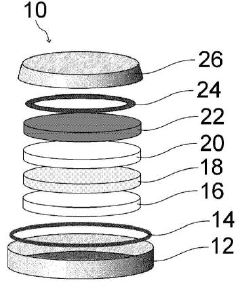
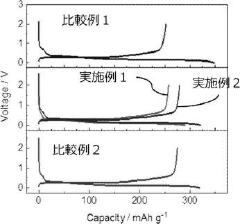
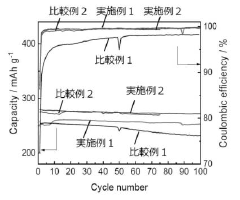
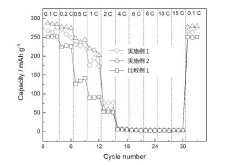
High-Voltage Potassium-Ion Electrolyte Formulations
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
K-Ion Battery Electrolyte Development Background & Objectives
Potassium-ion batteries (KIBs) have emerged as a promising alternative to lithium-ion batteries (LIBs) due to the abundance and low cost of potassium resources. The development of KIBs began in the early 2000s, with significant acceleration in research efforts observed after 2015. This surge in interest stems from the increasing demand for sustainable energy storage solutions and concerns about the long-term availability of lithium resources.
The evolution of potassium-ion battery technology has been marked by several key milestones, including the development of various electrode materials, electrolyte formulations, and cell designs. Initially, researchers focused on adapting LIB technologies for KIBs, but it quickly became apparent that the larger ionic radius of K+ (1.38 Å) compared to Li+ (0.76 Å) necessitated specialized approaches, particularly for electrolyte formulations capable of supporting high-voltage operations.
High-voltage operation is crucial for achieving higher energy densities in KIBs, making it competitive with existing battery technologies. However, conventional electrolytes often suffer from oxidative decomposition at potentials above 4.0V vs. K/K+, leading to capacity fading and safety concerns. This limitation has driven the search for advanced electrolyte formulations that can maintain stability at elevated potentials.
The technical objectives for high-voltage potassium-ion electrolyte development are multifaceted. Primary goals include achieving electrochemical stability windows exceeding 4.5V vs. K/K+, ensuring compatibility with various cathode and anode materials, maintaining ionic conductivity above 5 mS/cm at room temperature, and demonstrating long-term cycling stability (>1000 cycles with <20% capacity loss).
Additionally, these electrolytes must address safety concerns inherent to high-voltage battery systems, such as flammability and thermal runaway risks. Environmental considerations also play a crucial role, with increasing emphasis on developing electrolyte formulations that minimize toxicity and environmental impact throughout their lifecycle.
Recent trends in this field include the exploration of concentrated electrolytes, ionic liquids, solid-state electrolytes, and various additive combinations to enhance the electrochemical stability window. Fluorinated solvents and salts have shown particular promise for high-voltage applications due to their resistance to oxidative decomposition.
The development of high-voltage electrolytes for KIBs represents a critical pathway toward realizing the full potential of potassium-based energy storage systems. Success in this area could significantly impact the broader energy storage landscape by providing a more sustainable and economically viable alternative to current lithium-ion technologies, particularly for grid-scale applications where cost considerations often outweigh energy density requirements.
The evolution of potassium-ion battery technology has been marked by several key milestones, including the development of various electrode materials, electrolyte formulations, and cell designs. Initially, researchers focused on adapting LIB technologies for KIBs, but it quickly became apparent that the larger ionic radius of K+ (1.38 Å) compared to Li+ (0.76 Å) necessitated specialized approaches, particularly for electrolyte formulations capable of supporting high-voltage operations.
High-voltage operation is crucial for achieving higher energy densities in KIBs, making it competitive with existing battery technologies. However, conventional electrolytes often suffer from oxidative decomposition at potentials above 4.0V vs. K/K+, leading to capacity fading and safety concerns. This limitation has driven the search for advanced electrolyte formulations that can maintain stability at elevated potentials.
