SEI Formation And Control In Potassium-Ion Batteries
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
SEI Formation Mechanisms and Research Objectives
The Solid Electrolyte Interphase (SEI) formation in potassium-ion batteries (PIBs) represents a critical yet complex electrochemical process that significantly influences battery performance and longevity. Unlike the relatively well-understood SEI in lithium-ion batteries, potassium's larger ionic radius and distinct chemical properties create unique challenges and mechanisms during interface formation.
SEI in PIBs typically forms during the initial charging cycles when the electrolyte components decompose at the electrode surface, particularly at the anode. This decomposition is primarily driven by the highly reactive nature of potassium metal and the instability of conventional electrolytes at low reduction potentials. The resulting SEI layer consists of inorganic compounds (such as K2CO3, KF, and various potassium salts) and organic components derived from solvent decomposition.
The formation mechanism follows several pathways: direct electrochemical reduction of electrolyte components, chemical reactions between reduced species and electrolyte molecules, and dissolution-precipitation processes. These mechanisms are heavily influenced by the electrolyte composition, electrode materials, and operating conditions including temperature and current density during formation cycles.
A distinctive characteristic of SEI in PIBs is its typically thicker and less uniform structure compared to lithium-ion counterparts, attributed to potassium's higher reactivity and larger ionic size. This often results in greater irreversible capacity loss during initial cycles and presents challenges for long-term cycling stability.
Research objectives in this field focus on several key areas. First, developing fundamental understanding of the composition-structure-property relationships of SEI layers in various PIB systems through advanced characterization techniques such as XPS, TEM, and in-situ spectroscopic methods. Second, engineering stable and ion-conductive SEI layers through electrolyte additives specifically designed for potassium chemistry.
Additionally, researchers aim to establish correlations between SEI formation conditions and battery performance metrics, particularly coulombic efficiency and cycle life. This includes optimizing formation protocols that balance SEI quality with practical manufacturing considerations.
Another critical objective involves computational modeling of SEI formation processes to predict optimal electrolyte formulations and formation conditions, potentially accelerating experimental progress through guided design principles.
The ultimate goal of SEI research in PIBs is to develop formation strategies that yield protective interfaces with high potassium-ion conductivity, minimal electronic conductivity, good mechanical stability against volume changes, and chemical resistance to continuous electrolyte decomposition—all critical factors for enabling commercially viable potassium-ion battery technology.
SEI in PIBs typically forms during the initial charging cycles when the electrolyte components decompose at the electrode surface, particularly at the anode. This decomposition is primarily driven by the highly reactive nature of potassium metal and the instability of conventional electrolytes at low reduction potentials. The resulting SEI layer consists of inorganic compounds (such as K2CO3, KF, and various potassium salts) and organic components derived from solvent decomposition.
The formation mechanism follows several pathways: direct electrochemical reduction of electrolyte components, chemical reactions between reduced species and electrolyte molecules, and dissolution-precipitation processes. These mechanisms are heavily influenced by the electrolyte composition, electrode materials, and operating conditions including temperature and current density during formation cycles.
A distinctive characteristic of SEI in PIBs is its typically thicker and less uniform structure compared to lithium-ion counterparts, attributed to potassium's higher reactivity and larger ionic size. This often results in greater irreversible capacity loss during initial cycles and presents challenges for long-term cycling stability.
Research objectives in this field focus on several key areas. First, developing fundamental understanding of the composition-structure-property relationships of SEI layers in various PIB systems through advanced characterization techniques such as XPS, TEM, and in-situ spectroscopic methods. Second, engineering stable and ion-conductive SEI layers through electrolyte additives specifically designed for potassium chemistry.
Additionally, researchers aim to establish correlations between SEI formation conditions and battery performance metrics, particularly coulombic efficiency and cycle life. This includes optimizing formation protocols that balance SEI quality with practical manufacturing considerations.
