Electrolyte Engineering For Stable Potassium-Ion Cells
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Potassium-Ion Battery Electrolyte Development Background and Objectives
Potassium-ion batteries (PIBs) have emerged as a promising alternative to lithium-ion batteries (LIBs) due to the abundance and low cost of potassium resources. The development of PIBs can be traced back to the 1970s when initial investigations into alkali metal-ion batteries began. However, significant research momentum only gathered in the 2010s as concerns about lithium supply chain sustainability intensified. The evolution of PIB technology has been marked by incremental improvements in electrode materials, but electrolyte engineering has remained a critical bottleneck limiting commercial viability.
The fundamental challenge in PIB electrolyte development stems from the larger ionic radius of K+ (1.38 Å) compared to Li+ (0.76 Å), resulting in weaker coordination with solvent molecules and different solvation structures. This characteristic leads to unique interfacial chemistry that conventional electrolyte formulations fail to adequately address. Historical approaches borrowed heavily from LIB electrolyte designs, which proved insufficient for the distinct electrochemical environment of potassium systems.
Recent technological trends indicate a shift toward multifunctional electrolyte engineering, incorporating additives that simultaneously address multiple failure mechanisms. The development trajectory has evolved from simple salt-solvent combinations to complex formulations featuring solvation structure regulators, interface stabilizers, and cathode protection agents. This holistic approach represents the current frontier in PIB electrolyte research.
The global research landscape shows accelerating publication rates in PIB electrolyte studies, with annual output increasing approximately 300% between 2015 and 2022. Patent filings have similarly surged, particularly from East Asian research institutions and companies, signaling growing commercial interest. Bibliometric analysis reveals that electrolyte engineering now constitutes approximately 35% of all PIB research publications, highlighting its recognized importance in the field.
The primary technical objectives for PIB electrolyte development include: extending cycle life beyond 1000 cycles at 80% capacity retention; widening the electrochemical stability window to enable high-voltage cathode materials (>4.0V vs. K/K+); mitigating dendrite formation to improve safety profiles; and enhancing low-temperature performance for broader application scenarios. Secondary objectives focus on developing environmentally benign formulations and reducing production costs to maintain the inherent cost advantages of potassium-based systems.
Achieving these objectives would position PIBs as viable alternatives in stationary energy storage applications, where cost considerations often outweigh energy density requirements. The ultimate goal is to develop electrolyte systems that enable PIBs to reach performance parity with LIBs in specific application niches while maintaining their fundamental cost advantages, thereby diversifying the battery technology landscape and reducing pressure on lithium supply chains.
The fundamental challenge in PIB electrolyte development stems from the larger ionic radius of K+ (1.38 Å) compared to Li+ (0.76 Å), resulting in weaker coordination with solvent molecules and different solvation structures. This characteristic leads to unique interfacial chemistry that conventional electrolyte formulations fail to adequately address. Historical approaches borrowed heavily from LIB electrolyte designs, which proved insufficient for the distinct electrochemical environment of potassium systems.
Recent technological trends indicate a shift toward multifunctional electrolyte engineering, incorporating additives that simultaneously address multiple failure mechanisms. The development trajectory has evolved from simple salt-solvent combinations to complex formulations featuring solvation structure regulators, interface stabilizers, and cathode protection agents. This holistic approach represents the current frontier in PIB electrolyte research.
The global research landscape shows accelerating publication rates in PIB electrolyte studies, with annual output increasing approximately 300% between 2015 and 2022. Patent filings have similarly surged, particularly from East Asian research institutions and companies, signaling growing commercial interest. Bibliometric analysis reveals that electrolyte engineering now constitutes approximately 35% of all PIB research publications, highlighting its recognized importance in the field.
The primary technical objectives for PIB electrolyte development include: extending cycle life beyond 1000 cycles at 80% capacity retention; widening the electrochemical stability window to enable high-voltage cathode materials (>4.0V vs. K/K+); mitigating dendrite formation to improve safety profiles; and enhancing low-temperature performance for broader application scenarios. Secondary objectives focus on developing environmentally benign formulations and reducing production costs to maintain the inherent cost advantages of potassium-based systems.
