Advanced Characterization Techniques For Potassium-Ion Electrode Interfaces
AUG 21, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
K-ion Battery Interface Characterization Background & Objectives
Potassium-ion batteries (PIBs) have emerged as a promising alternative to lithium-ion batteries due to the abundance and low cost of potassium resources. The development of PIBs has gained significant momentum over the past decade, with research focusing on addressing key challenges related to electrode materials, electrolytes, and interfacial phenomena. The electrode-electrolyte interface plays a crucial role in determining battery performance, safety, and longevity, making advanced characterization techniques for these interfaces a critical area of investigation.
The evolution of interface characterization techniques for potassium-ion batteries has followed a trajectory similar to that of lithium-ion batteries, but with unique challenges specific to potassium chemistry. Early research relied primarily on ex-situ techniques, which provided limited insights into the dynamic processes occurring at the electrode interfaces. As the field has matured, in-situ and operando characterization methods have become increasingly important for understanding the complex interfacial reactions in real-time.
The larger ionic radius of potassium (1.38 Å) compared to lithium (0.76 Å) leads to distinct interfacial behaviors, including different solid electrolyte interphase (SEI) formation mechanisms and composition. This fundamental difference necessitates specialized characterization approaches tailored to potassium-ion systems. Traditional techniques developed for lithium-ion batteries often require significant modifications or novel methodologies when applied to PIBs.
Recent advances in spectroscopic, microscopic, and electrochemical techniques have enabled more detailed investigations of potassium-ion electrode interfaces. These include synchrotron-based X-ray techniques, advanced electron microscopy, nuclear magnetic resonance spectroscopy, and various surface-sensitive analytical methods. The integration of computational modeling with experimental characterization has also emerged as a powerful approach for elucidating interfacial phenomena at atomic and molecular levels.
The primary objective of advanced characterization for PIB interfaces is to establish fundamental understanding of the formation, evolution, and degradation mechanisms of the electrode-electrolyte interfaces. This includes identifying the chemical composition and structural properties of the SEI layer, understanding ion transport mechanisms across interfaces, and elucidating the role of interfacial phenomena in capacity fading and cell failure.
Another key goal is to develop standardized protocols for interface characterization that can facilitate comparison across different PIB systems and accelerate materials discovery and optimization. By establishing correlations between interfacial properties and battery performance metrics, researchers aim to design interfaces with enhanced stability, conductivity, and compatibility with potassium-ion chemistry.
Ultimately, these characterization efforts are directed toward enabling the development of high-performance, long-lasting, and safe potassium-ion batteries that can complement or potentially replace lithium-ion technology in specific applications, particularly in large-scale energy storage systems where cost considerations are paramount.
The evolution of interface characterization techniques for potassium-ion batteries has followed a trajectory similar to that of lithium-ion batteries, but with unique challenges specific to potassium chemistry. Early research relied primarily on ex-situ techniques, which provided limited insights into the dynamic processes occurring at the electrode interfaces. As the field has matured, in-situ and operando characterization methods have become increasingly important for understanding the complex interfacial reactions in real-time.
The larger ionic radius of potassium (1.38 Å) compared to lithium (0.76 Å) leads to distinct interfacial behaviors, including different solid electrolyte interphase (SEI) formation mechanisms and composition. This fundamental difference necessitates specialized characterization approaches tailored to potassium-ion systems. Traditional techniques developed for lithium-ion batteries often require significant modifications or novel methodologies when applied to PIBs.
Recent advances in spectroscopic, microscopic, and electrochemical techniques have enabled more detailed investigations of potassium-ion electrode interfaces. These include synchrotron-based X-ray techniques, advanced electron microscopy, nuclear magnetic resonance spectroscopy, and various surface-sensitive analytical methods. The integration of computational modeling with experimental characterization has also emerged as a powerful approach for elucidating interfacial phenomena at atomic and molecular levels.