Another critical objective involves computational modeling of SEI formation processes to predict optimal electrolyte formulations and formation conditions, potentially accelerating experimental progress through guided design principles.
The ultimate goal of SEI research in PIBs is to develop formation strategies that yield protective interfaces with high potassium-ion conductivity, minimal electronic conductivity, good mechanical stability against volume changes, and chemical resistance to continuous electrolyte decomposition—all critical factors for enabling commercially viable potassium-ion battery technology.
Market Analysis for Potassium-Ion Battery Technologies
The global energy storage market is witnessing a significant shift towards more sustainable and cost-effective solutions, creating substantial opportunities for potassium-ion battery technologies. Current market projections indicate that the global battery market, valued at approximately $120 billion in 2022, is expected to grow at a compound annual growth rate of 18% through 2030, with alternative battery chemistries like potassium-ion batteries poised to capture an increasing share.
Potassium-ion batteries (PIBs) are emerging as a compelling alternative to lithium-ion batteries (LIBs) due to the abundant and widely distributed nature of potassium resources. Potassium is approximately 1,000 times more abundant in the Earth's crust than lithium, resulting in significantly lower raw material costs. This cost advantage positions PIBs as particularly attractive for large-scale energy storage applications where cost per kilowatt-hour is a critical factor.
Market segmentation analysis reveals several key application areas for PIBs. Grid-scale energy storage represents the largest potential market, with an estimated value of $40 billion by 2030. The renewable energy sector's growth, particularly solar and wind installations, is driving demand for cost-effective storage solutions that can address intermittency issues. PIBs, with their potentially lower cost profile, are well-positioned to serve this market segment.
Consumer electronics constitutes another significant market opportunity, projected to reach $15 billion for alternative battery technologies by 2028. However, penetration in this segment will require PIBs to overcome energy density limitations compared to established LIB technologies. The electric vehicle sector presents a longer-term opportunity, particularly for entry-level vehicles and markets where cost sensitivity outweighs performance requirements.
Regional market analysis indicates that Asia-Pacific, particularly China, South Korea, and Japan, leads in PIB research and development investments. These countries have established strategic initiatives to reduce dependence on lithium resources. Europe follows with significant research funding through programs like Horizon Europe, while North America shows growing interest primarily through university and national laboratory research programs.
Market adoption barriers for PIBs include technical challenges related to SEI formation and control, which directly impact battery cycle life and performance stability. Current market penetration remains limited to niche applications and research projects, with commercial deployment at scale anticipated within 3-5 years as technical hurdles are overcome.
Competitive analysis reveals that several battery manufacturers and energy storage companies are actively developing PIB technologies, including CATL, Samsung SDI, and several emerging startups. These companies are strategically positioning themselves to capitalize on the growing demand for cost-effective energy storage solutions as technical challenges related to SEI formation are resolved.
Potassium-ion batteries (PIBs) are emerging as a compelling alternative to lithium-ion batteries (LIBs) due to the abundant and widely distributed nature of potassium resources. Potassium is approximately 1,000 times more abundant in the Earth's crust than lithium, resulting in significantly lower raw material costs. This cost advantage positions PIBs as particularly attractive for large-scale energy storage applications where cost per kilowatt-hour is a critical factor.
Market segmentation analysis reveals several key application areas for PIBs. Grid-scale energy storage represents the largest potential market, with an estimated value of $40 billion by 2030. The renewable energy sector's growth, particularly solar and wind installations, is driving demand for cost-effective storage solutions that can address intermittency issues. PIBs, with their potentially lower cost profile, are well-positioned to serve this market segment.
Consumer electronics constitutes another significant market opportunity, projected to reach $15 billion for alternative battery technologies by 2028. However, penetration in this segment will require PIBs to overcome energy density limitations compared to established LIB technologies. The electric vehicle sector presents a longer-term opportunity, particularly for entry-level vehicles and markets where cost sensitivity outweighs performance requirements.