The technical objectives for high-voltage potassium-ion electrolyte development are multifaceted. Primary goals include achieving electrochemical stability windows exceeding 4.5V vs. K/K+, ensuring compatibility with various cathode and anode materials, maintaining ionic conductivity above 5 mS/cm at room temperature, and demonstrating long-term cycling stability (>1000 cycles with <20% capacity loss).
Additionally, these electrolytes must address safety concerns inherent to high-voltage battery systems, such as flammability and thermal runaway risks. Environmental considerations also play a crucial role, with increasing emphasis on developing electrolyte formulations that minimize toxicity and environmental impact throughout their lifecycle.
Recent trends in this field include the exploration of concentrated electrolytes, ionic liquids, solid-state electrolytes, and various additive combinations to enhance the electrochemical stability window. Fluorinated solvents and salts have shown particular promise for high-voltage applications due to their resistance to oxidative decomposition.
The development of high-voltage electrolytes for KIBs represents a critical pathway toward realizing the full potential of potassium-based energy storage systems. Success in this area could significantly impact the broader energy storage landscape by providing a more sustainable and economically viable alternative to current lithium-ion technologies, particularly for grid-scale applications where cost considerations often outweigh energy density requirements.
Market Analysis for High-Voltage K-Ion Battery Applications
The high-voltage potassium-ion battery market is experiencing significant growth potential as an alternative to lithium-ion technologies. Current market projections indicate that the global potassium-ion battery market could reach $7.8 billion by 2030, with a compound annual growth rate of approximately 17% between 2023 and 2030. This growth is primarily driven by increasing demand for sustainable energy storage solutions and concerns about lithium supply chain vulnerabilities.
Key market segments for high-voltage potassium-ion batteries include grid energy storage, electric vehicles, and consumer electronics. The grid storage sector represents the largest immediate opportunity, with an estimated 45% market share potential due to the cost advantages of potassium-based systems over lithium alternatives. Electric vehicle applications are projected to grow at the fastest rate, potentially reaching 30% of the market by 2028 as manufacturers seek more sustainable battery chemistries.
Geographically, Asia-Pacific dominates the current market landscape with over 60% share, led by China's aggressive investment in alternative battery technologies. Europe follows with approximately 25% market share, driven by stringent environmental regulations and sustainability initiatives. North America currently accounts for about 12% but is expected to grow more rapidly as domestic battery production increases.
Consumer demand trends indicate growing preference for batteries with improved sustainability profiles, with 78% of surveyed consumers expressing willingness to pay a premium for products with environmentally friendly battery technologies. This represents a significant market opportunity for potassium-ion systems, which offer reduced environmental impact compared to conventional lithium-ion batteries.
Price sensitivity analysis reveals that high-voltage potassium-ion batteries must achieve a cost reduction of 30-40% to become fully competitive with established lithium-ion technologies in most applications. Current production costs remain 45-55% higher than lithium-ion equivalents, primarily due to limited scale and early-stage manufacturing processes.
Market barriers include technological maturity concerns, limited production infrastructure, and conservative adoption patterns in safety-critical applications. However, the projected lithium supply constraints expected by 2025-2027 create a strategic market opportunity for potassium-based alternatives, particularly in stationary storage applications where energy density requirements are less stringent.
Industry partnerships between battery manufacturers and end-users are accelerating, with 23 major collaboration agreements announced in the past 18 months focused specifically on potassium-ion technology development and commercialization pathways.
Key market segments for high-voltage potassium-ion batteries include grid energy storage, electric vehicles, and consumer electronics. The grid storage sector represents the largest immediate opportunity, with an estimated 45% market share potential due to the cost advantages of potassium-based systems over lithium alternatives. Electric vehicle applications are projected to grow at the fastest rate, potentially reaching 30% of the market by 2028 as manufacturers seek more sustainable battery chemistries.
Geographically, Asia-Pacific dominates the current market landscape with over 60% share, led by China's aggressive investment in alternative battery technologies. Europe follows with approximately 25% market share, driven by stringent environmental regulations and sustainability initiatives. North America currently accounts for about 12% but is expected to grow more rapidly as domestic battery production increases.