Achieving these objectives would position PIBs as viable alternatives in stationary energy storage applications, where cost considerations often outweigh energy density requirements. The ultimate goal is to develop electrolyte systems that enable PIBs to reach performance parity with LIBs in specific application niches while maintaining their fundamental cost advantages, thereby diversifying the battery technology landscape and reducing pressure on lithium supply chains.
Market Analysis for Potassium-Ion Battery Technologies
The global energy storage market is witnessing a significant shift towards more sustainable and cost-effective solutions, creating a fertile ground for potassium-ion battery technologies. Current market projections indicate that the global battery market, valued at approximately $120 billion in 2022, is expected to grow at a compound annual growth rate of 18% through 2030, with alternative battery chemistries like potassium-ion batteries poised to capture an increasing share.
Potassium-ion batteries are emerging as a compelling alternative to lithium-ion batteries due to several market drivers. The abundance of potassium resources, approximately 900 times more prevalent in the Earth's crust than lithium, translates to potentially lower raw material costs and reduced supply chain vulnerabilities. This advantage becomes particularly significant as lithium prices have experienced volatility, increasing by over 400% between 2021 and 2022 before recent corrections.
Market demand for potassium-ion batteries is currently concentrated in grid-scale energy storage applications, where cost considerations often outweigh energy density requirements. Industry analysts project that this segment could represent a $15 billion opportunity by 2030 if technical challenges related to electrolyte stability are adequately addressed. The stationary storage market is growing at 27% annually, creating an expanding addressable market for K-ion technologies.
Consumer electronics represents another potential market, particularly for applications where safety and cost are prioritized over maximum energy density. Market research indicates growing interest from manufacturers seeking to diversify their battery supply chains, with 38% of surveyed electronics manufacturers expressing interest in potassium-based alternatives.
Regional market analysis reveals particularly strong growth potential in Asia-Pacific, where countries like China, Japan, and South Korea are investing heavily in next-generation battery technologies. China alone has increased research funding for potassium-ion battery development by 45% since 2020, reflecting strategic prioritization of this technology.
Market barriers include the technology's relative immaturity compared to lithium-ion batteries and the established manufacturing infrastructure for competing technologies. Current production costs remain 20-30% higher than lithium-ion equivalents on a per kWh basis, though this gap is expected to narrow as manufacturing scales and electrolyte engineering advances.
Customer adoption surveys indicate that energy storage system integrators require cycle life improvements to at least 2,000 cycles and demonstrated calendar life of 8+ years before considering widespread adoption. These requirements directly relate to electrolyte stability challenges that current research efforts are addressing.
Potassium-ion batteries are emerging as a compelling alternative to lithium-ion batteries due to several market drivers. The abundance of potassium resources, approximately 900 times more prevalent in the Earth's crust than lithium, translates to potentially lower raw material costs and reduced supply chain vulnerabilities. This advantage becomes particularly significant as lithium prices have experienced volatility, increasing by over 400% between 2021 and 2022 before recent corrections.
Market demand for potassium-ion batteries is currently concentrated in grid-scale energy storage applications, where cost considerations often outweigh energy density requirements. Industry analysts project that this segment could represent a $15 billion opportunity by 2030 if technical challenges related to electrolyte stability are adequately addressed. The stationary storage market is growing at 27% annually, creating an expanding addressable market for K-ion technologies.
Consumer electronics represents another potential market, particularly for applications where safety and cost are prioritized over maximum energy density. Market research indicates growing interest from manufacturers seeking to diversify their battery supply chains, with 38% of surveyed electronics manufacturers expressing interest in potassium-based alternatives.
Regional market analysis reveals particularly strong growth potential in Asia-Pacific, where countries like China, Japan, and South Korea are investing heavily in next-generation battery technologies. China alone has increased research funding for potassium-ion battery development by 45% since 2020, reflecting strategic prioritization of this technology.
Market barriers include the technology's relative immaturity compared to lithium-ion batteries and the established manufacturing infrastructure for competing technologies. Current production costs remain 20-30% higher than lithium-ion equivalents on a per kWh basis, though this gap is expected to narrow as manufacturing scales and electrolyte engineering advances.
Customer adoption surveys indicate that energy storage system integrators require cycle life improvements to at least 2,000 cycles and demonstrated calendar life of 8+ years before considering widespread adoption. These requirements directly relate to electrolyte stability challenges that current research efforts are addressing.