Regional market analysis indicates that Asia-Pacific, particularly China, South Korea, and Japan, leads in PIB research and development investments. These countries have established strategic initiatives to reduce dependence on lithium resources. Europe follows with significant research funding through programs like Horizon Europe, while North America shows growing interest primarily through university and national laboratory research programs.
Market adoption barriers for PIBs include technical challenges related to SEI formation and control, which directly impact battery cycle life and performance stability. Current market penetration remains limited to niche applications and research projects, with commercial deployment at scale anticipated within 3-5 years as technical hurdles are overcome.
Competitive analysis reveals that several battery manufacturers and energy storage companies are actively developing PIB technologies, including CATL, Samsung SDI, and several emerging startups. These companies are strategically positioning themselves to capitalize on the growing demand for cost-effective energy storage solutions as technical challenges related to SEI formation are resolved.
Current Challenges in K-Ion Battery SEI Development
Despite the promising potential of potassium-ion batteries (KIBs) as alternatives to lithium-ion batteries, their development faces significant challenges related to solid electrolyte interphase (SEI) formation and control. The highly reactive nature of potassium metal with conventional electrolytes leads to unstable and non-uniform SEI layers, resulting in poor cycling performance and safety concerns. This instability stems from potassium's lower reduction potential compared to lithium, causing more aggressive reactions with electrolyte components.
The larger ionic radius of K+ (1.38 Å) compared to Li+ (0.76 Å) creates substantial volume changes during cycling, which continuously damages the SEI layer. This mechanical stress leads to repeated SEI formation, consuming active potassium and electrolyte while increasing cell impedance. The resulting "thick but fragile" SEI layers fail to provide adequate protection for the electrode surface.
Electrolyte decomposition products in KIBs differ significantly from those in lithium-ion systems. Research indicates that KIBs tend to form more inorganic-rich SEI components like K2CO3 and KF, which exhibit different mechanical properties and ion conductivity compared to their lithium counterparts. These differences necessitate specialized electrolyte formulations rather than simply adopting lithium-ion battery solutions.
Temperature sensitivity presents another critical challenge. KIB SEI layers show greater instability across operating temperature ranges, with accelerated degradation at elevated temperatures and significantly reduced ionic conductivity at lower temperatures. This temperature-dependent behavior limits the practical application scenarios for KIB technology.
The lack of standardized characterization methodologies specifically designed for KIB SEI analysis hinders systematic research progress. Current analytical techniques often derive from lithium-ion battery research and may not adequately capture the unique characteristics of potassium-based systems. This knowledge gap impedes the development of targeted solutions for KIB-specific SEI issues.
Dendrite formation, exacerbated by unstable SEI layers, remains a persistent safety concern. Potassium dendrites can grow more rapidly and with different morphologies compared to lithium dendrites, increasing the risk of internal short circuits. Effective SEI engineering strategies must address this dendrite growth mechanism unique to potassium-based systems.
Finally, the environmental stability of KIB SEI layers presents challenges for manufacturing and long-term storage. Potassium's higher reactivity with atmospheric components like moisture and oxygen requires more stringent production environments and packaging solutions, adding complexity and cost to commercial implementation.
The larger ionic radius of K+ (1.38 Å) compared to Li+ (0.76 Å) creates substantial volume changes during cycling, which continuously damages the SEI layer. This mechanical stress leads to repeated SEI formation, consuming active potassium and electrolyte while increasing cell impedance. The resulting "thick but fragile" SEI layers fail to provide adequate protection for the electrode surface.
Electrolyte decomposition products in KIBs differ significantly from those in lithium-ion systems. Research indicates that KIBs tend to form more inorganic-rich SEI components like K2CO3 and KF, which exhibit different mechanical properties and ion conductivity compared to their lithium counterparts. These differences necessitate specialized electrolyte formulations rather than simply adopting lithium-ion battery solutions.