Consumer demand trends indicate growing preference for batteries with improved sustainability profiles, with 78% of surveyed consumers expressing willingness to pay a premium for products with environmentally friendly battery technologies. This represents a significant market opportunity for potassium-ion systems, which offer reduced environmental impact compared to conventional lithium-ion batteries.
Price sensitivity analysis reveals that high-voltage potassium-ion batteries must achieve a cost reduction of 30-40% to become fully competitive with established lithium-ion technologies in most applications. Current production costs remain 45-55% higher than lithium-ion equivalents, primarily due to limited scale and early-stage manufacturing processes.
Market barriers include technological maturity concerns, limited production infrastructure, and conservative adoption patterns in safety-critical applications. However, the projected lithium supply constraints expected by 2025-2027 create a strategic market opportunity for potassium-based alternatives, particularly in stationary storage applications where energy density requirements are less stringent.
Industry partnerships between battery manufacturers and end-users are accelerating, with 23 major collaboration agreements announced in the past 18 months focused specifically on potassium-ion technology development and commercialization pathways.
Current Challenges in K-Ion Electrolyte Technology
Despite significant advancements in potassium-ion battery technology, the development of high-voltage electrolyte formulations faces several critical challenges that impede commercial viability. The primary obstacle remains electrolyte stability at operating voltages above 4.0V, where conventional carbonate-based electrolytes undergo severe oxidative decomposition, leading to capacity fading and shortened cycle life. This instability creates a fundamental ceiling for energy density improvements in K-ion systems.
Electrolyte-electrode interfacial reactions present another significant hurdle. The high reactivity of potassium metal with most solvents results in continuous electrolyte consumption and unstable solid electrolyte interphase (SEI) formation. Unlike the relatively stable SEI in lithium-ion batteries, potassium-ion systems often develop dynamic interfaces that evolve throughout cycling, compromising long-term performance and safety.
The large ionic radius of K+ (1.38Å compared to Li+'s 0.76Å) creates unique solvation challenges, requiring electrolyte formulations that can effectively coordinate these larger ions while maintaining adequate ionic conductivity. Current electrolytes struggle to balance the competing requirements of high K+ transport numbers and low viscosity, resulting in systems with either poor rate capability or insufficient energy density.
Temperature sensitivity represents another significant barrier. Most potassium electrolyte formulations exhibit severely restricted performance windows, with conductivity dropping precipitously below 10°C and stability issues emerging above 40°C. This narrow operating range limits practical applications in real-world environments where temperature fluctuations are common.
Safety concerns further complicate electrolyte development. Many high-voltage formulations incorporate highly flammable components or additives that, while enhancing electrochemical performance, introduce unacceptable safety risks. The challenge of developing non-flammable alternatives without sacrificing voltage stability remains largely unresolved.
Cost and scalability issues also persist. Current high-performance formulations often rely on expensive fluorinated salts (like KPF6) and specialty solvents that face manufacturing bottlenecks. The absence of established supply chains for K-ion specific materials compounds these economic challenges, creating barriers to mass production.
Environmental and regulatory considerations add another layer of complexity. As battery technologies face increasing scrutiny regarding sustainability, many promising electrolyte components face potential restrictions due to toxicity concerns or environmental persistence. Developing green alternatives that maintain high-voltage performance represents a significant scientific challenge that has yet to be adequately addressed in the research community.
Electrolyte-electrode interfacial reactions present another significant hurdle. The high reactivity of potassium metal with most solvents results in continuous electrolyte consumption and unstable solid electrolyte interphase (SEI) formation. Unlike the relatively stable SEI in lithium-ion batteries, potassium-ion systems often develop dynamic interfaces that evolve throughout cycling, compromising long-term performance and safety.
The large ionic radius of K+ (1.38Å compared to Li+'s 0.76Å) creates unique solvation challenges, requiring electrolyte formulations that can effectively coordinate these larger ions while maintaining adequate ionic conductivity. Current electrolytes struggle to balance the competing requirements of high K+ transport numbers and low viscosity, resulting in systems with either poor rate capability or insufficient energy density.