The primary objective of advanced characterization for PIB interfaces is to establish fundamental understanding of the formation, evolution, and degradation mechanisms of the electrode-electrolyte interfaces. This includes identifying the chemical composition and structural properties of the SEI layer, understanding ion transport mechanisms across interfaces, and elucidating the role of interfacial phenomena in capacity fading and cell failure.
Another key goal is to develop standardized protocols for interface characterization that can facilitate comparison across different PIB systems and accelerate materials discovery and optimization. By establishing correlations between interfacial properties and battery performance metrics, researchers aim to design interfaces with enhanced stability, conductivity, and compatibility with potassium-ion chemistry.
Ultimately, these characterization efforts are directed toward enabling the development of high-performance, long-lasting, and safe potassium-ion batteries that can complement or potentially replace lithium-ion technology in specific applications, particularly in large-scale energy storage systems where cost considerations are paramount.
Market Analysis for K-ion Battery Technologies
The global potassium-ion battery market is experiencing significant growth, driven by increasing demand for sustainable energy storage solutions. Current market valuations indicate a compound annual growth rate of approximately 7-8% between 2023 and 2030, with the market expected to reach substantial commercial viability by mid-decade. This growth trajectory is primarily fueled by the inherent advantages of potassium-ion technology, particularly its cost-effectiveness compared to lithium-ion alternatives.
The abundance of potassium resources represents a key market driver, with potassium being approximately 1000 times more abundant in the Earth's crust than lithium. This abundance translates to potentially lower raw material costs and reduced supply chain vulnerabilities. Market analysis indicates that regions with established battery manufacturing infrastructure, particularly in East Asia, Europe, and North America, are positioning themselves as early adopters of K-ion technology.
From an application perspective, the market for advanced characterization techniques for potassium-ion electrode interfaces is segmented across several sectors. Grid-scale energy storage represents the largest potential market segment, where cost considerations often outweigh energy density requirements. The electric vehicle sector presents a secondary market, particularly for applications where moderate energy density is acceptable, such as urban mobility solutions and commercial fleet vehicles.
Consumer electronics manufacturers are also showing increasing interest in K-ion technology as a potential alternative to lithium-ion batteries, especially for applications where cost sensitivity is high. Market research indicates that approximately 15-20% of current lithium-ion applications could potentially be served by potassium-ion alternatives within the next decade, contingent upon continued improvements in electrode interface characterization and overall performance metrics.
The competitive landscape features both established battery manufacturers expanding their research portfolios and specialized startups focused exclusively on potassium-ion technology. Major battery manufacturers are allocating increasing portions of their R&D budgets toward alternative battery chemistries, including potassium-ion systems, with particular emphasis on advanced characterization techniques for electrode interfaces.
Market barriers include the current technological maturity gap between K-ion and more established battery technologies, as well as the significant capital investment required for manufacturing scale-up. However, the economic advantages of potassium-based systems, particularly in terms of raw material costs, are expected to drive continued market expansion as characterization techniques improve and performance metrics approach those of competing technologies.
The abundance of potassium resources represents a key market driver, with potassium being approximately 1000 times more abundant in the Earth's crust than lithium. This abundance translates to potentially lower raw material costs and reduced supply chain vulnerabilities. Market analysis indicates that regions with established battery manufacturing infrastructure, particularly in East Asia, Europe, and North America, are positioning themselves as early adopters of K-ion technology.
From an application perspective, the market for advanced characterization techniques for potassium-ion electrode interfaces is segmented across several sectors. Grid-scale energy storage represents the largest potential market segment, where cost considerations often outweigh energy density requirements. The electric vehicle sector presents a secondary market, particularly for applications where moderate energy density is acceptable, such as urban mobility solutions and commercial fleet vehicles.
Consumer electronics manufacturers are also showing increasing interest in K-ion technology as a potential alternative to lithium-ion batteries, especially for applications where cost sensitivity is high. Market research indicates that approximately 15-20% of current lithium-ion applications could potentially be served by potassium-ion alternatives within the next decade, contingent upon continued improvements in electrode interface characterization and overall performance metrics.
