through extended product lifespans and enabling more efficient recycling processes for aged Nylon 6 materials.
Market Analysis of UV-Resistant Nylon 6 Applications
The global market for UV-resistant Nylon 6 applications has experienced significant growth in recent years, driven primarily by increasing demand in automotive, construction, and outdoor equipment sectors. The market size for UV-resistant polyamides, including Nylon 6, was valued at approximately $1.2 billion in 2022 and is projected to grow at a compound annual growth rate of 6.8% through 2028.
Automotive applications represent the largest market segment, accounting for nearly 38% of the total UV-resistant Nylon 6 consumption. This dominance stems from the material's ability to withstand prolonged sun exposure while maintaining structural integrity in exterior components such as mirror housings, grilles, and under-hood applications. The automotive industry's shift toward lightweight materials for fuel efficiency has further accelerated adoption.
The construction sector follows as the second-largest consumer, utilizing UV-resistant Nylon 6 in outdoor fixtures, roofing materials, and solar panel components. This segment has shown the fastest growth rate at 8.2% annually, propelled by expanding solar energy installations worldwide and increasing awareness of material degradation costs in building maintenance.
Consumer goods and outdoor equipment manufacturers constitute another significant market, with applications in sporting goods, garden equipment, and marine components. This segment values the combination of mechanical strength and UV resistance that modified Nylon 6 offers compared to alternative materials.
Geographically, Asia-Pacific dominates the market with a 42% share, led by China's manufacturing capabilities and growing domestic consumption. North America and Europe follow with 28% and 24% market shares respectively, with particularly strong demand in premium automotive and high-performance outdoor applications.
Market analysis reveals a price premium of 15-30% for UV-resistant Nylon 6 compared to standard variants, reflecting the added value of extended service life in outdoor applications. This premium has been gradually decreasing as manufacturing processes improve and competition increases among material suppliers.
Customer requirements are increasingly focused on longer warranty periods, with expectations for outdoor durability extending from 5-7 years historically to 10-15 years currently. This trend directly correlates with improved understanding of aging mechanisms and more effective UV stabilization technologies.
Market forecasts indicate particularly strong growth potential in emerging applications such as electric vehicle components, renewable energy infrastructure, and advanced 3D-printed outdoor structures, where the combination of lightweight properties and environmental resistance provides significant advantages over traditional materials.
Automotive applications represent the largest market segment, accounting for nearly 38% of the total UV-resistant Nylon 6 consumption. This dominance stems from the material's ability to withstand prolonged sun exposure while maintaining structural integrity in exterior components such as mirror housings, grilles, and under-hood applications. The automotive industry's shift toward lightweight materials for fuel efficiency has further accelerated adoption.
The construction sector follows as the second-largest consumer, utilizing UV-resistant Nylon 6 in outdoor fixtures, roofing materials, and solar panel components. This segment has shown the fastest growth rate at 8.2% annually, propelled by expanding solar energy installations worldwide and increasing awareness of material degradation costs in building maintenance.
Consumer goods and outdoor equipment manufacturers constitute another significant market, with applications in sporting goods, garden equipment, and marine components. This segment values the combination of mechanical strength and UV resistance that modified Nylon 6 offers compared to alternative materials.
Geographically, Asia-Pacific dominates the market with a 42% share, led by China's manufacturing capabilities and growing domestic consumption. North America and Europe follow with 28% and 24% market shares respectively, with particularly strong demand in premium automotive and high-performance outdoor applications.
Market analysis reveals a price premium of 15-30% for UV-resistant Nylon 6 compared to standard variants, reflecting the added value of extended service life in outdoor applications. This premium has been gradually decreasing as manufacturing processes improve and competition increases among material suppliers.
Customer requirements are increasingly focused on longer warranty periods, with expectations for outdoor durability extending from 5-7 years historically to 10-15 years currently. This trend directly correlates with improved understanding of aging mechanisms and more effective UV stabilization technologies.
Market forecasts indicate particularly strong growth potential in emerging applications such as electric vehicle components, renewable energy infrastructure, and advanced 3D-printed outdoor structures, where the combination of lightweight properties and environmental resistance provides significant advantages over traditional materials.
Current Understanding and Challenges in Polymer Photodegradation
The field of polymer photodegradation has seen significant advancements in recent decades, yet numerous challenges persist in fully understanding the complex mechanisms involved. For Nylon 6 specifically, researchers have established that UV radiation triggers a series of photochemical reactions leading to material degradation. The primary mechanism involves the absorption of UV photons by chromophoric groups within the polymer structure, initiating the formation of free radicals through homolytic bond cleavage.