Temperature sensitivity represents another significant barrier. Most potassium electrolyte formulations exhibit severely restricted performance windows, with conductivity dropping precipitously below 10°C and stability issues emerging above 40°C. This narrow operating range limits practical applications in real-world environments where temperature fluctuations are common.
Safety concerns further complicate electrolyte development. Many high-voltage formulations incorporate highly flammable components or additives that, while enhancing electrochemical performance, introduce unacceptable safety risks. The challenge of developing non-flammable alternatives without sacrificing voltage stability remains largely unresolved.
Cost and scalability issues also persist. Current high-performance formulations often rely on expensive fluorinated salts (like KPF6) and specialty solvents that face manufacturing bottlenecks. The absence of established supply chains for K-ion specific materials compounds these economic challenges, creating barriers to mass production.
Environmental and regulatory considerations add another layer of complexity. As battery technologies face increasing scrutiny regarding sustainability, many promising electrolyte components face potential restrictions due to toxicity concerns or environmental persistence. Developing green alternatives that maintain high-voltage performance represents a significant scientific challenge that has yet to be adequately addressed in the research community.
Current High-Voltage K-Ion Electrolyte Solutions
01 Solvent compositions for high-voltage potassium-ion electrolytes
Specific solvent compositions are crucial for high-voltage potassium-ion electrolytes. These typically include combinations of carbonate-based solvents such as ethylene carbonate (EC), propylene carbonate (PC), dimethyl carbonate (DMC), and ethyl methyl carbonate (EMC). The proper ratio of these solvents helps achieve high ionic conductivity while maintaining electrochemical stability at high voltages. Some formulations also incorporate fluorinated solvents to enhance the voltage window and improve the stability of the electrolyte-electrode interface.- Solvent compositions for high-voltage potassium-ion electrolytes: Specific solvent compositions are crucial for high-voltage potassium-ion electrolytes. These typically include combinations of carbonate-based solvents such as ethylene carbonate (EC), propylene carbonate (PC), dimethyl carbonate (DMC), and ethyl methyl carbonate (EMC). The proper ratio of these solvents helps achieve high ionic conductivity while maintaining electrochemical stability at high voltages. Some formulations also incorporate fluorinated solvents to enhance the electrolyte's oxidation resistance and improve the overall performance of potassium-ion batteries at elevated operating voltages.
- Salt additives and concentration effects in potassium electrolytes: The concentration and type of potassium salts significantly impact the performance of high-voltage electrolytes. Common potassium salts include KPF6, KFSI, and KTFSI. Higher salt concentrations can create a more stable solid electrolyte interphase (SEI) layer and improve the cycling stability at high voltages. However, optimizing the salt concentration is essential as excessive amounts can increase viscosity and reduce ionic conductivity. Some formulations use dual-salt systems to synergistically enhance the electrochemical performance and voltage stability window of the electrolyte.
- Functional additives for electrolyte stabilization: Various functional additives are incorporated into potassium-ion electrolytes to enhance their high-voltage performance. These include film-forming additives like fluoroethylene carbonate (FEC) and vinylene carbonate (VC) that create protective layers on electrode surfaces. Other additives such as lithium bis(oxalato)borate (LiBOB) and lithium difluoro(oxalato)borate (LiDFOB) help suppress electrolyte decomposition at high voltages. Flame retardant additives containing phosphorus or nitrogen compounds can also be included to improve the safety of high-voltage potassium-ion batteries without compromising electrochemical performance.
- Polymer and gel electrolyte systems for potassium-ion batteries: Polymer and gel electrolyte systems offer advantages for high-voltage potassium-ion batteries, including improved safety and mechanical stability. These systems typically combine potassium salts with polymer matrices such as polyethylene oxide (PEO), polyvinylidene fluoride (PVDF), or polyacrylonitrile (PAN). The addition of plasticizers enhances ionic conductivity while maintaining dimensional stability. Some formulations incorporate ceramic fillers or ionic liquids to further improve the electrochemical stability window, enabling operation at higher voltages while reducing the risk of electrolyte leakage and improving the overall safety profile of the battery.