The competitive landscape features both established battery manufacturers expanding their research portfolios and specialized startups focused exclusively on potassium-ion technology. Major battery manufacturers are allocating increasing portions of their R&D budgets toward alternative battery chemistries, including potassium-ion systems, with particular emphasis on advanced characterization techniques for electrode interfaces.
Market barriers include the current technological maturity gap between K-ion and more established battery technologies, as well as the significant capital investment required for manufacturing scale-up. However, the economic advantages of potassium-based systems, particularly in terms of raw material costs, are expected to drive continued market expansion as characterization techniques improve and performance metrics approach those of competing technologies.
Current Challenges in K-ion Electrode Interface Analysis
Despite significant advancements in potassium-ion battery (PIB) research, the analysis of electrode-electrolyte interfaces presents formidable challenges that impede further development. The larger ionic radius of K+ (1.38 Å) compared to Li+ (0.76 Å) and Na+ (1.02 Å) creates unique interfacial phenomena that cannot be fully characterized using techniques optimized for lithium-ion systems. This fundamental difference necessitates specialized characterization approaches tailored specifically for potassium-ion electrode interfaces.
A primary challenge lies in the high reactivity of potassium metal with conventional electrolytes, resulting in complex and dynamic solid electrolyte interphase (SEI) formations that are difficult to analyze in situ. The SEI in PIBs tends to be thicker and more heterogeneous than in lithium-ion batteries, complicating efforts to obtain clear spectroscopic and microscopic data. Current analytical techniques struggle to capture the rapid evolution of these interfaces during cycling without disrupting the very phenomena being studied.
The characterization of K+ ion transport mechanisms across these interfaces presents another significant hurdle. Traditional electrochemical impedance spectroscopy (EIS) methods often fail to deconvolute the various interfacial processes specific to potassium systems, particularly the distinction between bulk and interfacial transport phenomena. This limitation creates substantial gaps in understanding the rate-limiting steps in K+ ion insertion and extraction processes.
Spatial resolution constraints in current imaging techniques further complicate interface analysis. Even advanced techniques such as transmission electron microscopy (TEM) and scanning electron microscopy (SEM) face challenges in resolving the nanoscale features of PIB interfaces while maintaining the native chemical environment. Sample preparation methods frequently alter the original interface structure, leading to potentially misleading observations.
The detection and quantification of trace elements at these interfaces represent another critical challenge. Conventional surface analysis techniques like X-ray photoelectron spectroscopy (XPS) and time-of-flight secondary ion mass spectrometry (ToF-SIMS) often lack the sensitivity required to detect low-concentration species that may significantly impact interfacial properties in potassium systems.
Furthermore, the correlation between interfacial properties and electrochemical performance remains poorly understood due to limitations in operando characterization capabilities. Current techniques struggle to simultaneously track structural, chemical, and electrochemical changes at the electrode-electrolyte interface during battery operation, particularly under realistic cycling conditions and timeframes relevant to practical applications.
Addressing these challenges requires developing novel characterization methodologies specifically designed for potassium systems, combining multiple complementary techniques, and advancing in situ and operando capabilities that can capture the dynamic nature of these interfaces without perturbing their natural state.
A primary challenge lies in the high reactivity of potassium metal with conventional electrolytes, resulting in complex and dynamic solid electrolyte interphase (SEI) formations that are difficult to analyze in situ. The SEI in PIBs tends to be thicker and more heterogeneous than in lithium-ion batteries, complicating efforts to obtain clear spectroscopic and microscopic data. Current analytical techniques struggle to capture the rapid evolution of these interfaces during cycling without disrupting the very phenomena being studied.
The characterization of K+ ion transport mechanisms across these interfaces presents another significant hurdle. Traditional electrochemical impedance spectroscopy (EIS) methods often fail to deconvolute the various interfacial processes specific to potassium systems, particularly the distinction between bulk and interfacial transport phenomena. This limitation creates substantial gaps in understanding the rate-limiting steps in K+ ion insertion and extraction processes.