Temperature sensitivity presents another critical challenge. KIB SEI layers show greater instability across operating temperature ranges, with accelerated degradation at elevated temperatures and significantly reduced ionic conductivity at lower temperatures. This temperature-dependent behavior limits the practical application scenarios for KIB technology.
The lack of standardized characterization methodologies specifically designed for KIB SEI analysis hinders systematic research progress. Current analytical techniques often derive from lithium-ion battery research and may not adequately capture the unique characteristics of potassium-based systems. This knowledge gap impedes the development of targeted solutions for KIB-specific SEI issues.
Dendrite formation, exacerbated by unstable SEI layers, remains a persistent safety concern. Potassium dendrites can grow more rapidly and with different morphologies compared to lithium dendrites, increasing the risk of internal short circuits. Effective SEI engineering strategies must address this dendrite growth mechanism unique to potassium-based systems.
Finally, the environmental stability of KIB SEI layers presents challenges for manufacturing and long-term storage. Potassium's higher reactivity with atmospheric components like moisture and oxygen requires more stringent production environments and packaging solutions, adding complexity and cost to commercial implementation.
Current SEI Control Strategies for K-Ion Batteries
01 Electrolyte additives for SEI formation in potassium-ion batteries
Various electrolyte additives can be incorporated into potassium-ion batteries to promote the formation of a stable solid electrolyte interphase (SEI) layer. These additives undergo preferential reduction at the electrode surface, forming protective films that prevent continuous electrolyte decomposition. Common additives include fluorinated compounds, carbonates, and specific salts that contribute to forming a more uniform and stable SEI layer, thereby improving battery cycling performance and longevity.- Electrolyte additives for SEI formation in potassium-ion batteries: Various electrolyte additives can be incorporated into potassium-ion batteries to promote the formation of a stable solid electrolyte interphase (SEI) layer. These additives undergo preferential reduction at the electrode surface, forming protective films that prevent continuous electrolyte decomposition. Effective additives include fluorinated compounds, carbonates, and specific salts that contribute to a more uniform and stable SEI layer, improving battery cycling performance and reducing capacity fade.
- Electrode surface modifications for controlled SEI formation: Surface modifications of potassium-ion battery electrodes can significantly influence SEI formation and properties. Techniques include coating electrodes with protective layers, surface functionalization with specific chemical groups, and controlled pre-lithiation processes. These modifications create favorable interfaces for stable SEI development, reducing unwanted side reactions and improving the electrochemical performance of potassium-ion batteries by controlling the composition and morphology of the SEI layer.
- Novel electrolyte systems for enhanced SEI stability: Advanced electrolyte formulations play a crucial role in controlling SEI formation in potassium-ion batteries. These include ionic liquid-based electrolytes, concentrated electrolytes, and hybrid electrolyte systems that promote the formation of more stable and flexible SEI layers. The composition of these electrolytes affects the chemical makeup of the SEI, resulting in improved ionic conductivity while minimizing continuous electrolyte decomposition during cycling, thereby enhancing battery longevity and performance.
- Temperature and cycling protocols for optimized SEI formation: Controlled temperature conditions and specific cycling protocols significantly impact SEI formation in potassium-ion batteries. Formation cycles conducted at carefully selected temperatures and current densities can lead to more stable and uniform SEI layers. Pre-conditioning steps, gradual activation protocols, and tailored charge-discharge regimes help optimize the SEI structure and composition, resulting in improved battery performance, reduced irreversible capacity loss, and enhanced cycle life.
- Advanced characterization and modeling of SEI in potassium-ion systems: Advanced analytical techniques and computational modeling approaches are essential for understanding and controlling SEI formation in potassium-ion batteries. In-situ and ex-situ characterization methods, including spectroscopic and microscopic techniques, provide insights into SEI composition, structure, and evolution during cycling. Computational models help predict SEI formation mechanisms and properties, enabling the rational design of electrode materials and electrolyte formulations for optimized SEI layers with enhanced stability and functionality.
