Solid-State Potassium-Ion Electrolytes: Materials And Prospects
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Potassium-Ion Electrolytes Background and Objectives
Potassium-ion battery technology has emerged as a promising alternative to lithium-ion batteries due to the abundance and low cost of potassium resources. The development of potassium-ion batteries can be traced back to the early 1990s, but significant research momentum has only been gained in the past decade. This renewed interest stems from growing concerns about the long-term sustainability of lithium resources and the increasing demand for energy storage solutions.
The evolution of potassium-ion battery technology has followed a trajectory similar to that of lithium-ion batteries, with initial focus on liquid electrolytes. However, safety concerns, limited electrochemical stability windows, and poor cycling performance have driven researchers toward solid-state electrolyte solutions. Solid-state potassium-ion electrolytes represent a critical advancement in this field, offering potential improvements in safety, stability, and energy density.
Historical development of solid-state potassium-ion electrolytes has progressed through several key phases. Early research primarily focused on polymer-based electrolytes, followed by ceramic and glass-ceramic materials. Recent years have witnessed significant advancements in composite electrolytes that combine the advantages of different material classes to overcome inherent limitations of single-component systems.
Current technological trends indicate a growing interest in developing novel materials with high ionic conductivity at room temperature, which remains one of the primary challenges in this field. Research is increasingly focused on understanding ion transport mechanisms at atomic and molecular levels, enabling rational design of materials with optimized structures for potassium-ion conduction.
The primary technical objectives for solid-state potassium-ion electrolytes include achieving room-temperature ionic conductivity exceeding 10^-3 S/cm, maintaining stable interfaces with electrode materials, demonstrating mechanical stability during cycling, and ensuring compatibility with scalable manufacturing processes. These objectives are driven by the need to develop electrolytes that can enable potassium-ion batteries with energy densities comparable to or exceeding those of current lithium-ion technologies.
Additionally, researchers aim to develop electrolytes that can effectively suppress potassium dendrite formation, which presents a significant safety concern similar to lithium dendrites in lithium-ion batteries. The larger ionic radius of potassium introduces unique challenges and opportunities in electrolyte design that differ from those encountered in lithium-ion systems.
The ultimate goal of research in this field is to establish solid-state potassium-ion batteries as a viable, sustainable, and cost-effective alternative to current energy storage technologies, particularly for grid-scale applications where cost considerations outweigh energy density requirements. This aligns with global efforts to transition toward renewable energy sources and reduce dependence on critical materials with limited geographical distribution.
The evolution of potassium-ion battery technology has followed a trajectory similar to that of lithium-ion batteries, with initial focus on liquid electrolytes. However, safety concerns, limited electrochemical stability windows, and poor cycling performance have driven researchers toward solid-state electrolyte solutions. Solid-state potassium-ion electrolytes represent a critical advancement in this field, offering potential improvements in safety, stability, and energy density.
Historical development of solid-state potassium-ion electrolytes has progressed through several key phases. Early research primarily focused on polymer-based electrolytes, followed by ceramic and glass-ceramic materials. Recent years have witnessed significant advancements in composite electrolytes that combine the advantages of different material classes to overcome inherent limitations of single-component systems.
Current technological trends indicate a growing interest in developing novel materials with high ionic conductivity at room temperature, which remains one of the primary challenges in this field. Research is increasingly focused on understanding ion transport mechanisms at atomic and molecular levels, enabling rational design of materials with optimized structures for potassium-ion conduction.
The primary technical objectives for solid-state potassium-ion electrolytes include achieving room-temperature ionic conductivity exceeding 10^-3 S/cm, maintaining stable interfaces with electrode materials, demonstrating mechanical stability during cycling, and ensuring compatibility with scalable manufacturing processes. These objectives are driven by the need to develop electrolytes that can enable potassium-ion batteries with energy densities comparable to or exceeding those of current lithium-ion technologies.
Additionally, researchers aim to develop electrolytes that can effectively suppress potassium dendrite formation, which presents a significant safety concern similar to lithium dendrites in lithium-ion batteries. The larger ionic radius of potassium introduces unique challenges and opportunities in electrolyte design that differ from those encountered in lithium-ion systems.
The ultimate goal of research in this field is to establish solid-state potassium-ion batteries as a viable, sustainable, and cost-effective alternative to current energy storage technologies, particularly for grid-scale applications where cost considerations outweigh energy density requirements. This aligns with global efforts to transition toward renewable energy sources and reduce dependence on critical materials with limited geographical distribution.