Spatial resolution constraints in current imaging techniques further complicate interface analysis. Even advanced techniques such as transmission electron microscopy (TEM) and scanning electron microscopy (SEM) face challenges in resolving the nanoscale features of PIB interfaces while maintaining the native chemical environment. Sample preparation methods frequently alter the original interface structure, leading to potentially misleading observations.
The detection and quantification of trace elements at these interfaces represent another critical challenge. Conventional surface analysis techniques like X-ray photoelectron spectroscopy (XPS) and time-of-flight secondary ion mass spectrometry (ToF-SIMS) often lack the sensitivity required to detect low-concentration species that may significantly impact interfacial properties in potassium systems.
Furthermore, the correlation between interfacial properties and electrochemical performance remains poorly understood due to limitations in operando characterization capabilities. Current techniques struggle to simultaneously track structural, chemical, and electrochemical changes at the electrode-electrolyte interface during battery operation, particularly under realistic cycling conditions and timeframes relevant to practical applications.
Addressing these challenges requires developing novel characterization methodologies specifically designed for potassium systems, combining multiple complementary techniques, and advancing in situ and operando capabilities that can capture the dynamic nature of these interfaces without perturbing their natural state.
State-of-the-Art Characterization Techniques
01 Spectroscopic techniques for electrode interface analysis
Various spectroscopic methods are employed to characterize potassium-ion electrode interfaces, including X-ray photoelectron spectroscopy (XPS), Raman spectroscopy, and Fourier transform infrared spectroscopy (FTIR). These techniques provide valuable information about the chemical composition, bonding states, and structural changes at the electrode-electrolyte interface during cycling. Spectroscopic analysis helps identify the formation and evolution of the solid electrolyte interphase (SEI) layer, which is crucial for understanding the performance and stability of potassium-ion batteries.- Spectroscopic techniques for electrode interface analysis: Various spectroscopic methods are employed to characterize potassium-ion electrode interfaces, including X-ray photoelectron spectroscopy (XPS), Raman spectroscopy, and Fourier transform infrared spectroscopy (FTIR). These techniques provide valuable information about the chemical composition, bonding states, and structural changes at the electrode-electrolyte interface during cycling. Spectroscopic analysis helps in understanding the formation and evolution of the solid electrolyte interphase (SEI) layer, which is crucial for the performance and stability of potassium-ion batteries.
- Microscopy and imaging techniques for interface visualization: Advanced microscopy techniques are utilized to visualize and analyze the morphology and structure of potassium-ion electrode interfaces. These include scanning electron microscopy (SEM), transmission electron microscopy (TEM), atomic force microscopy (AFM), and focused ion beam (FIB) techniques. These methods provide high-resolution images of the interface, revealing features such as surface roughness, porosity, and the presence of defects or cracks. Such visualization is essential for understanding how the interface morphology affects ion transport and battery performance.
- Electrochemical characterization methods: Electrochemical techniques play a vital role in characterizing potassium-ion electrode interfaces. Methods such as electrochemical impedance spectroscopy (EIS), cyclic voltammetry (CV), galvanostatic intermittent titration technique (GITT), and potentiostatic intermittent titration technique (PITT) provide insights into interface resistance, charge transfer kinetics, and ion diffusion processes. These techniques help in evaluating the electrochemical stability of the interface and understanding how it evolves during battery operation, which is crucial for improving battery performance and longevity.
- In-situ and operando characterization approaches: In-situ and operando characterization techniques allow for real-time monitoring of potassium-ion electrode interfaces during battery operation. These approaches include in-situ XRD, in-situ Raman spectroscopy, and operando TEM. By observing the interface changes under actual operating conditions, researchers can gain deeper insights into degradation mechanisms, phase transformations, and the dynamics of the solid electrolyte interphase formation. This real-time information is invaluable for developing strategies to enhance interface stability and battery performance.