02 Novel electrode materials for controlled SEI formation
Specialized electrode materials can be designed to facilitate controlled SEI formation in potassium-ion batteries. These materials include carbon-based anodes with specific surface modifications, composite electrodes incorporating protective coatings, and structurally engineered materials that promote uniform potassium-ion intercalation. By controlling the surface chemistry and structure of electrode materials, more stable and functional SEI layers can be formed, leading to improved battery performance and reduced capacity fading during cycling.Expand Specific Solutions03 Pre-formation treatments for optimized SEI layers
Pre-formation treatments can be applied to potassium-ion battery components to optimize SEI layer properties before regular battery operation. These treatments include controlled pre-cycling protocols, surface conditioning of electrodes, and specialized formation cycles at specific temperatures and current densities. Such pre-formation strategies help establish more stable and protective SEI layers from the beginning, reducing initial capacity loss and improving the long-term cycling stability of potassium-ion batteries.Expand Specific Solutions04 Advanced electrolyte formulations for SEI control
Advanced electrolyte formulations can be developed to control SEI formation in potassium-ion batteries. These formulations may include optimized solvent mixtures, novel potassium salts, and carefully balanced concentrations of components to influence the composition and morphology of the resulting SEI layer. Concentrated electrolytes, ionic liquids, and solvent-in-salt systems have shown promise in forming more stable SEI layers that effectively protect electrodes while maintaining good ionic conductivity.Expand Specific Solutions05 In-situ SEI modification and repair mechanisms
In-situ SEI modification and repair mechanisms can be incorporated into potassium-ion batteries to maintain SEI integrity during cycling. These approaches include self-healing additives that can repair damaged SEI regions, sacrificial components that continuously reinforce the SEI layer, and responsive materials that adapt to changing electrochemical conditions. Such dynamic SEI control strategies help extend battery lifespan by addressing the challenges of SEI degradation during long-term cycling and under demanding operating conditions.Expand Specific Solutions
Leading Research Groups and Industrial Players
The SEI formation and control in potassium-ion batteries market is in an early growth phase, characterized by intensive research rather than commercial maturity. The global market remains relatively small but shows promising expansion potential as potassium-ion technology offers a cost-effective alternative to lithium-ion batteries. From a technical maturity perspective, significant challenges persist in controlling the solid electrolyte interphase formation. Leading research institutions like MIT, Monash University, and Jilin University are advancing fundamental understanding, while commercial players including Samsung SDI, CATL (Ningde Amperex), and Capchem are developing practical solutions. Automotive manufacturers such as Toyota, BMW, and GM are increasingly investing in this technology, recognizing its potential for next-generation energy storage applications.
Monash University
Technical Solution: Monash University has developed innovative approaches to SEI formation and control in potassium-ion batteries, focusing on both fundamental understanding and practical solutions. Their research team has pioneered the use of in-situ characterization techniques including synchrotron-based X-ray diffraction and advanced spectroscopy to monitor SEI formation in real-time during battery operation. This has led to groundbreaking insights into the dynamic nature of SEI layers in potassium systems. Monash researchers have developed novel electrolyte formulations incorporating ionic liquids, particularly those based on pyrrolidinium and piperidinium cations, which demonstrate significantly improved compatibility with potassium metal anodes[4]. Their work has shown that these ionic liquid electrolytes promote the formation of more uniform and stable SEI layers compared to conventional carbonate-based electrolytes. Additionally, the university has explored the use of biomass-derived additives as sustainable SEI-forming agents, demonstrating that certain natural polymers can be functionalized to participate beneficially in SEI formation processes, creating more flexible and self-healing interfaces that accommodate the large volume changes associated with potassium insertion/extraction.