Current research indicates that Nylon 6 degradation under UV exposure follows multiple pathways simultaneously. The most documented process is chain scission, where the polymer backbone breaks down into smaller fragments, resulting in decreased molecular weight and mechanical strength. Concurrently, photo-oxidation occurs when atmospheric oxygen reacts with the generated free radicals, forming peroxy radicals and subsequently hydroperoxides, which further decompose to create carbonyl compounds.
Despite these established pathways, significant gaps remain in understanding the complete degradation kinetics. The rate-determining steps and the influence of environmental factors such as humidity, temperature, and contaminants on degradation progression are not fully characterized. This presents challenges for accurately predicting service life in real-world applications where multiple stressors act simultaneously.
Another major challenge lies in the heterogeneous nature of the degradation process. Studies have shown that photodegradation in Nylon 6 often begins at the surface and progresses inward, creating a gradient of degradation. This spatial variation complicates both analysis and the development of protective strategies, as surface modifications may not adequately protect the bulk material over extended periods.
The role of additives and impurities presents another area of incomplete understanding. While stabilizers like hindered amine light stabilizers (HALS) and UV absorbers are commonly employed to mitigate degradation, their long-term effectiveness and potential secondary reactions remain subjects of ongoing research. Similarly, the impact of processing-induced impurities on photodegradation pathways requires further investigation.
Advanced characterization techniques have revealed that crystallinity changes during UV exposure significantly affect degradation rates, with amorphous regions typically degrading more rapidly than crystalline domains. However, the dynamic relationship between crystallinity evolution and ongoing degradation represents a complex feedback system that current models struggle to fully capture.
The development of standardized accelerated aging protocols that accurately correlate with real-world performance remains an elusive goal. Current methods often fail to reproduce the complex interplay of factors present in natural weathering, leading to discrepancies between laboratory predictions and field observations of Nylon 6 performance under UV exposure.
Current research indicates that Nylon 6 degradation under UV exposure follows multiple pathways simultaneously. The most documented process is chain scission, where the polymer backbone breaks down into smaller fragments, resulting in decreased molecular weight and mechanical strength. Concurrently, photo-oxidation occurs when atmospheric oxygen reacts with the generated free radicals, forming peroxy radicals and subsequently hydroperoxides, which further decompose to create carbonyl compounds.
Despite these established pathways, significant gaps remain in understanding the complete degradation kinetics. The rate-determining steps and the influence of environmental factors such as humidity, temperature, and contaminants on degradation progression are not fully characterized. This presents challenges for accurately predicting service life in real-world applications where multiple stressors act simultaneously.
Another major challenge lies in the heterogeneous nature of the degradation process. Studies have shown that photodegradation in Nylon 6 often begins at the surface and progresses inward, creating a gradient of degradation. This spatial variation complicates both analysis and the development of protective strategies, as surface modifications may not adequately protect the bulk material over extended periods.
The role of additives and impurities presents another area of incomplete understanding. While stabilizers like hindered amine light stabilizers (HALS) and UV absorbers are commonly employed to mitigate degradation, their long-term effectiveness and potential secondary reactions remain subjects of ongoing research. Similarly, the impact of processing-induced impurities on photodegradation pathways requires further investigation.
Advanced characterization techniques have revealed that crystallinity changes during UV exposure significantly affect degradation rates, with amorphous regions typically degrading more rapidly than crystalline domains. However, the dynamic relationship between crystallinity evolution and ongoing degradation represents a complex feedback system that current models struggle to fully capture.
The development of standardized accelerated aging protocols that accurately correlate with real-world performance remains an elusive goal. Current methods often fail to reproduce the complex interplay of factors present in natural weathering, leading to discrepancies between laboratory predictions and field observations of Nylon 6 performance under UV exposure.
