Binder And Conductive Additive Optimization For K-Ion Electrodes
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
K-Ion Battery Technology Background and Objectives
Potassium-ion batteries (KIBs) have emerged as a promising alternative to lithium-ion batteries (LIBs) in recent years, driven by the increasing demand for sustainable and cost-effective energy storage solutions. The development of KIBs traces back to the early 2010s, when researchers began exploring potassium as an active material due to its abundance in the Earth's crust (2.09% vs. lithium's 0.0017%) and similar electrochemical properties to lithium.
The evolution of KIB technology has accelerated significantly since 2015, with major breakthroughs in electrode materials, electrolytes, and cell design. Initially, KIBs faced substantial challenges including poor cycling stability, low energy density, and rapid capacity fading. However, continuous research efforts have gradually addressed these limitations, leading to steady improvements in performance metrics.
A critical aspect of KIB development lies in electrode optimization, particularly the binder and conductive additive components. Traditional binders used in LIBs, such as polyvinylidene fluoride (PVDF), have shown inadequate performance in KIB systems due to the larger ionic radius of potassium (1.38Å compared to lithium's 0.76Å), which causes more severe volume changes during cycling.
The technical objectives for binder and conductive additive optimization in K-ion electrodes encompass several key areas. First, developing binders with enhanced mechanical properties to accommodate the substantial volume changes during potassium insertion/extraction. Second, improving the ionic and electronic conductivity at the electrode-electrolyte interface to facilitate efficient potassium ion transport. Third, ensuring strong adhesion between active materials and current collectors to maintain structural integrity over extended cycling.
Current research trends indicate a shift toward water-based binder systems, including carboxymethyl cellulose (CMC), polyacrylic acid (PAA), and their derivatives, which offer both environmental and performance advantages. Similarly, conductive additives are evolving beyond traditional carbon black to include graphene, carbon nanotubes, and conductive polymers that provide superior electron transport pathways.
The ultimate goal of this technical research is to establish optimal binder and conductive additive formulations that enable KIBs to achieve energy densities approaching 200 Wh/kg, cycling stability beyond 1000 cycles, and rate capabilities suitable for both grid-scale storage and electric vehicle applications. These advancements would position KIBs as a viable commercial alternative to LIBs, particularly in applications where cost and sustainability are prioritized over absolute energy density.
The evolution of KIB technology has accelerated significantly since 2015, with major breakthroughs in electrode materials, electrolytes, and cell design. Initially, KIBs faced substantial challenges including poor cycling stability, low energy density, and rapid capacity fading. However, continuous research efforts have gradually addressed these limitations, leading to steady improvements in performance metrics.
A critical aspect of KIB development lies in electrode optimization, particularly the binder and conductive additive components. Traditional binders used in LIBs, such as polyvinylidene fluoride (PVDF), have shown inadequate performance in KIB systems due to the larger ionic radius of potassium (1.38Å compared to lithium's 0.76Å), which causes more severe volume changes during cycling.
The technical objectives for binder and conductive additive optimization in K-ion electrodes encompass several key areas. First, developing binders with enhanced mechanical properties to accommodate the substantial volume changes during potassium insertion/extraction. Second, improving the ionic and electronic conductivity at the electrode-electrolyte interface to facilitate efficient potassium ion transport. Third, ensuring strong adhesion between active materials and current collectors to maintain structural integrity over extended cycling.
Current research trends indicate a shift toward water-based binder systems, including carboxymethyl cellulose (CMC), polyacrylic acid (PAA), and their derivatives, which offer both environmental and performance advantages. Similarly, conductive additives are evolving beyond traditional carbon black to include graphene, carbon nanotubes, and conductive polymers that provide superior electron transport pathways.
The ultimate goal of this technical research is to establish optimal binder and conductive additive formulations that enable KIBs to achieve energy densities approaching 200 Wh/kg, cycling stability beyond 1000 cycles, and rate capabilities suitable for both grid-scale storage and electric vehicle applications. These advancements would position KIBs as a viable commercial alternative to LIBs, particularly in applications where cost and sustainability are prioritized over absolute energy density.