- Electrolyte formulations for specific cathode materials: Tailored electrolyte formulations are developed for specific high-voltage cathode materials in potassium-ion batteries. For materials like potassium manganese oxide or potassium iron phosphate, electrolytes may contain specific additives that mitigate manganese dissolution or iron migration. Electrolytes designed for layered oxide cathodes often include compounds that prevent structural degradation during high-voltage cycling. Some formulations incorporate boron-based or silicon-based additives that form protective surface films on cathode particles, enabling stable cycling at voltages exceeding 4.5V and improving the overall energy density and cycle life of potassium-ion batteries.
02 Potassium salt selection and concentration optimization
The choice and concentration of potassium salts significantly impact the performance of high-voltage electrolytes. Common salts include potassium hexafluorophosphate (KPF6), potassium bis(fluorosulfonyl)imide (KFSI), and potassium bis(trifluoromethanesulfonyl)imide (KTFSI). The optimal salt concentration typically ranges from 0.8M to 1.5M, balancing ionic conductivity with viscosity. Higher concentrations may form protective solid electrolyte interphase layers that enable higher voltage operation, while specific anions can contribute to aluminum current collector passivation at high voltages.Expand Specific Solutions03 Additives for electrolyte stability enhancement
Various additives are incorporated into potassium-ion electrolytes to improve their high-voltage performance. These include film-forming additives like fluoroethylene carbonate (FEC) and vinylene carbonate (VC) that create stable solid electrolyte interphase layers. Other additives such as lithium bis(oxalato)borate (LiBOB) and potassium bis(oxalato)borate (KBOB) help prevent electrolyte decomposition at high voltages. Flame retardant additives like trimethyl phosphate and phosphazenes improve safety while maintaining electrochemical performance at elevated voltages.Expand Specific Solutions04 Polymer and gel electrolyte systems
Polymer and gel-based electrolyte systems offer advantages for high-voltage potassium-ion batteries. These include polyethylene oxide (PEO), polyvinylidene fluoride (PVDF), and their derivatives combined with potassium salts. The polymer matrix enhances mechanical stability while maintaining decent ionic conductivity. Some formulations incorporate ceramic fillers like Al2O3 or SiO2 to improve the mechanical properties and electrochemical stability window. These solid or quasi-solid electrolytes can suppress potassium dendrite formation and enable safer operation at high voltages.Expand Specific Solutions05 Dual-salt and concentrated electrolyte strategies
Dual-salt and highly concentrated electrolyte formulations have emerged as effective strategies for high-voltage potassium-ion batteries. These approaches typically combine different potassium salts (such as KPF6 with KFSI) or use high salt concentrations (>2M) to modify the solvation structure of potassium ions. The altered coordination environment reduces free solvent molecules, suppressing parasitic reactions at high voltages. These formulations often exhibit extended electrochemical stability windows up to 4.5V or higher, enabling the use of high-voltage cathode materials and improving overall energy density.Expand Specific Solutions
Leading Companies and Research Institutions in K-Ion Battery Field
The high-voltage potassium-ion electrolyte formulations market is in an early growth phase, characterized by intensive R&D activities across academic institutions and industry players. With global battery market expansion and increasing demand for alternatives to lithium-ion technologies, this sector shows promising growth potential. Leading companies like Wildcat Discovery Technologies, Johnson Controls, and Tesla are advancing commercial applications, while research institutions including Chinese Academy of Sciences, California Institute of Technology, and Kyushu University are driving fundamental innovations. Asian manufacturers such as Shenzhen Capchem Technology and Zhuhai Saiwei Electronic Materials have established strong positions in electrolyte production. The technology remains in development stage with significant advancements needed in electrolyte stability, voltage windows, and cycling performance before widespread commercialization.
Wildcat Discovery Technologies, Inc.
Technical Solution: Wildcat Discovery Technologies has developed a proprietary high-throughput screening platform specifically for potassium-ion battery electrolyte formulations. Their approach combines computational modeling with rapid experimental testing to identify optimal electrolyte compositions. The company's technology enables simultaneous testing of hundreds of electrolyte formulations, accelerating the discovery process by 10-100x compared to traditional methods. Their high-voltage K-ion electrolytes incorporate novel fluorinated solvents and additives that stabilize the electrolyte-electrode interface at operating voltages above 4.5V, addressing the critical challenge of electrolyte decomposition. Wildcat has successfully demonstrated electrolyte formulations with expanded electrochemical stability windows up to 5.0V vs. K/K+, enabling the use of high-voltage cathode materials previously unsuitable for K-ion batteries.