Established Methodologies for UV Aging Assessment
01 Thermal oxidation mechanisms in Nylon 6
Thermal oxidation is a primary aging mechanism for Nylon 6, involving the breakdown of polymer chains due to heat exposure in the presence of oxygen. This process leads to the formation of free radicals, which propagate chain reactions resulting in molecular weight reduction, embrittlement, and loss of mechanical properties. The oxidation typically begins at vulnerable sites in the polymer structure and can be accelerated by UV radiation, mechanical stress, or contaminants.- Thermal oxidation mechanisms in Nylon 6: Thermal oxidation is a primary aging mechanism for Nylon 6, involving the breakdown of polymer chains due to heat exposure in the presence of oxygen. This process leads to chain scission, cross-linking, and the formation of carbonyl groups, which significantly deteriorate the mechanical properties of the material. The oxidation typically begins at the amorphous regions of the polymer and progresses to more crystalline areas, causing embrittlement, discoloration, and reduced tensile strength over time.
- Hydrolytic degradation of Nylon 6: Hydrolytic degradation occurs when Nylon 6 is exposed to moisture or water, particularly at elevated temperatures. Water molecules attack the amide bonds in the polymer chain, causing them to break and form carboxylic acid and amine end groups. This process reduces molecular weight, decreases mechanical strength, and increases brittleness. The rate of hydrolysis is influenced by temperature, pH, and the presence of acids or bases that can catalyze the reaction.
- UV radiation effects on Nylon 6 aging: Ultraviolet radiation causes photodegradation of Nylon 6 through the formation of free radicals that initiate chain scission and oxidation reactions. This leads to surface cracking, yellowing, and loss of mechanical properties. The chromophoric groups in Nylon 6 absorb UV energy, leading to the breaking of chemical bonds and subsequent reactions with atmospheric oxygen. The degradation typically begins at the surface and gradually progresses inward, creating a gradient of degradation through the material thickness.
- Stabilization methods against Nylon 6 aging: Various stabilization methods can be employed to mitigate the aging of Nylon 6. These include the incorporation of antioxidants to prevent thermal oxidation, UV stabilizers to absorb harmful radiation, and metal deactivators to prevent catalytic degradation by metal ions. Additionally, physical modifications such as blending with other polymers or adding reinforcing fillers can improve resistance to environmental factors. These stabilization techniques extend the service life of Nylon 6 products by inhibiting the primary degradation mechanisms.
- Environmental stress cracking and fatigue in Nylon 6: Environmental stress cracking occurs when Nylon 6 is simultaneously exposed to mechanical stress and aggressive chemical environments. This synergistic effect accelerates the formation and propagation of cracks, leading to premature failure. Similarly, mechanical fatigue from cyclic loading causes progressive damage through the initiation and growth of microcracks. These phenomena are particularly relevant in applications where Nylon 6 components are subjected to dynamic loads or chemical exposure, such as in automotive parts or industrial equipment.
02 Hydrolytic degradation of Nylon 6
Hydrolytic degradation occurs when Nylon 6 is exposed to moisture or humid environments, causing the amide bonds in the polymer chain to break down. This process is particularly significant because Nylon 6 is hygroscopic and can absorb water from the surrounding environment. The hydrolysis reaction leads to chain scission, reducing molecular weight and deteriorating mechanical properties such as tensile strength and impact resistance. Temperature increases can significantly accelerate this degradation mechanism.Expand Specific Solutions03 UV radiation effects on Nylon 6 aging
Ultraviolet radiation causes photodegradation of Nylon 6, leading to yellowing, surface cracking, and loss of mechanical properties. The UV radiation breaks chemical bonds in the polymer structure, creating free radicals that initiate chain reactions. These reactions can cause crosslinking or chain scission, both of which alter the material's properties. The photodegradation process is often accompanied by oxidation reactions, which further accelerate the aging process and can lead to significant deterioration of the polymer's performance characteristics.Expand Specific Solutions04 Stabilization methods against Nylon 6 aging
Various stabilization methods can be employed to mitigate the aging of Nylon 6. These include the addition of antioxidants to prevent thermal oxidation, UV stabilizers to protect against photodegradation, and metal deactivators to prevent catalytic degradation by metal ions. Physical methods such as barrier coatings can also be applied to reduce exposure to degradation factors. Additionally, chemical modifications of the polymer structure can enhance resistance to various aging mechanisms, thereby extending the service life of Nylon 6 products.Expand Specific Solutions05 Physical aging and mechanical stress effects
Physical aging in Nylon 6 involves structural reorganization over time, leading to increased density, reduced free volume, and changes in mechanical properties without chemical changes. Simultaneously, mechanical stress can accelerate aging through stress cracking, fatigue, and creep mechanisms. Under load, polymer chains realign and may form microcracks that propagate with continued stress. These physical aging processes are temperature-dependent and can significantly affect the long-term performance of Nylon 6 components, particularly in applications involving cyclic loading or constant stress.Expand Specific Solutions
Leading Organizations in Polymer Aging Research
The UV aging of Nylon 6 represents a mature research field within polymer degradation science, currently in a growth phase with an estimated global market value of $2.5 billion for UV-resistant polyamides. The competitive landscape features established chemical companies like DuPont and Solutia developing proprietary stabilization technologies, while cosmetic giants including L'Oréal, Johnson & Johnson, and Colgate-Palmolive focus on applications in personal care products. Academic institutions such as Wuhan Textile University and East China University of Science & Technology are advancing fundamental research on degradation mechanisms. The technology has reached commercial maturity for standard applications, though innovations in nano-additives and multi-functional stabilizers from companies like Samsung Electronics and Torrent Pharmaceuticals are driving new market opportunities.