- Computational and modeling techniques for interface studies: Computational methods and modeling techniques are employed to complement experimental characterization of potassium-ion electrode interfaces. These include density functional theory (DFT) calculations, molecular dynamics (MD) simulations, and finite element analysis. These approaches help in predicting interface properties, understanding ion transport mechanisms, and elucidating reaction pathways that are difficult to observe experimentally. By combining computational studies with experimental characterization, researchers can develop a more comprehensive understanding of interface phenomena and design improved electrode materials and electrolytes.
02 Microscopy and imaging techniques for interface visualization
Advanced microscopy techniques are utilized to visualize and analyze the morphology and structure of potassium-ion electrode interfaces. These include scanning electron microscopy (SEM), transmission electron microscopy (TEM), atomic force microscopy (AFM), and focused ion beam (FIB) techniques. These imaging methods provide high-resolution visualization of interface features, allowing researchers to observe dendrite formation, surface roughness, and structural changes during battery operation. Such observations are essential for developing strategies to improve interface stability and battery performance.Expand Specific Solutions03 Electrochemical characterization methods
Electrochemical techniques play a vital role in characterizing potassium-ion electrode interfaces. Methods such as electrochemical impedance spectroscopy (EIS), cyclic voltammetry (CV), galvanostatic intermittent titration technique (GITT), and potentiostatic intermittent titration technique (PITT) provide insights into interface resistance, charge transfer kinetics, and ion diffusion processes. These techniques help monitor the formation and growth of interfacial layers, assess electrode stability, and evaluate the impact of different electrolyte compositions on interface properties during battery cycling.Expand Specific Solutions04 In-situ and operando characterization approaches
In-situ and operando characterization techniques enable real-time monitoring of potassium-ion electrode interfaces during battery operation. These approaches include in-situ X-ray diffraction (XRD), in-situ Raman spectroscopy, operando atomic force microscopy, and synchrotron-based techniques. By observing interface phenomena as they occur under actual operating conditions, researchers can gain deeper insights into degradation mechanisms, phase transformations, and interfacial reactions that affect battery performance and longevity.Expand Specific Solutions05 Computational and modeling techniques for interface studies
Computational methods and modeling approaches are increasingly used to study potassium-ion electrode interfaces at the atomic and molecular levels. Density functional theory (DFT) calculations, molecular dynamics (MD) simulations, and machine learning algorithms help predict interface properties, reaction mechanisms, and degradation pathways. These computational techniques complement experimental methods by providing theoretical insights into interface phenomena that may be difficult to observe directly, guiding the design of improved electrode materials and electrolyte formulations.Expand Specific Solutions
Leading Research Groups and Industrial Players
The potassium-ion electrode interface characterization technology landscape is currently in an early growth phase, with academic institutions leading fundamental research while commercial entities focus on practical applications. The market is expanding as potassium-ion batteries emerge as alternatives to lithium-ion technologies, driven by cost advantages and resource abundance. Universities including Tsinghua, Zhejiang, and Tianjin are pioneering advanced characterization methods, while companies like Waters Technology Corp. and Micromass UK Ltd. contribute specialized analytical instrumentation. Research institutions such as Draper Laboratory provide interdisciplinary expertise. The ecosystem demonstrates varying technical maturity, with academic research at TRL 3-4 and commercial applications approaching TRL 6, creating opportunities for industry-academia partnerships to accelerate commercialization of these critical characterization techniques.
Zhejiang University
Technical Solution: Zhejiang University has developed multi-modal characterization techniques for potassium-ion electrode interfaces combining in-situ X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and advanced electron microscopy. Their approach integrates time-resolved spectroscopic methods with electrochemical measurements to monitor interface evolution during cycling. They've pioneered the use of synchrotron-based techniques to capture the dynamic formation of solid-electrolyte interphase (SEI) layers on potassium-ion electrodes with nanoscale resolution. Their research has revealed critical insights into the unique degradation mechanisms specific to K-ion systems, particularly the larger ionic radius effects compared to Li-ion systems. The university has also developed specialized sample preparation protocols that preserve the native state of these highly reactive interfaces for more accurate characterization.