Market Analysis for Solid-State K-Ion Battery Applications
The solid-state potassium-ion battery market is experiencing significant growth potential due to increasing demand for sustainable energy storage solutions. Current market projections indicate that the global potassium-ion battery market could reach $2.5 billion by 2030, with solid-state variants potentially capturing 15-20% of this emerging segment. This growth is primarily driven by the abundant nature of potassium resources, which are approximately 1,000 times more plentiful than lithium in the Earth's crust, offering a cost-effective alternative to conventional lithium-ion technologies.
The market for solid-state K-ion batteries is segmented across several key application areas. Grid energy storage represents the largest potential market, with an estimated 40% share of future applications. The inherent safety advantages of solid-state electrolytes make these batteries particularly suitable for large-scale stationary storage systems where safety concerns are paramount. Additionally, the lower cost structure of potassium-based systems compared to lithium alternatives provides a compelling economic advantage for utility-scale deployments.
Consumer electronics constitutes another significant market segment, projected to account for approximately 25% of applications. The higher theoretical capacity of potassium-ion systems (theoretical specific capacity of K metal: 685 mAh/g) compared to sodium alternatives makes them attractive for portable devices where energy density remains important but cost considerations are increasingly critical.
Electric vehicles represent a developing but potentially substantial market, estimated at 20% of future applications. While current solid-state K-ion technologies face challenges in power density compared to lithium-ion counterparts, their improved safety profile and potentially lower manufacturing costs are driving research interest from automotive manufacturers seeking diversification in battery technologies.
Regional market analysis reveals Asia-Pacific as the dominant market for solid-state K-ion battery development, accounting for approximately 60% of research activities and commercial initiatives. China leads this regional focus, leveraging its abundant potassium resources and established battery manufacturing infrastructure. North America and Europe follow with approximately 25% and 15% market activity respectively, with particular focus on grid storage applications.
Market adoption faces several challenges, including competition from more established lithium and sodium-ion technologies. Current market penetration remains below 1% of the total battery market, reflecting the early-stage nature of this technology. However, the compound annual growth rate for research funding in this sector has exceeded 35% over the past five years, indicating strong institutional and commercial interest in accelerating development.
The market for solid-state K-ion batteries is segmented across several key application areas. Grid energy storage represents the largest potential market, with an estimated 40% share of future applications. The inherent safety advantages of solid-state electrolytes make these batteries particularly suitable for large-scale stationary storage systems where safety concerns are paramount. Additionally, the lower cost structure of potassium-based systems compared to lithium alternatives provides a compelling economic advantage for utility-scale deployments.
Consumer electronics constitutes another significant market segment, projected to account for approximately 25% of applications. The higher theoretical capacity of potassium-ion systems (theoretical specific capacity of K metal: 685 mAh/g) compared to sodium alternatives makes them attractive for portable devices where energy density remains important but cost considerations are increasingly critical.
Electric vehicles represent a developing but potentially substantial market, estimated at 20% of future applications. While current solid-state K-ion technologies face challenges in power density compared to lithium-ion counterparts, their improved safety profile and potentially lower manufacturing costs are driving research interest from automotive manufacturers seeking diversification in battery technologies.
Regional market analysis reveals Asia-Pacific as the dominant market for solid-state K-ion battery development, accounting for approximately 60% of research activities and commercial initiatives. China leads this regional focus, leveraging its abundant potassium resources and established battery manufacturing infrastructure. North America and Europe follow with approximately 25% and 15% market activity respectively, with particular focus on grid storage applications.
Market adoption faces several challenges, including competition from more established lithium and sodium-ion technologies. Current market penetration remains below 1% of the total battery market, reflecting the early-stage nature of this technology. However, the compound annual growth rate for research funding in this sector has exceeded 35% over the past five years, indicating strong institutional and commercial interest in accelerating development.
Current Status and Technical Challenges of K-Ion Electrolytes
The global research on solid-state potassium-ion electrolytes has witnessed significant growth in recent years, driven by the increasing demand for sustainable and cost-effective energy storage solutions. Currently, the development of K-ion electrolytes is at a nascent stage compared to lithium and sodium counterparts, with most research concentrated in Asia, particularly China, Japan, and South Korea, followed by North America and Europe.