Current Challenges in K-Ion Electrolyte Stability
Potassium-ion batteries (KIBs) face significant electrolyte stability challenges that currently limit their commercial viability. The primary issue stems from the highly reactive nature of potassium metal, which possesses a standard electrode potential of -2.93V vs. SHE, making it even more reactive than lithium (-3.04V) in practical battery environments. This reactivity leads to aggressive side reactions with conventional electrolyte components, resulting in continuous electrolyte decomposition and rapid capacity fading.
A major challenge is the formation of unstable solid electrolyte interphase (SEI) layers on electrode surfaces. Unlike the relatively stable SEI in lithium-ion systems, potassium-based SEI layers tend to be mechanically fragile and chemically unstable. This is exacerbated by the larger ionic radius of K+ (1.38Å) compared to Li+ (0.76Å), causing more significant volume changes during cycling and mechanical stress on the SEI layer.
Conventional carbonate-based electrolytes (such as EC/DEC, EC/DMC) that perform adequately in lithium-ion systems show poor compatibility with potassium-based electrodes. These electrolytes undergo excessive decomposition, particularly at the anode interface, generating gaseous products that lead to cell swelling and safety concerns. The decomposition products often include potassium alkyl carbonates and various organic compounds that do not form cohesive protective layers.
Electrolyte salt stability presents another significant hurdle. Common salts like KPF6 suffer from lower chemical stability compared to their lithium counterparts, with increased susceptibility to hydrolysis and thermal decomposition. This leads to the formation of harmful byproducts including HF, which attacks both electrode materials and cell components, accelerating degradation mechanisms.
The narrow electrochemical stability window of current K-ion electrolytes severely limits the operating voltage range of KIBs. Most conventional electrolyte formulations begin decomposing at potentials above 4.0V vs. K/K+, restricting the use of high-voltage cathode materials that could potentially increase energy density.
Temperature sensitivity further complicates electrolyte engineering for KIBs. Current formulations exhibit poor performance at temperature extremes, with accelerated decomposition at elevated temperatures and significantly reduced ionic conductivity at lower temperatures, limiting practical application scenarios.
Concentration polarization effects are more pronounced in K-ion systems due to the larger size and lower mobility of potassium ions in conventional solvent systems. This leads to concentration gradients during high-rate operation, promoting dendritic growth and further destabilizing the electrolyte-electrode interfaces.
A major challenge is the formation of unstable solid electrolyte interphase (SEI) layers on electrode surfaces. Unlike the relatively stable SEI in lithium-ion systems, potassium-based SEI layers tend to be mechanically fragile and chemically unstable. This is exacerbated by the larger ionic radius of K+ (1.38Å) compared to Li+ (0.76Å), causing more significant volume changes during cycling and mechanical stress on the SEI layer.
Conventional carbonate-based electrolytes (such as EC/DEC, EC/DMC) that perform adequately in lithium-ion systems show poor compatibility with potassium-based electrodes. These electrolytes undergo excessive decomposition, particularly at the anode interface, generating gaseous products that lead to cell swelling and safety concerns. The decomposition products often include potassium alkyl carbonates and various organic compounds that do not form cohesive protective layers.
Electrolyte salt stability presents another significant hurdle. Common salts like KPF6 suffer from lower chemical stability compared to their lithium counterparts, with increased susceptibility to hydrolysis and thermal decomposition. This leads to the formation of harmful byproducts including HF, which attacks both electrode materials and cell components, accelerating degradation mechanisms.
The narrow electrochemical stability window of current K-ion electrolytes severely limits the operating voltage range of KIBs. Most conventional electrolyte formulations begin decomposing at potentials above 4.0V vs. K/K+, restricting the use of high-voltage cathode materials that could potentially increase energy density.
Temperature sensitivity further complicates electrolyte engineering for KIBs. Current formulations exhibit poor performance at temperature extremes, with accelerated decomposition at elevated temperatures and significantly reduced ionic conductivity at lower temperatures, limiting practical application scenarios.
Concentration polarization effects are more pronounced in K-ion systems due to the larger size and lower mobility of potassium ions in conventional solvent systems. This leads to concentration gradients during high-rate operation, promoting dendritic growth and further destabilizing the electrolyte-electrode interfaces.