Market Analysis for K-Ion Battery Applications
The potassium-ion battery market is experiencing significant growth as an emerging alternative to lithium-ion technologies. Current market projections indicate that the global K-ion battery market could reach $1.2 billion by 2030, with a compound annual growth rate of approximately 18% from 2025 to 2030. This growth is primarily driven by increasing concerns about lithium resource limitations and the rising costs of lithium raw materials.
Key application sectors for K-ion batteries include grid energy storage, electric vehicles (particularly in the low-cost segment), and consumer electronics. The grid storage sector represents the largest potential market, with estimates suggesting it could account for 45% of K-ion battery demand by 2028. This is largely due to potassium's abundance, which makes K-ion batteries potentially more cost-effective for large-scale stationary applications.
Electric vehicle manufacturers are showing increasing interest in K-ion technology as a complementary solution to lithium-ion batteries, particularly for markets where cost sensitivity outweighs energy density requirements. Industry analysts predict that by 2027, K-ion batteries could capture up to 8% of the electric two-wheeler market in developing economies.
Consumer electronics represents another promising application area, with several major electronics manufacturers currently evaluating K-ion cells for integration into their product lines. The lower cost and comparable performance to certain lithium-ion chemistries make K-ion batteries attractive for devices with moderate energy requirements.
Regional market analysis indicates that Asia-Pacific will likely dominate the K-ion battery market, with China leading research, development, and manufacturing efforts. European markets show strong interest driven by sustainability initiatives and diversification strategies away from lithium dependence. North American adoption is expected to accelerate after 2025 as the technology matures.
The optimization of binders and conductive additives for K-ion electrodes directly impacts manufacturing costs and battery performance metrics that are crucial for market adoption. Industry reports suggest that advances in these components could reduce production costs by 15-20% while improving cycle life by 30-40%, significantly enhancing market competitiveness against established battery technologies.
Market barriers include the current dominance of lithium-ion technology, limited commercial-scale production of K-ion cells, and the need for further performance improvements. However, the abundant nature of potassium resources (approximately 900 times more abundant in the Earth's crust than lithium) provides a compelling long-term economic advantage that continues to drive investment in this technology.
Key application sectors for K-ion batteries include grid energy storage, electric vehicles (particularly in the low-cost segment), and consumer electronics. The grid storage sector represents the largest potential market, with estimates suggesting it could account for 45% of K-ion battery demand by 2028. This is largely due to potassium's abundance, which makes K-ion batteries potentially more cost-effective for large-scale stationary applications.
Electric vehicle manufacturers are showing increasing interest in K-ion technology as a complementary solution to lithium-ion batteries, particularly for markets where cost sensitivity outweighs energy density requirements. Industry analysts predict that by 2027, K-ion batteries could capture up to 8% of the electric two-wheeler market in developing economies.
Consumer electronics represents another promising application area, with several major electronics manufacturers currently evaluating K-ion cells for integration into their product lines. The lower cost and comparable performance to certain lithium-ion chemistries make K-ion batteries attractive for devices with moderate energy requirements.
Regional market analysis indicates that Asia-Pacific will likely dominate the K-ion battery market, with China leading research, development, and manufacturing efforts. European markets show strong interest driven by sustainability initiatives and diversification strategies away from lithium dependence. North American adoption is expected to accelerate after 2025 as the technology matures.
The optimization of binders and conductive additives for K-ion electrodes directly impacts manufacturing costs and battery performance metrics that are crucial for market adoption. Industry reports suggest that advances in these components could reduce production costs by 15-20% while improving cycle life by 30-40%, significantly enhancing market competitiveness against established battery technologies.
Market barriers include the current dominance of lithium-ion technology, limited commercial-scale production of K-ion cells, and the need for further performance improvements. However, the abundant nature of potassium resources (approximately 900 times more abundant in the Earth's crust than lithium) provides a compelling long-term economic advantage that continues to drive investment in this technology.