Strengths: Their advanced characterization capabilities provide unprecedented insights into SEI formation mechanisms specific to potassium systems. Their ionic liquid electrolytes demonstrate superior thermal stability and safety characteristics compared to conventional formulations. Weaknesses: The ionic liquid electrolytes typically exhibit lower ionic conductivity at room temperature, potentially limiting power performance. The biomass-derived additives show batch-to-batch variability that could complicate quality control in manufacturing settings.
Massachusetts Institute of Technology
Technical Solution: MIT has developed a multifaceted approach to SEI formation and control in potassium-ion batteries, focusing on artificial SEI engineering and electrolyte design. Their research team has pioneered the use of atomic layer deposition (ALD) to create ultrathin protective layers on electrode surfaces prior to battery assembly, effectively pre-engineering the SEI composition and structure. This technique allows precise control over the SEI properties, resulting in significantly improved cycling stability. MIT researchers have also developed novel dual-salt electrolyte systems combining potassium hexafluorophosphate (KPF6) and potassium bis(fluorosulfonyl)imide (KFSI) that work synergistically to form robust SEI layers[2]. Their computational modeling capabilities have enabled atomic-level understanding of SEI formation mechanisms in PIBs, revealing that the larger ionic radius of K+ compared to Li+ fundamentally alters SEI composition and structure. Additionally, MIT has explored the use of ionic liquids as electrolyte components to mitigate the high reactivity of potassium with conventional carbonate solvents.
Strengths: Their ALD approach enables unprecedented control over SEI properties and composition, resulting in superior cycling performance. Their computational models provide fundamental insights that guide rational electrolyte design. Weaknesses: The ALD technique adds significant manufacturing complexity and cost that may limit commercial scalability. Some of their advanced electrolyte formulations involve expensive components that could challenge mass production economics.
Key Patents and Publications on K-Ion Battery SEI
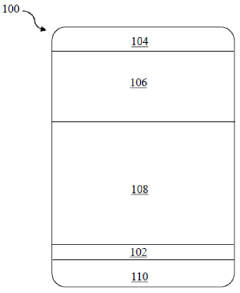
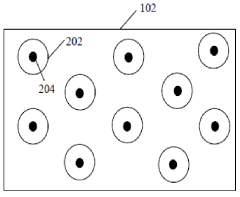
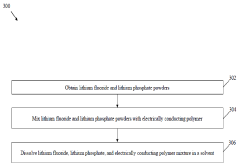
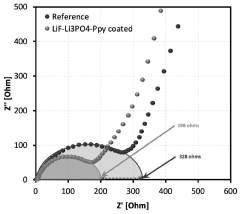
Solid electrolyte interphase (SEI) and a method for its preparation
PatentActiveIN202141055967A
Innovation
- A solid electrolyte interphase (SEI) comprising a porous polymer matrix embedded with metal precursors like lithium fluoride and lithium phosphate, coated on the current collector to prevent dendrite formation and enhance battery performance and safety, prepared through a method involving mixing metal precursors with an electrically conducting polymer and solvent, followed by solvent removal.
Materials Sustainability and Resource Considerations
The sustainability aspects of potassium-ion battery (PIB) technology present significant advantages over conventional lithium-ion systems, particularly regarding SEI formation and control. Potassium resources are approximately 1000 times more abundant in the Earth's crust than lithium, with widespread global distribution that reduces geopolitical supply risks. This abundance translates to potentially lower raw material costs and reduced environmental impact from mining operations when compared to lithium extraction, which often involves water-intensive brine processing or environmentally disruptive hard-rock mining.
The SEI formation process in PIBs utilizes electrolyte components that can be selected with sustainability considerations in mind. Recent research has focused on developing bio-derived electrolyte additives and solvents that can participate in SEI formation while reducing dependence on fluorinated compounds and other environmentally persistent chemicals commonly used in lithium-ion batteries. These green electrolyte systems show promise in forming stable SEIs while minimizing environmental footprint.