State-of-the-Art Electrolyte Engineering Solutions for K-Ion Cells
01 Electrolyte additives for enhanced stability
Various additives can be incorporated into potassium-ion cell electrolytes to improve their stability. These additives can include fluorinated compounds, film-forming agents, and stabilizing salts that create protective interfaces on electrode surfaces. Such additives help prevent electrolyte decomposition during cycling, reduce side reactions with electrode materials, and enhance the overall electrochemical stability window of the electrolyte system.- Electrolyte additives for improved stability: Various additives can be incorporated into potassium-ion cell electrolytes to enhance their stability. These additives can include fluorinated compounds, film-forming agents, and stabilizing salts that create protective interfaces on electrode surfaces. Such additives help prevent electrolyte decomposition during cycling, reduce side reactions, and improve the overall electrochemical performance and longevity of potassium-ion batteries.
- Novel electrolyte solvent systems: Innovative solvent combinations and formulations can significantly improve the stability of potassium-ion cell electrolytes. These include mixed carbonate systems, ether-based solvents, and ionic liquids that offer wider electrochemical windows and better thermal stability. The careful selection and optimization of solvent systems can mitigate decomposition issues, enhance ion transport, and improve compatibility with electrode materials in potassium-ion batteries.
- Solid-state and gel polymer electrolytes: Solid-state and gel polymer electrolytes represent an alternative approach to improving stability in potassium-ion cells. These electrolytes eliminate or reduce the flammable liquid component, enhancing safety and stability. Polymer matrices, ceramic materials, and composite structures can be engineered to provide mechanical strength while maintaining adequate ionic conductivity, resulting in potassium-ion batteries with improved cycle life and thermal stability.
- Potassium salt concentration and formulation: The concentration and type of potassium salts used in electrolytes significantly impact stability. Highly concentrated electrolytes, dual-salt systems, and specially formulated potassium salts can reduce unwanted side reactions and improve the formation of stable solid electrolyte interphases. Optimizing salt concentration and composition helps address issues related to potassium dendrite formation and electrolyte degradation during long-term cycling.
- Interface engineering and protective coatings: Engineering stable interfaces between electrolytes and electrodes is crucial for potassium-ion cell stability. This can be achieved through electrode surface modifications, artificial SEI formation, and protective coatings that minimize direct contact between reactive electrode materials and the electrolyte. These approaches help prevent continuous electrolyte decomposition, reduce irreversible capacity loss, and enhance the overall electrochemical performance and lifespan of potassium-ion batteries.
02 Novel electrolyte solvent systems
Innovative solvent combinations and formulations can significantly improve the stability of potassium-ion cell electrolytes. These may include mixed carbonate systems, ether-based solvents, or ionic liquids that offer better thermal stability and reduced volatility. The careful selection of solvent systems can minimize decomposition at electrode interfaces, improve potassium ion transport, and enhance cycling performance at wider temperature ranges.Expand Specific Solutions03 Solid-state and gel polymer electrolytes
Solid-state and gel polymer electrolytes represent an alternative approach to improving stability in potassium-ion cells. These electrolytes can eliminate or reduce the flammable liquid component, enhancing safety and stability. Polymer matrices, ceramic materials, or composite systems can be designed to provide mechanical stability while maintaining adequate ionic conductivity for potassium ions, resulting in batteries with longer cycle life and improved thermal stability.Expand Specific Solutions04 Potassium salt concentration and formulation
The concentration and type of potassium salts used in electrolytes significantly impact stability. Highly concentrated electrolytes or localized high-concentration electrolyte systems can suppress solvent decomposition and aluminum corrosion. Various potassium salts including KFSI, KPF6, and KTFSI can be formulated to optimize the balance between ionic conductivity, electrochemical stability, and interfacial compatibility with electrode materials.Expand Specific Solutions05 Interface engineering for electrolyte stability
Engineering stable interfaces between the electrolyte and electrodes is crucial for potassium-ion cell performance. This can be achieved through surface coatings on electrodes, electrolyte additives that form stable solid electrolyte interphases (SEI), or pre-treatment of electrode materials. These approaches help prevent continuous electrolyte decomposition, mitigate potassium dendrite formation, and create stable passivation layers that enhance long-term cycling stability and coulombic efficiency.Expand Specific Solutions
Leading Companies and Research Institutions in K-Ion Battery Development
The potassium-ion battery market is currently in its early growth phase, characterized by intensive R&D efforts rather than widespread commercialization. The global market size remains relatively small compared to lithium-ion technologies but is projected to expand significantly due to potassium's abundance and cost advantages. Technologically, electrolyte engineering for stable K-ion cells is advancing through collaborative efforts between academic institutions and industry players. Leading organizations like Chinese Academy of Sciences, Toyota Motor Corp., and LG Energy Solution are making significant progress in addressing electrolyte stability challenges. Research institutions including MIT, Cornell University, and multiple Chinese universities are developing innovative electrolyte formulations, while automotive companies (BMW, GM) are exploring commercial applications. The technology is approaching commercial viability, with several companies transitioning from laboratory research to pilot production phases.