Strengths: Superior integration of multiple characterization techniques providing comprehensive interface analysis; strong expertise in synchrotron-based methods. Weakness: Their techniques often require specialized equipment with limited accessibility, potentially limiting widespread adoption in industrial applications.
Tianjin University
Technical Solution: Tianjin University has developed an innovative suite of operando characterization techniques specifically optimized for potassium-ion electrode interfaces. Their approach combines electrochemical atomic force microscopy (EC-AFM) with in-situ Raman spectroscopy to simultaneously track morphological changes and chemical transformations at electrode surfaces during cycling. They've created custom-designed electrochemical cells that enable real-time observation of interface phenomena while maintaining controlled environments to prevent contamination. Their research has particularly focused on understanding the mechanical stress and strain effects unique to K-ion intercalation, which causes more significant volume changes than Li-ion systems. The university has also pioneered the use of isotope labeling techniques combined with secondary ion mass spectrometry (SIMS) to track potassium ion transport pathways across interfaces with unprecedented precision.
Strengths: Exceptional capability to correlate mechanical and chemical changes at interfaces in real-time; innovative cell designs that enable multiple simultaneous measurements. Weakness: Their techniques require complex data integration and analysis workflows that demand significant expertise to implement effectively.
Key Scientific Breakthroughs in Interface Analysis
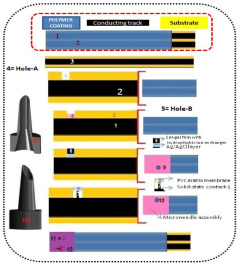
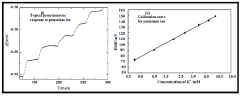
A process for making solid-state ion sensor for on-chip determination of potassium ion in body fluid
PatentInactiveIN201811041978A
Innovation
- Development of all-solid-state potentiometric sensors using functional alkoxysilane-derived nanodispersions for creating ion-selective membranes and reference electrodes, integrated within hollow microneedle assemblies, allowing for transdermal sensing of potassium ions without internal filling solutions and enabling miniaturization and wearability.
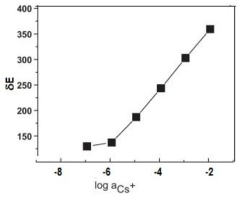
A process for making all solid-state cesium ion sensor
PatentInactiveIN201911048932A
Innovation
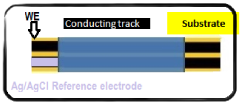
- The development of a solid-state cesium ion sensor using a cesium green ionophore with a solid-state Ag/AgCl reference electrode, where a colloidal suspension of siloxane-polyindole-gold nanoparticles and PVC matrix membranes are used to create a non-specific ion exchanger, eliminating the need for internal filling solutions and enabling selective cesium ion sensing and removal in any given sample.
Safety and Performance Standards for K-ion Batteries
The development of potassium-ion batteries necessitates robust safety and performance standards to ensure their reliable integration into various applications. Currently, the standards specifically designed for K-ion batteries remain in nascent stages, with most regulatory frameworks adapting existing lithium-ion battery standards. This gap presents both challenges and opportunities for establishing comprehensive guidelines tailored to the unique characteristics of potassium-ion systems.
Safety standards must address the distinct reactivity profiles of potassium electrodes, which differ significantly from lithium counterparts. Potassium's higher reactivity with electrolytes demands more stringent safety protocols, particularly regarding thermal runaway prevention and management. Testing procedures need modification to account for the larger ionic radius of potassium and its impact on electrode interface stability during cycling.
Performance standardization requires metrics specifically calibrated for K-ion technology. While energy density benchmarks may initially be lower than those established for Li-ion systems, standards should emphasize potassium's advantages in power capability, cycle life at high discharge rates, and performance at extreme temperatures. The development of Coulombic efficiency standards must consider the unique solid electrolyte interphase (SEI) formation dynamics at potassium electrode interfaces.