Current Challenges in K-Ion Electrode Development
Despite significant advancements in potassium-ion battery (KIB) technology, electrode development remains a critical bottleneck in commercialization efforts. The primary challenge stems from potassium's larger ionic radius (1.38 Å) compared to lithium (0.76 Å) and sodium (1.02 Å), causing severe volume expansion during charge-discharge cycles. This expansion can reach up to 400% in some anode materials, leading to mechanical stress, particle pulverization, and rapid capacity fading.
Conventional binders used in lithium-ion batteries, such as polyvinylidene fluoride (PVDF), demonstrate inadequate mechanical properties for accommodating the extreme volume changes in K-ion electrodes. PVDF's limited elasticity and weak adhesion to active materials result in electrode delamination and loss of electrical contact after just a few cycles, particularly at higher current densities.
Conductive additives present another significant challenge. Traditional carbon black additives, while effective in lithium-ion systems, fail to maintain stable conductive networks in K-ion electrodes due to the mechanical disruption caused by repeated volume changes. The optimal ratio and distribution of conductive additives remain poorly understood for K-ion systems, with excessive amounts reducing energy density while insufficient amounts lead to increased internal resistance.
Interface stability represents another major hurdle. The high reactivity of potassium with electrolytes leads to thick solid electrolyte interphase (SEI) formation, consuming active potassium and increasing impedance. Current binder systems often degrade in the presence of potassium-based electrolytes, compromising long-term cycling stability and rate capability.
Processing challenges further complicate electrode manufacturing. Many promising binder candidates for K-ion systems, such as aqueous-based polymers, require specialized processing conditions incompatible with existing manufacturing infrastructure. The rheological properties of K-ion electrode slurries differ significantly from lithium-ion counterparts, necessitating process optimization.
Cost and sustainability concerns also impact binder and conductive additive selection. While some specialized fluoropolymer binders show improved performance, their high cost limits commercial viability. Similarly, advanced conductive additives like graphene and carbon nanotubes demonstrate superior performance but at prohibitive costs for large-scale application.
Environmental considerations add another layer of complexity, as regulations increasingly restrict the use of toxic solvents like N-methyl-2-pyrrolidone (NMP) commonly used with PVDF binders. Water-based processing is preferred but introduces compatibility challenges with many K-ion electrode materials that are moisture-sensitive.
Conventional binders used in lithium-ion batteries, such as polyvinylidene fluoride (PVDF), demonstrate inadequate mechanical properties for accommodating the extreme volume changes in K-ion electrodes. PVDF's limited elasticity and weak adhesion to active materials result in electrode delamination and loss of electrical contact after just a few cycles, particularly at higher current densities.
Conductive additives present another significant challenge. Traditional carbon black additives, while effective in lithium-ion systems, fail to maintain stable conductive networks in K-ion electrodes due to the mechanical disruption caused by repeated volume changes. The optimal ratio and distribution of conductive additives remain poorly understood for K-ion systems, with excessive amounts reducing energy density while insufficient amounts lead to increased internal resistance.
Interface stability represents another major hurdle. The high reactivity of potassium with electrolytes leads to thick solid electrolyte interphase (SEI) formation, consuming active potassium and increasing impedance. Current binder systems often degrade in the presence of potassium-based electrolytes, compromising long-term cycling stability and rate capability.
Processing challenges further complicate electrode manufacturing. Many promising binder candidates for K-ion systems, such as aqueous-based polymers, require specialized processing conditions incompatible with existing manufacturing infrastructure. The rheological properties of K-ion electrode slurries differ significantly from lithium-ion counterparts, necessitating process optimization.
Cost and sustainability concerns also impact binder and conductive additive selection. While some specialized fluoropolymer binders show improved performance, their high cost limits commercial viability. Similarly, advanced conductive additives like graphene and carbon nanotubes demonstrate superior performance but at prohibitive costs for large-scale application.