East China University of Science & Technology
Technical Solution: East China University of Science & Technology has conducted extensive research on the molecular-level aging mechanisms of Nylon 6 under UV radiation. Their studies have revealed that UV exposure initiates a series of photochemical reactions in Nylon 6, beginning with the formation of free radicals through hydrogen abstraction, particularly at the α-position to the nitrogen atom. These radicals subsequently react with oxygen to form peroxy radicals, leading to chain scission and the formation of carbonyl groups. The university's research team has developed novel characterization methodologies combining FTIR spectroscopy, gel permeation chromatography, and mechanical testing to quantitatively track degradation progression. Their technical solution involves a multi-component stabilization system incorporating phenolic antioxidants and specialized UV absorbers with high extinction coefficients in the 290-350nm range. Additionally, they've pioneered the use of nanocomposite technology, incorporating modified clay nanoparticles that serve both as UV shields and reinforcing agents, effectively addressing both the chemical degradation and physical property deterioration aspects of UV aging.
Strengths: Fundamental understanding of degradation mechanisms at the molecular level; innovative characterization methodologies; dual-function nanocomposite approach. Weaknesses: Some solutions remain at laboratory scale; potential challenges in commercial-scale implementation; higher cost compared to conventional stabilization methods.
Technische Universität Braunschweig
Technical Solution: Technische Universität Braunschweig has conducted pioneering research on the photodegradation kinetics of Nylon 6 under various UV exposure conditions. Their studies have identified that the primary aging mechanism involves photolysis of the amide bonds, leading to chain scission and formation of various degradation products including aldehydes, ketones, and carboxylic acids. The university's research team has developed advanced analytical protocols combining chemiluminescence, FTIR spectroscopy with multivariate analysis, and rheological measurements to characterize the complex degradation pathways. Their technical solution focuses on understanding the relationship between polymer morphology and degradation susceptibility, revealing that crystalline regions exhibit significantly higher resistance to UV degradation compared to amorphous regions. Based on this insight, they've developed processing methodologies that optimize crystallinity distribution to enhance overall UV resistance. Additionally, they've investigated the role of moisture in accelerating photodegradation through hydrolysis reactions that work synergistically with photolytic processes, leading to the development of moisture-resistant coating technologies that significantly improve UV stability.
Strengths: Sophisticated analytical approaches to degradation characterization; fundamental understanding of structure-property-degradation relationships; innovative processing-based solutions. Weaknesses: Some approaches require specialized processing equipment; limited focus on additive-based stabilization; solutions may be more applicable to high-value applications than mass-market products.
Critical Photochemical Mechanisms in Nylon 6 Degradation
Novel peptides, use thereof in cosmetic and cosmeceutic applications, and compositions comprising same
PatentInactiveUS20100310484A1
Innovation
- A family of peptides, specifically peptides of Formulae I and II, which include lipoyl residues and histidine residues, are used to regenerate glutathione and provide internal photoprotection against UV-induced DNA modifications, combined with lipoic acid and human growth factor or alpha-MSH, to prevent, delay, or treat skin aging effects.