Chinese Academy of Sciences Institute of Physics
Technical Solution: The Chinese Academy of Sciences Institute of Physics has developed an innovative electrolyte engineering approach for potassium-ion batteries focusing on ionic liquid-based systems. Their research team has created a custom-designed electrolyte combining potassium bis(trifluoromethanesulfonyl)imide (KTFSI) with pyrrolidinium-based ionic liquids, specifically 1-butyl-1-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide (Pyr14TFSI). This formulation is supplemented with a small percentage of ethylene carbonate as a co-solvent to enhance ionic conductivity. Their electrolyte design leverages the wide electrochemical stability window of ionic liquids (exceeding 5V vs. K/K+) while addressing the typically high viscosity through careful co-solvent selection. Extensive electrochemical impedance spectroscopy and surface analysis reveal that their electrolyte forms a highly stable interface on both graphite anodes and Prussian blue analog cathodes, effectively suppressing the parasitic reactions that typically plague potassium-ion systems.
Strengths: Exceptional thermal stability and safety characteristics, wide electrochemical stability window allowing for high-voltage operation, and minimal electrolyte decomposition during cycling. Weaknesses: Higher production costs compared to conventional electrolytes, increased viscosity affecting rate capability, and potential challenges in low-temperature applications due to the inherent properties of ionic liquids.
Chinese Academy of Science Institute of Chemistry
Technical Solution: The Chinese Academy of Science Institute of Chemistry has developed a breakthrough concentrated electrolyte system for potassium-ion batteries based on potassium bis(fluorosulfonyl)imide (KFSI) salt in dimethyl ether (DME) at a high salt-to-solvent ratio. Their research demonstrates that this concentrated electrolyte fundamentally alters the solvation structure around potassium ions, creating a coordination environment that significantly reduces solvent decomposition at electrode surfaces. Through advanced spectroscopic analysis and computational modeling, they've shown that their electrolyte forms a fluorine-rich SEI layer that effectively passivates both anode and cathode surfaces. This protective layer substantially mitigates the dissolution of transition metal elements from cathode materials—a common failure mechanism in potassium-ion batteries. Their electrolyte engineering approach has enabled stable cycling of potassium-ion full cells with less than 0.04% capacity decay per cycle over 1000 cycles, representing a significant advancement in the field.
Strengths: Exceptional cycling stability, superior protection against transition metal dissolution, and excellent compatibility with various electrode materials including hard carbon anodes. Weaknesses: The high salt concentration increases electrolyte viscosity, potentially limiting ion transport at low temperatures, and the high cost of KFSI salt presents commercialization challenges.
Critical Patents and Research Breakthroughs in K-Ion Electrolytes
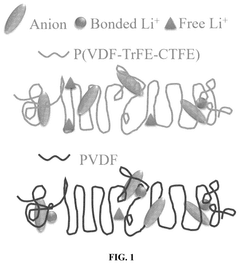
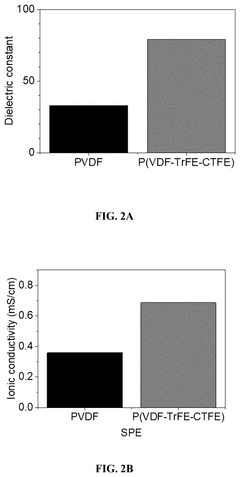
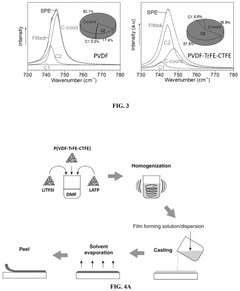
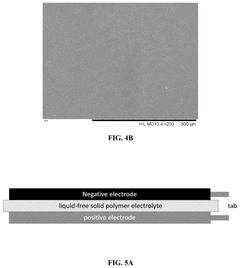
Flexible and liquid-free solid polymer electrolyte, methods for fabricating the same and its application thereof
PatentPendingUS20250239646A1
Innovation
- A flexible and liquid-free solid polymer electrolyte is developed using a mixture of crystalline and non-crystalline polymers with a high dielectric coefficient and active ceramic particles, enhancing ionic conductivity and mechanical strength, fabricated through a facile tape-casting method.