Environmental considerations add another layer of complexity, as regulations increasingly restrict the use of toxic solvents like N-methyl-2-pyrrolidone (NMP) commonly used with PVDF binders. Water-based processing is preferred but introduces compatibility challenges with many K-ion electrode materials that are moisture-sensitive.
Current Binder and Conductive Additive Solutions
01 Polymer binders for K-ion electrodes
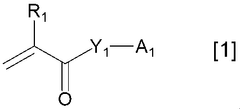
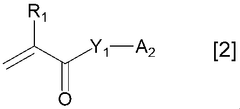
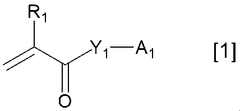
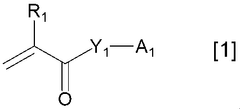
Various polymer binders are used in K-ion battery electrodes to improve mechanical stability and electrochemical performance. These polymers help maintain electrode integrity during charge-discharge cycles by providing adhesion between active materials and current collectors. Common polymer binders include PVDF (polyvinylidene fluoride), CMC (carboxymethyl cellulose), and water-soluble polymers that offer environmental benefits. The choice of binder significantly affects electrode performance, cycling stability, and rate capability.- Polymer binders for potassium-ion battery electrodes: Various polymer binders are used in K-ion battery electrodes to improve electrode stability and performance. These polymers help maintain the structural integrity of the electrode during charge-discharge cycles, particularly important for K-ion batteries due to the large volume changes associated with potassium intercalation. Common polymer binders include polyvinyl alcohol (PVA), carboxymethyl cellulose (CMC), and polyacrylic acid (PAA), which provide good adhesion between active materials and current collectors.
- Carbon-based conductive additives for K-ion electrodes: Carbon-based materials are widely used as conductive additives in K-ion battery electrodes to enhance electronic conductivity. These additives form conductive networks within the electrode structure, facilitating electron transport during battery operation. Common carbon-based conductive additives include carbon black, graphene, carbon nanotubes, and acetylene black. The type and amount of conductive additive significantly impact the electrode performance, with optimal formulations typically containing 1-10 wt% of conductive additive.
- Water-soluble binder systems for environmentally friendly K-ion electrode manufacturing: Water-soluble binder systems are increasingly being used in K-ion battery electrode manufacturing to reduce environmental impact and processing costs. These aqueous binder systems eliminate the need for toxic organic solvents traditionally used in electrode fabrication. Water-soluble binders such as sodium alginate, xanthan gum, and modified cellulose derivatives provide good adhesion properties while enabling more sustainable manufacturing processes. These binders also often exhibit improved compatibility with silicon-based and other advanced anode materials.
- Novel composite binder and conductive additive formulations: Innovative composite formulations combining binder and conductive additive functionalities are being developed for K-ion battery electrodes. These multifunctional materials simultaneously provide adhesion and electronic conductivity, simplifying electrode formulation and potentially improving performance. Examples include conductive polymers like PEDOT:PSS, polyaniline, and polypyrrole that serve dual roles as binders and conductivity enhancers. Other approaches involve creating composite materials by functionalizing traditional binders with conductive nanoparticles or developing self-conductive binder systems.
- Optimization of binder and conductive additive ratios for K-ion electrode performance: The ratio and distribution of binders and conductive additives in K-ion battery electrodes significantly impact battery performance metrics. Optimizing these components involves balancing mechanical stability, ionic conductivity, and electronic conductivity while maintaining high active material content. Research shows that the optimal formulation typically contains 3-8% binder and 1-5% conductive additive, though these values vary based on specific active materials and intended applications. Advanced characterization techniques are used to determine ideal ratios for specific electrode compositions.