Lactobacillus fermentum XJC48 with functions of prolonging life of dermal fibroblasts and resisting skin aging and application of lactobacillus fermentum XJC48
PatentActiveCN115960742A
Innovation
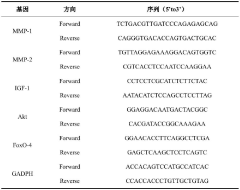
- 研发了一株发酵乳杆菌Limosilactobacillus fermentum XJC48,该菌株能够在体内外显著降低UV诱导的真皮成纤维细胞MMPs表达水平,延长真皮细胞寿命,并通过调节IGF-1/Akt/FOXO细胞寿命调节通路,增强皮肤细胞的抗老化能力。
Environmental Factors Influencing Degradation Kinetics
The degradation kinetics of Nylon 6 under UV radiation is significantly influenced by various environmental factors that can either accelerate or decelerate the aging process. Temperature plays a crucial role, as higher ambient temperatures typically accelerate photochemical reactions in polymers. Research indicates that for every 10°C increase in temperature, the rate of photodegradation in Nylon 6 can increase by 1.5-2 times, following an Arrhenius-type relationship.
Humidity levels also substantially impact degradation mechanisms. Water molecules can penetrate the amorphous regions of Nylon 6, causing hydrolysis of amide bonds which works synergistically with UV-induced oxidation. Studies have shown that relative humidity above 65% can increase the rate of chain scission by up to 40% compared to dry conditions, particularly when combined with UV exposure.
Oxygen concentration represents another critical environmental factor. The photo-oxidation of Nylon 6 involves free radical formation followed by reaction with atmospheric oxygen. Higher oxygen partial pressure accelerates oxidative degradation, with research demonstrating that oxygen diffusion into the polymer matrix becomes the rate-limiting step in thicker samples, creating degradation gradients from surface to core.
Pollutants and atmospheric contaminants further complicate degradation kinetics. Sulfur dioxide, nitrogen oxides, and ozone can react with polymer radicals formed during UV exposure, creating additional degradation pathways. Industrial environments with high concentrations of these pollutants have shown to increase degradation rates by 15-30% compared to clean rural environments.
The spectral distribution and intensity of UV radiation itself significantly affects degradation mechanisms. Shorter wavelengths (UVB: 280-315 nm) cause more severe damage than longer wavelengths (UVA: 315-400 nm) due to their higher energy content. Seasonal variations in solar radiation can lead to fluctuating degradation rates, with summer exposure causing approximately 2.5 times faster degradation than winter exposure at the same geographical location.
Cyclic environmental conditions, particularly wet-dry cycles and freeze-thaw cycles, create mechanical stresses that can enhance the effects of chemical degradation. These cycles can lead to microcracking that increases surface area exposed to UV radiation and oxygen, accelerating the overall degradation process by creating new reaction sites and pathways for oxygen diffusion.
Humidity levels also substantially impact degradation mechanisms. Water molecules can penetrate the amorphous regions of Nylon 6, causing hydrolysis of amide bonds which works synergistically with UV-induced oxidation. Studies have shown that relative humidity above 65% can increase the rate of chain scission by up to 40% compared to dry conditions, particularly when combined with UV exposure.
Oxygen concentration represents another critical environmental factor. The photo-oxidation of Nylon 6 involves free radical formation followed by reaction with atmospheric oxygen. Higher oxygen partial pressure accelerates oxidative degradation, with research demonstrating that oxygen diffusion into the polymer matrix becomes the rate-limiting step in thicker samples, creating degradation gradients from surface to core.
Pollutants and atmospheric contaminants further complicate degradation kinetics. Sulfur dioxide, nitrogen oxides, and ozone can react with polymer radicals formed during UV exposure, creating additional degradation pathways. Industrial environments with high concentrations of these pollutants have shown to increase degradation rates by 15-30% compared to clean rural environments.
The spectral distribution and intensity of UV radiation itself significantly affects degradation mechanisms. Shorter wavelengths (UVB: 280-315 nm) cause more severe damage than longer wavelengths (UVA: 315-400 nm) due to their higher energy content. Seasonal variations in solar radiation can lead to fluctuating degradation rates, with summer exposure causing approximately 2.5 times faster degradation than winter exposure at the same geographical location.
Cyclic environmental conditions, particularly wet-dry cycles and freeze-thaw cycles, create mechanical stresses that can enhance the effects of chemical degradation. These cycles can lead to microcracking that increases surface area exposed to UV radiation and oxygen, accelerating the overall degradation process by creating new reaction sites and pathways for oxygen diffusion.