Polymer-based electrolytes represent one of the most extensively studied categories, with polyethylene oxide (PEO) complexes showing promising ionic conductivities of 10^-5 to 10^-4 S/cm at elevated temperatures. However, their room temperature performance remains suboptimal for practical applications. Ceramic and glass-based solid electrolytes, including NASICON-type structures and potassium beta-alumina, have demonstrated enhanced thermal stability but suffer from brittleness and challenging manufacturing processes.
Composite electrolytes combining polymers with ceramic fillers have emerged as a promising direction, addressing the limitations of single-component systems. Recent advancements in this area have achieved conductivities approaching 10^-3 S/cm at room temperature, marking significant progress toward commercially viable systems.
Despite these advancements, K-ion electrolytes face several critical technical challenges. The larger ionic radius of K+ (1.38 Å) compared to Li+ (0.76 Å) results in slower diffusion kinetics and higher activation energies for ion transport. This fundamental limitation necessitates novel material design strategies to create optimized ion conduction pathways.
Interface stability represents another major hurdle, as many solid electrolytes exhibit high interfacial resistance with potassium metal anodes, leading to capacity fading and safety concerns. The high chemical reactivity of potassium further complicates the development of stable solid electrolyte interfaces.
Manufacturing scalability remains problematic, with many promising materials requiring complex synthesis procedures or expensive precursors. The transition from laboratory-scale production to industrial manufacturing presents significant engineering challenges that have yet to be adequately addressed.
Mechanical properties pose additional difficulties, as solid electrolytes must maintain good contact with electrodes during cycling while accommodating volume changes. The inherent brittleness of many ceramic-based systems and the poor mechanical strength of polymer electrolytes at elevated temperatures limit their practical application.
Environmental stability is another concern, as many potassium-containing compounds are hygroscopic and sensitive to moisture and air, necessitating stringent manufacturing conditions and packaging solutions that increase production costs and complexity.
Polymer-based electrolytes represent one of the most extensively studied categories, with polyethylene oxide (PEO) complexes showing promising ionic conductivities of 10^-5 to 10^-4 S/cm at elevated temperatures. However, their room temperature performance remains suboptimal for practical applications. Ceramic and glass-based solid electrolytes, including NASICON-type structures and potassium beta-alumina, have demonstrated enhanced thermal stability but suffer from brittleness and challenging manufacturing processes.
Composite electrolytes combining polymers with ceramic fillers have emerged as a promising direction, addressing the limitations of single-component systems. Recent advancements in this area have achieved conductivities approaching 10^-3 S/cm at room temperature, marking significant progress toward commercially viable systems.
Despite these advancements, K-ion electrolytes face several critical technical challenges. The larger ionic radius of K+ (1.38 Å) compared to Li+ (0.76 Å) results in slower diffusion kinetics and higher activation energies for ion transport. This fundamental limitation necessitates novel material design strategies to create optimized ion conduction pathways.
Interface stability represents another major hurdle, as many solid electrolytes exhibit high interfacial resistance with potassium metal anodes, leading to capacity fading and safety concerns. The high chemical reactivity of potassium further complicates the development of stable solid electrolyte interfaces.
Manufacturing scalability remains problematic, with many promising materials requiring complex synthesis procedures or expensive precursors. The transition from laboratory-scale production to industrial manufacturing presents significant engineering challenges that have yet to be adequately addressed.
Mechanical properties pose additional difficulties, as solid electrolytes must maintain good contact with electrodes during cycling while accommodating volume changes. The inherent brittleness of many ceramic-based systems and the poor mechanical strength of polymer electrolytes at elevated temperatures limit their practical application.
Environmental stability is another concern, as many potassium-containing compounds are hygroscopic and sensitive to moisture and air, necessitating stringent manufacturing conditions and packaging solutions that increase production costs and complexity.