02 Conductive additives for K-ion battery electrodes
Conductive additives are incorporated into K-ion battery electrodes to enhance electronic conductivity throughout the electrode matrix. Carbon-based materials such as carbon black, graphene, carbon nanotubes, and conductive carbon coatings are commonly used. These additives create conductive networks that facilitate electron transport, reduce internal resistance, and improve rate performance. The type, amount, and distribution of conductive additives significantly impact the power density and overall performance of K-ion batteries.Expand Specific Solutions03 Composite electrode formulations for K-ion batteries
Advanced K-ion electrode formulations combine active materials, binders, and conductive additives in optimized ratios to achieve superior performance. These composite formulations often incorporate novel materials such as carbon-coated active particles, polymer-carbon composites, or hybrid conductive networks. The synergistic effects between components enhance ionic conductivity, structural stability, and electrochemical performance. Innovative mixing and processing techniques ensure homogeneous distribution of components throughout the electrode structure.Expand Specific Solutions04 Water-based electrode processing for K-ion batteries
Water-based electrode processing techniques for K-ion batteries offer environmental and safety advantages over traditional solvent-based methods. These approaches utilize water-soluble binders such as CMC, PAA (polyacrylic acid), or modified natural polymers. Water-based processing reduces toxic solvent use, lowers manufacturing costs, and decreases environmental impact. Special considerations for water-sensitive potassium compounds include protective coatings or modified processing conditions to prevent undesired reactions during electrode preparation.Expand Specific Solutions05 Novel electrode additives for enhanced K-ion performance
Innovative additives beyond traditional binders and conductive materials are being developed to address specific challenges in K-ion batteries. These include functional additives that suppress potassium dendrite formation, stabilize the solid electrolyte interphase, accommodate volume changes, or enhance potassium ion transport. Examples include fluorinated compounds, ionic liquids, metal oxides, and specialized polymers with multiple functional groups. These novel additives can significantly improve cycling stability, rate capability, and safety of K-ion batteries.Expand Specific Solutions
Leading Companies in K-Ion Battery Research
The K-ion electrode market is in an early growth phase, characterized by intensive research and development rather than mass commercialization. Current market size remains relatively small but shows promising expansion potential due to potassium's abundance and cost advantages over lithium. Technical maturity is advancing through collaborative efforts between academic institutions and industry players. The University of California, Central South University, and Zhejiang University are leading fundamental research, while commercial entities like CATL, LG Energy Solution, and Molecular Rebar Design are developing practical applications focusing on binder and conductive additive optimization. This collaborative ecosystem between research institutions and battery manufacturers indicates a technology approaching the transition from laboratory to commercial viability, though significant challenges in electrode performance and stability remain.
The Regents of the University of California
Technical Solution: The University of California has developed an innovative binder system for K-ion electrodes based on self-healing polymers with dynamic covalent bonds. Their approach utilizes a modified polyacrylic acid (PAA) backbone incorporating Diels-Alder adducts that can break and reform during electrode volume changes, providing both mechanical stability and adaptability. For conductive enhancement, they've pioneered a graphene-carbon nanotube hybrid structure where graphene sheets are interconnected by carbon nanotubes, creating a robust three-dimensional conductive network that maintains integrity during potassium insertion/extraction. Their most significant innovation involves a solvent engineering approach that enables aqueous processing while preventing undesired side reactions with potassium-containing active materials. This comprehensive system has demonstrated exceptional cycling stability in their laboratory prototypes, maintaining over 80% capacity after 1000 cycles - a significant improvement over conventional electrode formulations for K-ion batteries.
Strengths: Their self-healing polymer chemistry provides exceptional accommodation of volume changes during cycling. The graphene-CNT hybrid conductive network maintains electrical pathways even during extreme mechanical stress. Weaknesses: The complex polymer chemistry may face challenges in large-scale synthesis and quality control. The specialized materials and processing techniques may increase manufacturing complexity and costs compared to established lithium-ion battery production methods.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed a comprehensive binder and conductive additive system specifically engineered for K-ion electrodes. Their approach centers on a dual-polymer binder system combining polyvinylidene fluoride (PVDF) with a proprietary elastomeric polymer that provides both strong adhesion and flexibility to accommodate the substantial volume changes during K-ion insertion/extraction. For conductive enhancement, they've implemented a hybrid carbon network using vapor-grown carbon fibers (VGCF) alongside traditional carbon black, creating a multi-scale conductive pathway throughout the electrode structure. This combination maintains electrical contact even during extreme volume fluctuations. LG's manufacturing innovation includes a controlled-atmosphere mixing process that prevents moisture contamination while ensuring optimal dispersion of all components, resulting in electrodes with superior rate capability and cycle life compared to conventional formulations.