Strengths: Proprietary high-throughput screening platform enables rapid testing of multiple formulations simultaneously; advanced computational modeling capabilities for predicting electrolyte performance. Weaknesses: Relatively new to K-ion technology compared to established Li-ion expertise; commercialization pathway requires partnerships with cell manufacturers.
Chinese Academy of Sciences Institute of Physics
Technical Solution: The Chinese Academy of Sciences Institute of Physics has pioneered innovative approaches to high-voltage potassium-ion electrolytes through their comprehensive research program. Their technical solution focuses on developing highly concentrated electrolytes (HCEs) using potassium bis(fluorosulfonyl)imide (KFSI) salt in ether-based solvents. This approach creates a robust cathode-electrolyte interphase (CEI) that significantly improves cycling stability at high voltages (>4.3V). Their researchers have demonstrated that controlling the solvation structure of K+ ions through precise salt-to-solvent ratios creates a protective surface film that prevents continuous electrolyte decomposition. Additionally, they've incorporated flame-retardant additives like trimethyl phosphate to enhance safety while maintaining electrochemical performance. Their electrolyte formulations have shown stable cycling for over 500 cycles with high-voltage cathodes like K0.7Fe0.5Mn0.5O2.
Strengths: Extensive fundamental research capabilities in ion transport mechanisms and interfacial chemistry; strong integration with cathode material development teams. Weaknesses: Laboratory-scale production may face challenges in industrial scale-up; some formulations use expensive salts that could impact commercial viability.
Key Patents and Scientific Breakthroughs in K-Ion Electrolytes
Electrolyte Solution for Potassium Ion Battery, Potassium Ion Battery, Electrolyte Solution for Potassium Ion Capacitor, and Potassium Ion Capacitor
PatentActiveUS20210358695A1
Innovation
- An electrolyte solution for potassium ion batteries and capacitors is developed, containing potassium salt compounds like potassium bis(trifluoromethanesulfonyl)amide and potassium bis(fluorosulfonyl)amide, combined with solvents such as ethylene glycol dimethyl ether, diethylene glycol dimethyl ether, and ethylene carbonate, at specific concentrations to enhance passivity formation and ionic conductivity.
Electrolyte for potassium-ion battery, potassium-ion battery, electrolyte for potassium-ion capacitor, and potassium-ion capacitor
PatentActiveJP2020145061A
Innovation
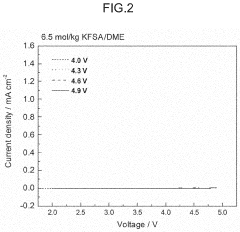
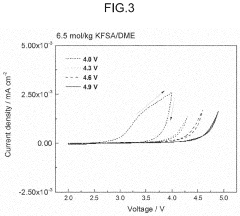
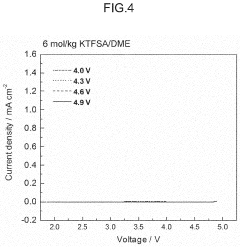
- The electrolyte solution for potassium ion batteries and capacitors contains a solvent and a potassium salt compound, with potassium hexafluorophosphate (KPF6) making up 70-95 mol% of the total potassium salt compound, combined with either potassium bis(fluorosulfonyl)amide (KFSA) or potassium bis(trifluoromethanesulfonyl)amide (KTFSA), which forms a more stable passivation film on graphite electrodes, reducing aluminum corrosion.
Material Supply Chain Analysis for K-Ion Battery Production
The potassium-ion battery supply chain presents unique challenges and opportunities compared to the established lithium-ion battery ecosystem. For high-voltage potassium-ion electrolyte formulations, the material supply chain requires careful consideration of both raw material availability and processing capabilities.