Current Material Solutions for Solid-State K-Ion Electrolytes
01 Polymer-based solid-state potassium-ion electrolytes
Polymer-based electrolytes offer advantages for potassium-ion batteries including flexibility, processability, and improved safety. These electrolytes typically incorporate potassium salts into polymer matrices such as polyethylene oxide (PEO), polyvinylidene fluoride (PVDF), or other polymer hosts. The addition of plasticizers or ceramic fillers can enhance ionic conductivity while maintaining mechanical stability. These materials address challenges of dendrite formation and interface stability in solid-state potassium batteries.- Polymer-based solid-state potassium-ion electrolytes: Polymer-based electrolytes represent a significant category of solid-state potassium-ion electrolytes. These materials typically incorporate potassium salts into polymer matrices such as polyethylene oxide (PEO) or polyvinylidene fluoride (PVDF). The polymer host provides mechanical flexibility while facilitating potassium ion transport through segmental motion of polymer chains. These electrolytes often exhibit improved safety characteristics compared to liquid electrolytes and can be manufactured as thin films, making them suitable for flexible battery applications.
- Ceramic and glass-based potassium-ion solid electrolytes: Ceramic and glass-based solid electrolytes for potassium-ion batteries typically consist of crystalline or amorphous materials with high ionic conductivity. These include NASICON-type structures, potassium beta-alumina, and various potassium-containing phosphates and silicates. These materials offer excellent thermal stability and negligible electronic conductivity, which helps prevent short circuits. Their rigid structure provides a stable framework for potassium ion migration through interconnected channels or vacancies in the crystal lattice, though they often face challenges related to brittleness and interfacial resistance.
- Composite solid-state potassium-ion electrolytes: Composite solid-state electrolytes combine different types of materials to achieve enhanced performance characteristics. These typically involve the integration of ceramic fillers within polymer matrices or the combination of different types of solid electrolytes. The composite approach aims to leverage the advantages of each component while mitigating their individual limitations. For example, adding ceramic particles to polymer electrolytes can improve mechanical strength and ionic conductivity, while the polymer component helps reduce interfacial resistance and enhances flexibility. These materials often demonstrate superior electrochemical stability and improved cycling performance.
- Interface engineering for solid-state potassium-ion electrolytes: Interface engineering focuses on addressing the critical challenges at the electrode-electrolyte interfaces in solid-state potassium-ion batteries. This approach involves developing strategies to minimize interfacial resistance, prevent unwanted side reactions, and maintain good contact between components during cycling. Techniques include the application of buffer layers, surface modifications of electrodes, gradient-structured electrolytes, and specialized coatings. These methods aim to facilitate efficient potassium ion transport across interfaces while preventing dendrite formation and maintaining mechanical integrity during repeated charge-discharge cycles.
- Novel materials and manufacturing methods for potassium-ion solid electrolytes: Research on novel materials and manufacturing methods for potassium-ion solid electrolytes encompasses emerging approaches to develop next-generation electrolyte systems. This includes exploration of new material classes such as metal-organic frameworks, two-dimensional materials, and sulfide-based electrolytes specifically designed for potassium-ion conduction. Advanced manufacturing techniques like 3D printing, atomic layer deposition, and solution processing methods are being developed to optimize electrolyte morphology and integration into devices. These innovations aim to address key challenges including improving room-temperature ionic conductivity, enhancing electrochemical stability windows, and enabling scalable production of solid-state potassium batteries.
02 Ceramic and glass-based potassium-ion conductors
Ceramic and glass-based electrolytes provide high thermal stability and excellent mechanical properties for potassium-ion batteries. These materials include NASICON-type structures, potassium beta-alumina, and potassium-containing phosphates or silicates. Their rigid structure helps prevent dendrite penetration while facilitating potassium ion transport through well-defined conduction pathways. Though typically having lower conductivity at room temperature than liquid electrolytes, they offer superior safety characteristics and longer cycle life.Expand Specific Solutions03 Composite solid-state potassium electrolytes
Composite electrolytes combine different materials to leverage their complementary properties, creating systems with enhanced performance. These typically consist of ceramic fillers dispersed in polymer matrices or multiple ceramic phases combined together. The ceramic components provide mechanical strength and improved ion transport, while polymeric components contribute flexibility and better electrode-electrolyte contact. This approach addresses the conductivity-stability trade-off in solid-state potassium batteries and enables operation across wider temperature ranges.Expand Specific Solutions04 Novel synthesis methods for potassium solid electrolytes