Stabilization Strategies and Additives for UV Protection
To effectively protect Nylon 6 from UV degradation, various stabilization strategies and additives have been developed. UV absorbers represent the first line of defense, functioning by absorbing harmful ultraviolet radiation and converting it into less damaging forms of energy, typically heat. Common UV absorbers for polyamide systems include benzotriazoles, benzophenones, and triazines, which can absorb radiation in the critical 290-400 nm wavelength range where Nylon 6 is most vulnerable.
Hindered Amine Light Stabilizers (HALS) offer another crucial protection mechanism. Unlike UV absorbers, HALS operate through a cyclic process that neutralizes free radicals formed during photo-oxidation. This regenerative capability allows HALS to provide long-term protection even at relatively low concentrations. Compounds such as Tinuvin 770 and Chimassorb 944 have demonstrated particular effectiveness in Nylon 6 applications.
Antioxidants serve as complementary additives in UV protection systems, preventing oxidative degradation that often accompanies photodegradation. Primary antioxidants like hindered phenols interrupt the free radical chain reaction, while secondary antioxidants such as phosphites decompose hydroperoxides. The synergistic combination of both types provides comprehensive protection against thermo-oxidative processes accelerated by UV exposure.
Nanomaterial-based solutions have emerged as advanced stabilization strategies. Nanoparticles of zinc oxide, titanium dioxide, and cerium oxide can effectively scatter and absorb UV radiation while maintaining the polymer's transparency to visible light. Additionally, nanoclays and carbon-based nanomaterials like graphene oxide have demonstrated multifunctional benefits, simultaneously enhancing UV resistance and mechanical properties.
Surface treatments and coatings represent external protection approaches. UV-resistant coatings containing stabilizers can be applied to Nylon 6 products, creating a sacrificial layer that absorbs radiation before it reaches the polymer substrate. Techniques such as plasma treatment can also modify the surface chemistry to improve compatibility with protective additives.
Formulation optimization remains critical for effective stabilization. The concentration, compatibility, and distribution of additives significantly impact protection efficiency. Typically, combinations of different stabilizer types (UV absorbers, HALS, and antioxidants) at carefully balanced ratios provide superior protection compared to single-component systems. Processing conditions must also be controlled to prevent premature consumption of stabilizers during manufacturing.
Recent innovations include reactive stabilizers that chemically bond to the polymer matrix, preventing migration and leaching during the product lifecycle. These covalently bound stabilizers offer promising solutions for applications requiring extended outdoor exposure or frequent cleaning cycles where conventional additives might be gradually lost.
Hindered Amine Light Stabilizers (HALS) offer another crucial protection mechanism. Unlike UV absorbers, HALS operate through a cyclic process that neutralizes free radicals formed during photo-oxidation. This regenerative capability allows HALS to provide long-term protection even at relatively low concentrations. Compounds such as Tinuvin 770 and Chimassorb 944 have demonstrated particular effectiveness in Nylon 6 applications.
Antioxidants serve as complementary additives in UV protection systems, preventing oxidative degradation that often accompanies photodegradation. Primary antioxidants like hindered phenols interrupt the free radical chain reaction, while secondary antioxidants such as phosphites decompose hydroperoxides. The synergistic combination of both types provides comprehensive protection against thermo-oxidative processes accelerated by UV exposure.
Nanomaterial-based solutions have emerged as advanced stabilization strategies. Nanoparticles of zinc oxide, titanium dioxide, and cerium oxide can effectively scatter and absorb UV radiation while maintaining the polymer's transparency to visible light. Additionally, nanoclays and carbon-based nanomaterials like graphene oxide have demonstrated multifunctional benefits, simultaneously enhancing UV resistance and mechanical properties.
Surface treatments and coatings represent external protection approaches. UV-resistant coatings containing stabilizers can be applied to Nylon 6 products, creating a sacrificial layer that absorbs radiation before it reaches the polymer substrate. Techniques such as plasma treatment can also modify the surface chemistry to improve compatibility with protective additives.
Formulation optimization remains critical for effective stabilization. The concentration, compatibility, and distribution of additives significantly impact protection efficiency. Typically, combinations of different stabilizer types (UV absorbers, HALS, and antioxidants) at carefully balanced ratios provide superior protection compared to single-component systems. Processing conditions must also be controlled to prevent premature consumption of stabilizers during manufacturing.
Recent innovations include reactive stabilizers that chemically bond to the polymer matrix, preventing migration and leaching during the product lifecycle. These covalently bound stabilizers offer promising solutions for applications requiring extended outdoor exposure or frequent cleaning cycles where conventional additives might be gradually lost.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!