International standardization bodies, including IEC, ISO, and UL, have begun preliminary discussions on K-ion battery standards. These efforts focus on establishing testing protocols for evaluating electrode interface stability, electrolyte compatibility, and long-term performance degradation mechanisms. Accelerated aging tests require recalibration to accurately predict K-ion battery lifespans in various application scenarios.
Environmental and sustainability standards represent another critical dimension, with emphasis on the recyclability of potassium-based electrode materials and the reduced environmental impact compared to lithium extraction. Standards should incorporate life cycle assessment methodologies specific to potassium resource utilization and processing.
For advanced characterization of electrode interfaces, standards must define protocols for in-situ and operando techniques that can accurately monitor interfacial phenomena during battery operation. These include standardized approaches for X-ray photoelectron spectroscopy (XPS), transmission electron microscopy (TEM), and electrochemical impedance spectroscopy (EIS) specifically optimized for potassium systems.
The establishment of comprehensive safety and performance standards will accelerate K-ion battery commercialization by providing manufacturers, researchers, and regulatory bodies with consistent evaluation frameworks. This standardization will ultimately facilitate market acceptance and integration of potassium-ion technology across diverse applications from grid storage to electric mobility.
Safety standards must address the distinct reactivity profiles of potassium electrodes, which differ significantly from lithium counterparts. Potassium's higher reactivity with electrolytes demands more stringent safety protocols, particularly regarding thermal runaway prevention and management. Testing procedures need modification to account for the larger ionic radius of potassium and its impact on electrode interface stability during cycling.
Performance standardization requires metrics specifically calibrated for K-ion technology. While energy density benchmarks may initially be lower than those established for Li-ion systems, standards should emphasize potassium's advantages in power capability, cycle life at high discharge rates, and performance at extreme temperatures. The development of Coulombic efficiency standards must consider the unique solid electrolyte interphase (SEI) formation dynamics at potassium electrode interfaces.
International standardization bodies, including IEC, ISO, and UL, have begun preliminary discussions on K-ion battery standards. These efforts focus on establishing testing protocols for evaluating electrode interface stability, electrolyte compatibility, and long-term performance degradation mechanisms. Accelerated aging tests require recalibration to accurately predict K-ion battery lifespans in various application scenarios.
Environmental and sustainability standards represent another critical dimension, with emphasis on the recyclability of potassium-based electrode materials and the reduced environmental impact compared to lithium extraction. Standards should incorporate life cycle assessment methodologies specific to potassium resource utilization and processing.
For advanced characterization of electrode interfaces, standards must define protocols for in-situ and operando techniques that can accurately monitor interfacial phenomena during battery operation. These include standardized approaches for X-ray photoelectron spectroscopy (XPS), transmission electron microscopy (TEM), and electrochemical impedance spectroscopy (EIS) specifically optimized for potassium systems.
The establishment of comprehensive safety and performance standards will accelerate K-ion battery commercialization by providing manufacturers, researchers, and regulatory bodies with consistent evaluation frameworks. This standardization will ultimately facilitate market acceptance and integration of potassium-ion technology across diverse applications from grid storage to electric mobility.
Environmental Impact and Sustainability Considerations
The development of potassium-ion batteries represents a significant advancement in sustainable energy storage technologies, particularly as an alternative to lithium-ion systems. When evaluating advanced characterization techniques for potassium-ion electrode interfaces, environmental impact and sustainability considerations must be thoroughly examined to ensure these emerging technologies align with global sustainability goals.
Potassium-ion battery systems offer inherent environmental advantages due to the abundance of potassium resources in the Earth's crust, approximately 900 times more abundant than lithium. This abundance translates to reduced mining impacts, lower resource depletion concerns, and potentially more geographically distributed supply chains that minimize transportation-related carbon emissions.