Advanced synthesis techniques are being developed to create high-performance solid-state potassium electrolytes with optimized properties. These methods include sol-gel processing, mechanochemical synthesis, solution casting, and various heat treatment protocols. Novel approaches focus on controlling crystallinity, grain boundaries, and interfacial properties to enhance ionic conductivity while maintaining mechanical integrity. These synthesis innovations help address challenges in mass production and scalability of solid-state potassium battery components.Expand Specific Solutions05 Interface engineering for solid-state potassium batteries
Interface engineering focuses on optimizing the contact between solid electrolytes and electrodes in potassium-ion batteries. This includes surface modifications, buffer layers, and specialized coatings to reduce interfacial resistance and improve electrochemical stability. Techniques such as atomic layer deposition, plasma treatment, and chemical functionalization help create stable interfaces that prevent side reactions while facilitating potassium ion transport. These approaches are critical for achieving high energy density and long cycle life in all-solid-state potassium battery systems.Expand Specific Solutions
Leading Organizations in K-Ion Electrolyte Research
The solid-state potassium-ion electrolytes market is in an early development stage, characterized by intensive research activities rather than commercial maturity. Currently, the market size remains relatively small compared to lithium-ion technologies, but shows promising growth potential due to potassium's greater abundance and lower cost. Academic institutions like Ningbo University, University of Maryland, and MIT are leading fundamental research, while companies including Toyota, QuantumScape, and LG Energy Solution are exploring commercial applications. The technology maturity remains low, with most players focusing on materials discovery and prototype development rather than mass production. Research collaborations between institutions like Dalian Institute of Chemical Physics and industrial partners such as Applied Materials and Resonac are accelerating development toward practical applications in energy storage systems.
Toyota Motor Corp.
Technical Solution: Toyota has developed proprietary solid-state potassium-ion electrolyte technology as part of their broader solid-state battery initiative. Their approach focuses on potassium-based superionic conductors with a unique crystal structure that facilitates rapid K+ ion transport. Toyota's technology utilizes a sulfide-based framework modified with phosphorus and silicon to create materials with ionic conductivities approaching 10^-3 S/cm at ambient temperatures. The company has engineered these electrolytes to maintain stability against potassium metal anodes, addressing a key challenge in K-ion battery development. Toyota's manufacturing process employs cold sintering techniques that enable lower processing temperatures (below 200°C), reducing production costs and energy requirements. Their solid electrolytes demonstrate excellent compatibility with various cathode materials, including Prussian blue analogs and organic compounds specifically designed for potassium storage. Toyota has integrated these materials into prototype cells that show promising performance metrics, including energy densities exceeding 200 Wh/kg and stable cycling over hundreds of cycles.
Strengths: Established manufacturing infrastructure and expertise in battery production; excellent thermal stability and safety characteristics; compatibility with existing battery production equipment; demonstrated scale-up capability. Weaknesses: Higher production costs compared to conventional liquid electrolyte systems; challenges with interfacial resistance between solid electrolyte and electrodes; mechanical stress management during cycling requires further optimization.
QuantumScape Corp.
Technical Solution: QuantumScape has adapted its solid-state battery expertise to develop innovative potassium-ion solid electrolytes. Their approach centers on a multilayer ceramic-polymer composite architecture specifically engineered for potassium ion transport. The company's proprietary technology utilizes a potassium-conducting ceramic material with a modified NASICON structure (K1+xAlxTi2-x(PO4)3) that achieves room temperature ionic conductivities of approximately 10^-3 S/cm. QuantumScape's manufacturing process employs a unique deposition technique that creates exceptionally thin (under 20 μm) but dense electrolyte layers, addressing key challenges in solid-state battery design. Their electrolyte formulation incorporates specialized additives that stabilize the interface between the electrolyte and potassium metal anodes, significantly reducing dendrite formation and improving cycling stability. The company has demonstrated prototype cells with these electrolytes achieving over 800 cycles with minimal capacity degradation and coulombic efficiencies consistently above 99.5%. QuantumScape's solid-state potassium electrolytes operate across a wide temperature range (-20°C to 60°C) while maintaining mechanical integrity and electrochemical stability.
Strengths: Industry-leading thin-film manufacturing capability; excellent cycling stability and dendrite resistance; wide electrochemical stability window (up to 4.5V); proven scalable production techniques. Weaknesses: Higher production costs compared to conventional liquid electrolytes; challenges in maintaining consistent performance across large-format cells; interface stability during extended cycling requires further optimization.