Material Supply Chain Analysis for K-Ion Battery Production
The potassium-ion battery supply chain presents unique challenges and opportunities compared to the established lithium-ion battery ecosystem. The electrolyte components for K-ion cells require specific raw materials that follow distinct sourcing, processing, and distribution pathways.
Potassium salts, primarily potassium hexafluorophosphate (KPF6), represent the foundation of K-ion electrolytes. Unlike lithium salts, potassium resources are abundantly available globally, with significant deposits in Canada, Russia, Germany, and Belarus. This geographical distribution offers greater supply security and potentially lower material costs compared to lithium resources. Current production capacity for battery-grade KPF6 remains limited, with only a handful of specialty chemical manufacturers equipped for high-purity production.
Organic solvents for K-ion electrolytes typically include ethylene carbonate (EC), propylene carbonate (PC), and dimethyl carbonate (DMC). These solvents share supply chains with the lithium-ion battery industry, creating potential competition for resources as K-ion technology scales. The solvent manufacturing industry is dominated by chemical companies in China, Japan, and South Korea, with emerging production in Europe and North America.
Additives for electrolyte stabilization represent a critical bottleneck in the K-ion supply chain. Fluoroethylene carbonate (FEC) and vinylene carbonate (VC), commonly used to form stable solid-electrolyte interphases in K-ion cells, face limited production capacity and high costs. The specialized nature of these additives creates vulnerability in the supply chain, with only a few manufacturers globally capable of meeting battery-grade specifications.
Packaging materials for electrolytes, including specialized containers and moisture-barrier systems, leverage existing infrastructure from the lithium-ion industry but require modifications to accommodate the higher reactivity of potassium-based systems. This segment of the supply chain benefits from established manufacturing capabilities but faces adaptation challenges.
The overall K-ion electrolyte supply chain currently lacks the vertical integration seen in mature lithium-ion production. Material transportation and handling present additional challenges due to the moisture sensitivity of potassium salts and certain additives. As K-ion technology advances toward commercialization, strategic investments in regional processing facilities and specialized logistics networks will be essential to support stable, cost-effective production.
Potassium salts, primarily potassium hexafluorophosphate (KPF6), represent the foundation of K-ion electrolytes. Unlike lithium salts, potassium resources are abundantly available globally, with significant deposits in Canada, Russia, Germany, and Belarus. This geographical distribution offers greater supply security and potentially lower material costs compared to lithium resources. Current production capacity for battery-grade KPF6 remains limited, with only a handful of specialty chemical manufacturers equipped for high-purity production.
Organic solvents for K-ion electrolytes typically include ethylene carbonate (EC), propylene carbonate (PC), and dimethyl carbonate (DMC). These solvents share supply chains with the lithium-ion battery industry, creating potential competition for resources as K-ion technology scales. The solvent manufacturing industry is dominated by chemical companies in China, Japan, and South Korea, with emerging production in Europe and North America.
Additives for electrolyte stabilization represent a critical bottleneck in the K-ion supply chain. Fluoroethylene carbonate (FEC) and vinylene carbonate (VC), commonly used to form stable solid-electrolyte interphases in K-ion cells, face limited production capacity and high costs. The specialized nature of these additives creates vulnerability in the supply chain, with only a few manufacturers globally capable of meeting battery-grade specifications.
Packaging materials for electrolytes, including specialized containers and moisture-barrier systems, leverage existing infrastructure from the lithium-ion industry but require modifications to accommodate the higher reactivity of potassium-based systems. This segment of the supply chain benefits from established manufacturing capabilities but faces adaptation challenges.