Characterization techniques themselves carry varying environmental footprints. Electron microscopy methods, while providing crucial interface insights, require significant energy inputs and often utilize environmentally sensitive chemicals for sample preparation. In contrast, spectroscopic techniques like Raman and FTIR generally present lower environmental impacts. Life cycle assessment (LCA) studies indicate that advanced synchrotron-based characterization methods, despite their high energy consumption, may ultimately contribute to sustainability by enabling more efficient battery designs.
The environmental implications of electrolyte systems used in potassium-ion batteries deserve particular attention. Many advanced characterization techniques have revealed that conventional organic electrolytes pose significant environmental and safety concerns. Recent research utilizing in-situ characterization has accelerated the development of water-based and bio-derived electrolyte systems that demonstrate reduced toxicity and improved biodegradability while maintaining acceptable electrochemical performance at the electrode interface.
Recycling considerations must be integrated into characterization research programs. Studies employing techniques such as X-ray absorption spectroscopy and neutron diffraction have provided critical insights into electrode interface degradation mechanisms, informing the development of more recyclable electrode materials and battery designs. These techniques help identify potential toxic byproducts formed during cycling and end-of-life processing.
Carbon footprint reduction strategies for potassium-ion battery production have been informed by interface characterization studies. Advanced techniques have enabled the optimization of low-temperature synthesis routes and aqueous processing methods that significantly reduce energy consumption and hazardous waste generation during manufacturing. Quantitative analyses suggest potential carbon emission reductions of 35-45% compared to conventional lithium-ion battery production processes.
The sustainability benefits of potassium-ion systems can only be fully realized through continued advancement of characterization techniques that enable longer cycle life and improved safety. Recent interface studies using operando techniques have contributed to extending cycle life from hundreds to thousands of cycles, dramatically improving the lifetime environmental profile of these energy storage systems.
Potassium-ion battery systems offer inherent environmental advantages due to the abundance of potassium resources in the Earth's crust, approximately 900 times more abundant than lithium. This abundance translates to reduced mining impacts, lower resource depletion concerns, and potentially more geographically distributed supply chains that minimize transportation-related carbon emissions.
Characterization techniques themselves carry varying environmental footprints. Electron microscopy methods, while providing crucial interface insights, require significant energy inputs and often utilize environmentally sensitive chemicals for sample preparation. In contrast, spectroscopic techniques like Raman and FTIR generally present lower environmental impacts. Life cycle assessment (LCA) studies indicate that advanced synchrotron-based characterization methods, despite their high energy consumption, may ultimately contribute to sustainability by enabling more efficient battery designs.
The environmental implications of electrolyte systems used in potassium-ion batteries deserve particular attention. Many advanced characterization techniques have revealed that conventional organic electrolytes pose significant environmental and safety concerns. Recent research utilizing in-situ characterization has accelerated the development of water-based and bio-derived electrolyte systems that demonstrate reduced toxicity and improved biodegradability while maintaining acceptable electrochemical performance at the electrode interface.
Recycling considerations must be integrated into characterization research programs. Studies employing techniques such as X-ray absorption spectroscopy and neutron diffraction have provided critical insights into electrode interface degradation mechanisms, informing the development of more recyclable electrode materials and battery designs. These techniques help identify potential toxic byproducts formed during cycling and end-of-life processing.
Carbon footprint reduction strategies for potassium-ion battery production have been informed by interface characterization studies. Advanced techniques have enabled the optimization of low-temperature synthesis routes and aqueous processing methods that significantly reduce energy consumption and hazardous waste generation during manufacturing. Quantitative analyses suggest potential carbon emission reductions of 35-45% compared to conventional lithium-ion battery production processes.
The sustainability benefits of potassium-ion systems can only be fully realized through continued advancement of characterization techniques that enable longer cycle life and improved safety. Recent interface studies using operando techniques have contributed to extending cycle life from hundreds to thousands of cycles, dramatically improving the lifetime environmental profile of these energy storage systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!