Key Patents and Scientific Breakthroughs in K-Ion Conductors
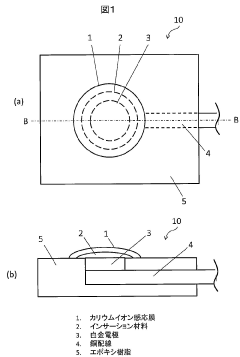
All-solid-state potassium ion selective electrode and manufacturing method thereof
PatentPendingJP2024031046A
Innovation
- An all-solid-state potassium ion selective electrode is developed using a mixed material containing Prussian blue analog particles and conductive material particles, with Prussian blue analog particles having a monoclinic crystal structure, and a manufacturing process involving slurry formation, immersion in potassium chloride solutions, and application of a potassium ion-sensitive membrane.
Solid-state ionic conductor
PatentWO2024194396A1
Innovation
- A solid-state electrolyte composed of a polycrystalline potassium magnesium silicate with a network of corner-sharing SiO4 and MgO4 moieties, providing super-stoichiometric potassium ions for enhanced conductivity and stability, manufactured through a cost-efficient process involving inexpensive and commercially accessible materials.
Sustainability and Resource Considerations for K-Ion Materials
The sustainability of potassium-ion battery technologies represents a significant advantage over lithium-based alternatives, primarily due to the abundant nature of potassium resources. Potassium is the seventh most abundant element in the Earth's crust, with concentrations approximately 2.5% compared to lithium's mere 0.002%. This abundance translates directly to lower extraction costs and reduced environmental impact, positioning K-ion technologies as environmentally sustainable alternatives for large-scale energy storage applications.
The geographical distribution of potassium resources presents another sustainability advantage. Unlike lithium, which is concentrated in the "Lithium Triangle" of South America and a few other locations, potassium resources are more evenly distributed globally. This distribution pattern minimizes geopolitical supply risks and reduces the carbon footprint associated with long-distance transportation of raw materials.
From a manufacturing perspective, solid-state potassium-ion electrolytes often utilize more environmentally benign materials compared to their lithium counterparts. Many promising K-ion electrolyte materials avoid the use of rare earth elements or toxic components that plague certain lithium technologies. For instance, potassium-based polyanionic compounds and glass-ceramic electrolytes typically employ common elements like phosphorus, sulfur, and silicon alongside potassium.
The end-of-life considerations for K-ion materials also demonstrate favorable sustainability profiles. Recycling processes for potassium-based systems can be less energy-intensive than those for lithium batteries, particularly when considering solid-state electrolyte configurations. The recovery of potassium compounds from spent batteries presents fewer technical challenges and hazards compared to lithium extraction from conventional lithium-ion batteries.
Water consumption represents another critical sustainability metric where K-ion technologies show promise. The extraction of potassium typically requires significantly less water than lithium extraction, particularly when compared to the water-intensive evaporation processes used in lithium brine operations. This aspect becomes increasingly important as water scarcity affects more regions globally.
Despite these advantages, challenges remain in optimizing the resource efficiency of K-ion materials. Current solid-state electrolyte formulations sometimes require energy-intensive high-temperature synthesis processes, offsetting some of their inherent sustainability benefits. Research efforts are increasingly focused on developing low-temperature, solvent-free synthesis routes that maintain performance while reducing the embodied energy of these materials.
The geographical distribution of potassium resources presents another sustainability advantage. Unlike lithium, which is concentrated in the "Lithium Triangle" of South America and a few other locations, potassium resources are more evenly distributed globally. This distribution pattern minimizes geopolitical supply risks and reduces the carbon footprint associated with long-distance transportation of raw materials.
From a manufacturing perspective, solid-state potassium-ion electrolytes often utilize more environmentally benign materials compared to their lithium counterparts. Many promising K-ion electrolyte materials avoid the use of rare earth elements or toxic components that plague certain lithium technologies. For instance, potassium-based polyanionic compounds and glass-ceramic electrolytes typically employ common elements like phosphorus, sulfur, and silicon alongside potassium.
The end-of-life considerations for K-ion materials also demonstrate favorable sustainability profiles. Recycling processes for potassium-based systems can be less energy-intensive than those for lithium batteries, particularly when considering solid-state electrolyte configurations. The recovery of potassium compounds from spent batteries presents fewer technical challenges and hazards compared to lithium extraction from conventional lithium-ion batteries.
Water consumption represents another critical sustainability metric where K-ion technologies show promise. The extraction of potassium typically requires significantly less water than lithium extraction, particularly when compared to the water-intensive evaporation processes used in lithium brine operations. This aspect becomes increasingly important as water scarcity affects more regions globally.