Recycling considerations for PIBs present both challenges and opportunities. The SEI layers formed in potassium systems contain different chemical compositions compared to lithium-ion batteries, potentially requiring adapted recycling processes. However, the less exotic nature of potassium compounds may simplify some aspects of the recycling chain. Research indicates that controlled SEI formation using sustainable additives can facilitate easier separation of components during end-of-life processing, enhancing material recovery rates.
Life cycle assessment (LCA) studies comparing PIBs with different SEI formation strategies reveal that electrolyte choices significantly impact the overall environmental footprint. Batteries utilizing naturally derived SEI-forming additives demonstrate reduced global warming potential and ecotoxicity scores. The trade-off between SEI stability and environmental impact remains a key research focus, with emerging data suggesting that optimized SEI formation can simultaneously address performance and sustainability goals.
Resource efficiency in PIB manufacturing can be enhanced through precise control of SEI formation processes. Techniques that enable thinner, more functional SEI layers reduce overall material consumption while improving battery performance. Advanced manufacturing approaches, such as dry electrode processing and solvent-free electrolyte systems, complement these SEI control strategies by minimizing waste generation and energy consumption during production.
The circular economy potential of PIBs is enhanced when SEI components are designed with end-of-life considerations. Research into self-healing SEI formulations that incorporate renewable materials shows promise for extending battery lifespan, thereby reducing resource demand through longer product cycles. These innovations align with broader sustainability goals while addressing the technical challenges of stable SEI formation in potassium-ion systems.
The SEI formation process in PIBs utilizes electrolyte components that can be selected with sustainability considerations in mind. Recent research has focused on developing bio-derived electrolyte additives and solvents that can participate in SEI formation while reducing dependence on fluorinated compounds and other environmentally persistent chemicals commonly used in lithium-ion batteries. These green electrolyte systems show promise in forming stable SEIs while minimizing environmental footprint.
Recycling considerations for PIBs present both challenges and opportunities. The SEI layers formed in potassium systems contain different chemical compositions compared to lithium-ion batteries, potentially requiring adapted recycling processes. However, the less exotic nature of potassium compounds may simplify some aspects of the recycling chain. Research indicates that controlled SEI formation using sustainable additives can facilitate easier separation of components during end-of-life processing, enhancing material recovery rates.
Life cycle assessment (LCA) studies comparing PIBs with different SEI formation strategies reveal that electrolyte choices significantly impact the overall environmental footprint. Batteries utilizing naturally derived SEI-forming additives demonstrate reduced global warming potential and ecotoxicity scores. The trade-off between SEI stability and environmental impact remains a key research focus, with emerging data suggesting that optimized SEI formation can simultaneously address performance and sustainability goals.
Resource efficiency in PIB manufacturing can be enhanced through precise control of SEI formation processes. Techniques that enable thinner, more functional SEI layers reduce overall material consumption while improving battery performance. Advanced manufacturing approaches, such as dry electrode processing and solvent-free electrolyte systems, complement these SEI control strategies by minimizing waste generation and energy consumption during production.
The circular economy potential of PIBs is enhanced when SEI components are designed with end-of-life considerations. Research into self-healing SEI formulations that incorporate renewable materials shows promise for extending battery lifespan, thereby reducing resource demand through longer product cycles. These innovations align with broader sustainability goals while addressing the technical challenges of stable SEI formation in potassium-ion systems.
Safety Standards and Performance Metrics
The development of safety standards and performance metrics for potassium-ion batteries (PIBs) is currently in its nascent stage compared to the well-established frameworks for lithium-ion batteries. The solid electrolyte interphase (SEI) formation in PIBs presents unique safety challenges that necessitate specialized standards and metrics.
Current safety evaluation protocols for PIBs primarily adapt existing lithium-ion battery standards, including those from organizations such as IEC, UL, and ISO. However, these adapted standards often fail to address the specific characteristics of potassium-ion chemistry, particularly regarding SEI formation dynamics and stability under various operating conditions.