Potassium resources are abundantly available in the Earth's crust at approximately 2.1% concentration, significantly higher than lithium's 0.006%. This abundance translates to lower extraction costs and reduced geopolitical supply risks. Major potassium sources include Canada, Russia, Belarus, and China, which collectively control over 80% of global potash production. Unlike lithium, which faces concentration in the "Lithium Triangle" of South America, potassium resources are more geographically distributed.
The electrolyte components for high-voltage potassium-ion batteries typically include potassium salts (such as KPF6, KFSI, or KTFSI), organic solvents (carbonates, ethers), and additives. The production of these specialized salts currently represents a bottleneck in the supply chain, with limited commercial-scale manufacturing facilities globally. Most production is concentrated in East Asia, particularly Japan, South Korea, and China.
Solvent production for electrolytes leverages existing infrastructure from the lithium-ion battery industry, providing some supply chain advantages. However, high-voltage formulations often require specialized additives that are currently produced in limited quantities, primarily for research purposes.
Processing equipment for electrolyte mixing and handling presents another critical component of the supply chain. While some equipment can be adapted from lithium-ion battery production, the different chemical properties of potassium-based electrolytes may necessitate specialized handling equipment, particularly for safety considerations related to reactivity.
Quality control infrastructure represents a developing area in the supply chain. The analytical techniques and equipment needed to ensure consistent electrolyte performance are still evolving as the technology matures. This includes specialized testing for potassium-ion conductivity, electrochemical stability windows, and compatibility with electrode materials.
Packaging and transportation logistics for potassium-based electrolytes benefit from established hazardous material handling protocols, though specific regulations for these newer formulations continue to develop. The moisture sensitivity of many potassium salts requires specialized packaging solutions to maintain quality during transport and storage.
The emerging nature of high-voltage potassium-ion technology means that supply chain resilience remains underdeveloped compared to lithium-ion batteries. Diversification of suppliers, particularly for specialized potassium salts and additives, will be crucial for commercial-scale production.
Potassium resources are abundantly available in the Earth's crust at approximately 2.1% concentration, significantly higher than lithium's 0.006%. This abundance translates to lower extraction costs and reduced geopolitical supply risks. Major potassium sources include Canada, Russia, Belarus, and China, which collectively control over 80% of global potash production. Unlike lithium, which faces concentration in the "Lithium Triangle" of South America, potassium resources are more geographically distributed.
The electrolyte components for high-voltage potassium-ion batteries typically include potassium salts (such as KPF6, KFSI, or KTFSI), organic solvents (carbonates, ethers), and additives. The production of these specialized salts currently represents a bottleneck in the supply chain, with limited commercial-scale manufacturing facilities globally. Most production is concentrated in East Asia, particularly Japan, South Korea, and China.
Solvent production for electrolytes leverages existing infrastructure from the lithium-ion battery industry, providing some supply chain advantages. However, high-voltage formulations often require specialized additives that are currently produced in limited quantities, primarily for research purposes.
Processing equipment for electrolyte mixing and handling presents another critical component of the supply chain. While some equipment can be adapted from lithium-ion battery production, the different chemical properties of potassium-based electrolytes may necessitate specialized handling equipment, particularly for safety considerations related to reactivity.
Quality control infrastructure represents a developing area in the supply chain. The analytical techniques and equipment needed to ensure consistent electrolyte performance are still evolving as the technology matures. This includes specialized testing for potassium-ion conductivity, electrochemical stability windows, and compatibility with electrode materials.
Packaging and transportation logistics for potassium-based electrolytes benefit from established hazardous material handling protocols, though specific regulations for these newer formulations continue to develop. The moisture sensitivity of many potassium salts requires specialized packaging solutions to maintain quality during transport and storage.
The emerging nature of high-voltage potassium-ion technology means that supply chain resilience remains underdeveloped compared to lithium-ion batteries. Diversification of suppliers, particularly for specialized potassium salts and additives, will be crucial for commercial-scale production.
Sustainability and Environmental Impact Assessment
The environmental impact of high-voltage potassium-ion electrolyte formulations represents a critical consideration in their development and implementation. Compared to traditional lithium-ion technologies, potassium-ion batteries offer significant sustainability advantages due to the greater natural abundance of potassium resources. Potassium is approximately 1,000 times more abundant in the Earth's crust than lithium, substantially reducing extraction-related environmental concerns and resource depletion risks.