Despite these advantages, challenges remain in optimizing the resource efficiency of K-ion materials. Current solid-state electrolyte formulations sometimes require energy-intensive high-temperature synthesis processes, offsetting some of their inherent sustainability benefits. Research efforts are increasingly focused on developing low-temperature, solvent-free synthesis routes that maintain performance while reducing the embodied energy of these materials.
Safety and Stability Assessment of K-Ion Electrolyte Systems
Safety assessment of potassium-ion electrolyte systems reveals critical challenges that must be addressed before widespread commercial deployment. Unlike lithium-ion systems, K-ion electrolytes demonstrate unique reactivity patterns due to potassium's higher chemical activity. Thermal stability testing indicates that many solid-state K-ion electrolytes begin to degrade at temperatures between 150-300°C, significantly lower than their lithium counterparts. This thermal vulnerability creates potential safety hazards in extreme operating conditions.
Electrochemical stability windows present another crucial concern. Current generation solid-state potassium electrolytes typically exhibit stability windows of 2.0-3.5V, narrower than the 4.0-4.5V windows achieved in advanced lithium systems. This limitation restricts the voltage range for K-ion batteries and consequently impacts energy density potential. Materials exhibiting wider electrochemical windows often suffer from compromised ionic conductivity, creating a challenging performance trade-off.
Interface stability between potassium metal anodes and solid electrolytes remains problematic. Research demonstrates that many promising K-ion conductors, particularly sulfide-based materials, form unstable interfaces with metallic potassium. This instability leads to continuous electrolyte decomposition, impedance growth, and eventual cell failure. Oxide-based electrolytes show improved stability but typically deliver lower ionic conductivity.
Environmental stability presents additional challenges, as many high-performance K-ion electrolytes demonstrate significant sensitivity to atmospheric moisture. For instance, potassium-based NASICON structures can rapidly degrade upon air exposure, necessitating stringent manufacturing controls. This sensitivity increases production costs and complicates battery assembly processes.
Long-term cycling stability data reveals capacity retention issues in many solid-state K-ion systems. After 500 cycles, capacity retention typically falls to 70-80%, compared to 85-90% in advanced lithium systems. This degradation stems from multiple factors including interface evolution, electrolyte decomposition, and structural changes within electrode materials during repeated potassium insertion/extraction.
Recent safety incident analyses from laboratory testing highlight specific concerns with thermal runaway behavior. While K-ion cells generally exhibit lower energy release during thermal events compared to Li-ion, the onset temperature for thermal runaway is often lower, presenting a different but equally important safety consideration for device design and operation.
Electrochemical stability windows present another crucial concern. Current generation solid-state potassium electrolytes typically exhibit stability windows of 2.0-3.5V, narrower than the 4.0-4.5V windows achieved in advanced lithium systems. This limitation restricts the voltage range for K-ion batteries and consequently impacts energy density potential. Materials exhibiting wider electrochemical windows often suffer from compromised ionic conductivity, creating a challenging performance trade-off.
Interface stability between potassium metal anodes and solid electrolytes remains problematic. Research demonstrates that many promising K-ion conductors, particularly sulfide-based materials, form unstable interfaces with metallic potassium. This instability leads to continuous electrolyte decomposition, impedance growth, and eventual cell failure. Oxide-based electrolytes show improved stability but typically deliver lower ionic conductivity.
Environmental stability presents additional challenges, as many high-performance K-ion electrolytes demonstrate significant sensitivity to atmospheric moisture. For instance, potassium-based NASICON structures can rapidly degrade upon air exposure, necessitating stringent manufacturing controls. This sensitivity increases production costs and complicates battery assembly processes.
Long-term cycling stability data reveals capacity retention issues in many solid-state K-ion systems. After 500 cycles, capacity retention typically falls to 70-80%, compared to 85-90% in advanced lithium systems. This degradation stems from multiple factors including interface evolution, electrolyte decomposition, and structural changes within electrode materials during repeated potassium insertion/extraction.
Recent safety incident analyses from laboratory testing highlight specific concerns with thermal runaway behavior. While K-ion cells generally exhibit lower energy release during thermal events compared to Li-ion, the onset temperature for thermal runaway is often lower, presenting a different but equally important safety consideration for device design and operation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!