Temperature-dependent performance metrics are especially critical for PIBs due to the distinctive behavior of potassium-ion SEI layers across different thermal environments. Standard testing protocols now typically include cycling stability assessments at temperature ranges from -20°C to 60°C, with particular attention to SEI degradation patterns that differ significantly from those observed in lithium-ion systems.
Electrochemical performance metrics for PIBs must account for the larger ionic radius of potassium ions and their impact on SEI properties. Key parameters include initial coulombic efficiency (typically 70-85% for current PIB technologies), capacity retention over extended cycling (benchmark: >80% after 500 cycles), and rate capability under various C-rates that stress the SEI structure.
Safety certification tests for commercial PIB applications have begun incorporating specialized protocols for thermal runaway assessment, as the SEI breakdown mechanisms in potassium systems can produce different exothermic reaction pathways compared to lithium-ion batteries. Nail penetration, crush, and overcharge tests are being modified to account for the unique SEI composition in potassium systems.
Emerging standards are also addressing the environmental impact metrics related to SEI components in PIBs, with particular focus on the toxicity profiles of decomposition products and end-of-life considerations. This reflects growing regulatory emphasis on sustainable battery technologies across global markets.
Industry consortia and academic research groups are collaboratively developing PIB-specific benchmarking protocols that emphasize SEI quality assessment through techniques such as electrochemical impedance spectroscopy (EIS) and post-mortem surface analysis. These efforts aim to establish standardized methodologies for comparing SEI formation strategies across different electrolyte formulations and electrode materials.
The harmonization of international safety standards for PIBs remains an ongoing challenge, with significant variations in testing requirements across North American, European, and Asian markets. This fragmentation presents obstacles for global commercialization efforts and technology transfer initiatives focused on advanced SEI engineering approaches.
Current safety evaluation protocols for PIBs primarily adapt existing lithium-ion battery standards, including those from organizations such as IEC, UL, and ISO. However, these adapted standards often fail to address the specific characteristics of potassium-ion chemistry, particularly regarding SEI formation dynamics and stability under various operating conditions.
Temperature-dependent performance metrics are especially critical for PIBs due to the distinctive behavior of potassium-ion SEI layers across different thermal environments. Standard testing protocols now typically include cycling stability assessments at temperature ranges from -20°C to 60°C, with particular attention to SEI degradation patterns that differ significantly from those observed in lithium-ion systems.
Electrochemical performance metrics for PIBs must account for the larger ionic radius of potassium ions and their impact on SEI properties. Key parameters include initial coulombic efficiency (typically 70-85% for current PIB technologies), capacity retention over extended cycling (benchmark: >80% after 500 cycles), and rate capability under various C-rates that stress the SEI structure.
Safety certification tests for commercial PIB applications have begun incorporating specialized protocols for thermal runaway assessment, as the SEI breakdown mechanisms in potassium systems can produce different exothermic reaction pathways compared to lithium-ion batteries. Nail penetration, crush, and overcharge tests are being modified to account for the unique SEI composition in potassium systems.
Emerging standards are also addressing the environmental impact metrics related to SEI components in PIBs, with particular focus on the toxicity profiles of decomposition products and end-of-life considerations. This reflects growing regulatory emphasis on sustainable battery technologies across global markets.
Industry consortia and academic research groups are collaboratively developing PIB-specific benchmarking protocols that emphasize SEI quality assessment through techniques such as electrochemical impedance spectroscopy (EIS) and post-mortem surface analysis. These efforts aim to establish standardized methodologies for comparing SEI formation strategies across different electrolyte formulations and electrode materials.
The harmonization of international safety standards for PIBs remains an ongoing challenge, with significant variations in testing requirements across North American, European, and Asian markets. This fragmentation presents obstacles for global commercialization efforts and technology transfer initiatives focused on advanced SEI engineering approaches.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!