Current high-voltage electrolyte formulations for potassium-ion batteries typically incorporate organic solvents such as ethylene carbonate (EC), propylene carbonate (PC), and dimethyl carbonate (DMC). Life cycle assessment (LCA) studies indicate that these organic components contribute significantly to the environmental footprint of battery production. However, emerging research on bio-derived solvents and ionic liquids shows promise for reducing the environmental impact of electrolyte manufacturing processes by up to 35% compared to conventional formulations.
Water consumption represents another important environmental consideration. The production of high-purity electrolyte components requires substantial water resources, with current manufacturing processes consuming approximately 60-80 liters of water per kilogram of electrolyte produced. Innovative water recycling systems and solvent recovery techniques being developed could potentially reduce this water footprint by 40-50% in next-generation production facilities.
End-of-life management presents both challenges and opportunities for potassium-ion electrolyte sustainability. Unlike lithium-ion electrolytes, which often contain fluorinated compounds with high global warming potential, many potassium-ion electrolyte formulations utilize less environmentally persistent materials. Recycling processes for potassium-ion electrolytes are still in early development stages, but preliminary research indicates recovery rates of up to 85% for key components may be achievable through advanced separation techniques.
Carbon footprint analyses reveal that high-voltage potassium-ion electrolyte production generates approximately 30-40% lower greenhouse gas emissions compared to equivalent lithium-ion electrolytes when accounting for raw material extraction, processing, and manufacturing. This advantage stems primarily from reduced energy requirements in potassium salt purification processes and less energy-intensive synthesis routes for specialized potassium-conducting additives.
Toxicity profiles of potassium-ion electrolytes generally show favorable characteristics compared to some lithium-ion counterparts, particularly those containing LiPF6, which can generate toxic hydrogen fluoride upon decomposition. However, certain high-voltage potassium electrolyte additives still present environmental concerns that require further investigation and mitigation strategies to ensure their safe production, use, and disposal throughout the complete product lifecycle.
Current high-voltage electrolyte formulations for potassium-ion batteries typically incorporate organic solvents such as ethylene carbonate (EC), propylene carbonate (PC), and dimethyl carbonate (DMC). Life cycle assessment (LCA) studies indicate that these organic components contribute significantly to the environmental footprint of battery production. However, emerging research on bio-derived solvents and ionic liquids shows promise for reducing the environmental impact of electrolyte manufacturing processes by up to 35% compared to conventional formulations.
Water consumption represents another important environmental consideration. The production of high-purity electrolyte components requires substantial water resources, with current manufacturing processes consuming approximately 60-80 liters of water per kilogram of electrolyte produced. Innovative water recycling systems and solvent recovery techniques being developed could potentially reduce this water footprint by 40-50% in next-generation production facilities.
End-of-life management presents both challenges and opportunities for potassium-ion electrolyte sustainability. Unlike lithium-ion electrolytes, which often contain fluorinated compounds with high global warming potential, many potassium-ion electrolyte formulations utilize less environmentally persistent materials. Recycling processes for potassium-ion electrolytes are still in early development stages, but preliminary research indicates recovery rates of up to 85% for key components may be achievable through advanced separation techniques.
Carbon footprint analyses reveal that high-voltage potassium-ion electrolyte production generates approximately 30-40% lower greenhouse gas emissions compared to equivalent lithium-ion electrolytes when accounting for raw material extraction, processing, and manufacturing. This advantage stems primarily from reduced energy requirements in potassium salt purification processes and less energy-intensive synthesis routes for specialized potassium-conducting additives.
Toxicity profiles of potassium-ion electrolytes generally show favorable characteristics compared to some lithium-ion counterparts, particularly those containing LiPF6, which can generate toxic hydrogen fluoride upon decomposition. However, certain high-voltage potassium electrolyte additives still present environmental concerns that require further investigation and mitigation strategies to ensure their safe production, use, and disposal throughout the complete product lifecycle.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!