The overall K-ion electrolyte supply chain currently lacks the vertical integration seen in mature lithium-ion production. Material transportation and handling present additional challenges due to the moisture sensitivity of potassium salts and certain additives. As K-ion technology advances toward commercialization, strategic investments in regional processing facilities and specialized logistics networks will be essential to support stable, cost-effective production.
Sustainability and Environmental Impact of K-Ion Battery Technologies
The sustainability profile of potassium-ion battery technologies represents a significant advantage over conventional lithium-ion systems. Potassium resources are approximately 1000 times more abundant than lithium in the Earth's crust, with widespread global distribution that reduces geopolitical supply risks. This abundance translates to lower extraction impacts and potentially more stable supply chains, addressing critical raw material concerns that currently plague lithium-based energy storage.
From a life-cycle perspective, K-ion batteries demonstrate promising environmental credentials. The extraction and processing of potassium compounds generally require less energy and water compared to lithium brine operations, which can consume up to 500,000 gallons of water per ton of lithium extracted. Additionally, potassium mining typically produces fewer toxic byproducts and causes less habitat disruption than lithium extraction, particularly when compared to hard-rock lithium mining operations.
Carbon footprint analyses indicate that K-ion battery production could potentially generate 15-25% lower greenhouse gas emissions compared to equivalent lithium-ion batteries, primarily due to reduced processing requirements and more accessible raw materials. This advantage becomes particularly significant when considering large-scale deployment scenarios for grid storage applications, where environmental impact scales proportionally with system size.
End-of-life management presents both challenges and opportunities for K-ion technologies. Current research indicates that potassium-based cathode materials may be more amenable to hydrometallurgical recycling processes than certain lithium compounds, potentially increasing recovery rates. However, specialized recycling infrastructure will need development as these batteries reach commercial scale, requiring investment in new separation and recovery technologies optimized for potassium chemistry.
Water usage represents another critical sustainability metric where K-ion systems demonstrate advantages. Conventional lithium extraction from salt flats in South America can require up to 2 million liters of water per ton of lithium carbonate, contributing to water scarcity in already arid regions. Potassium extraction typically demands significantly less water intensity, reducing pressure on local water resources in production regions.
The environmental impact of electrolyte engineering for K-ion cells warrants particular attention. While potassium salts themselves present lower toxicity profiles than some lithium compounds, certain advanced electrolyte additives being explored for stability enhancement may introduce new environmental concerns. Ongoing research must balance performance requirements with environmental considerations, prioritizing additives with favorable biodegradability and toxicity profiles.
From a life-cycle perspective, K-ion batteries demonstrate promising environmental credentials. The extraction and processing of potassium compounds generally require less energy and water compared to lithium brine operations, which can consume up to 500,000 gallons of water per ton of lithium extracted. Additionally, potassium mining typically produces fewer toxic byproducts and causes less habitat disruption than lithium extraction, particularly when compared to hard-rock lithium mining operations.
Carbon footprint analyses indicate that K-ion battery production could potentially generate 15-25% lower greenhouse gas emissions compared to equivalent lithium-ion batteries, primarily due to reduced processing requirements and more accessible raw materials. This advantage becomes particularly significant when considering large-scale deployment scenarios for grid storage applications, where environmental impact scales proportionally with system size.
End-of-life management presents both challenges and opportunities for K-ion technologies. Current research indicates that potassium-based cathode materials may be more amenable to hydrometallurgical recycling processes than certain lithium compounds, potentially increasing recovery rates. However, specialized recycling infrastructure will need development as these batteries reach commercial scale, requiring investment in new separation and recovery technologies optimized for potassium chemistry.
Water usage represents another critical sustainability metric where K-ion systems demonstrate advantages. Conventional lithium extraction from salt flats in South America can require up to 2 million liters of water per ton of lithium carbonate, contributing to water scarcity in already arid regions. Potassium extraction typically demands significantly less water intensity, reducing pressure on local water resources in production regions.
The environmental impact of electrolyte engineering for K-ion cells warrants particular attention. While potassium salts themselves present lower toxicity profiles than some lithium compounds, certain advanced electrolyte additives being explored for stability enhancement may introduce new environmental concerns. Ongoing research must balance performance requirements with environmental considerations, prioritizing additives with favorable biodegradability and toxicity profiles.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!