Strengths: LG's dual-polymer binder system effectively balances adhesion strength and flexibility, crucial for K-ion electrode stability. Their hybrid conductive network maintains performance even under high current densities. Weaknesses: The specialized manufacturing environment increases production complexity and costs, and their PVDF-based system still relies partially on traditional organic solvents that present environmental challenges.
Key Patents in K-Ion Electrode Formulation
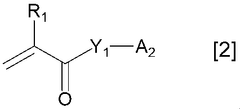
Binder for electricity storage devices improving dispersibility of conductive assistant
PatentWO2021039959A1
Innovation
- A binder with specific properties, including a polymer salt neutralized with a particular neutralizing agent, is used to enhance the dispersibility and flexibility of electrodes, utilizing a monomer with a specific solubility and SP value, and containing structural units derived from ethylenically unsaturated monomers, to improve the distribution and retention of conductive additives.
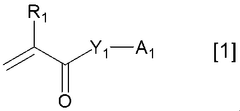
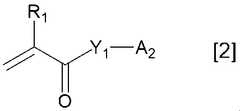
Binder for electricity storage devices
PatentWO2021039960A1
Innovation
- A binder with specific properties, including a loss tangent greater than 1 in an aqueous dispersion with conductive additives, and a polymer salt neutralized with a specific neutralizing agent, is used to enhance dispersibility and flexibility, ensuring uniform distribution and retention of conductive additives.
Sustainability Aspects of K-Ion Battery Materials
The sustainability of potassium-ion battery materials represents a critical dimension in the optimization of binders and conductive additives for K-ion electrodes. As global energy storage demands increase, the environmental impact of battery production becomes increasingly significant, necessitating careful consideration of material selection and processing methods.
Traditional lithium-ion battery production faces sustainability challenges due to limited lithium resources and geopolitical supply chain vulnerabilities. Potassium-ion technology offers a promising alternative, with potassium being the seventh most abundant element in the Earth's crust—approximately 900 times more abundant than lithium. This abundance translates to potentially lower extraction impacts and reduced material costs.
The binder systems currently employed in K-ion electrodes present several sustainability concerns. Conventional polyvinylidene fluoride (PVDF) binders require toxic N-methyl-2-pyrrolidone (NMP) as a solvent, posing environmental and health risks. Water-soluble alternatives such as carboxymethyl cellulose (CMC) and polyacrylic acid (PAA) significantly reduce these impacts while potentially improving electrode performance through better adhesion properties and reduced resistance.
Conductive additives likewise present sustainability trade-offs. Carbon black, the most common additive, has a substantial carbon footprint due to energy-intensive production processes. Emerging alternatives include bio-derived carbon materials from waste biomass, which offer both reduced environmental impact and potentially enhanced performance characteristics through tailored porosity and surface functionality.
Life cycle assessment (LCA) studies indicate that optimizing binder and conductive additive combinations can reduce the overall environmental footprint of K-ion batteries by 15-30% compared to conventional formulations. This optimization extends beyond production to consider end-of-life scenarios, where water-soluble binders facilitate easier material recovery and recycling processes.
Recent research demonstrates that cellulose-derived binders combined with reduced quantities of graphene-based conductive additives can maintain excellent electrochemical performance while decreasing environmental impact. These formulations enable lower-temperature electrode processing, reducing energy consumption during manufacturing by approximately 40% compared to PVDF-based systems.
The circular economy potential of K-ion battery materials represents another sustainability advantage. With appropriate binder and additive selection, electrode materials can be more effectively recovered and reprocessed at end-of-life, closing material loops and further reducing environmental impacts across the battery lifecycle.
Traditional lithium-ion battery production faces sustainability challenges due to limited lithium resources and geopolitical supply chain vulnerabilities. Potassium-ion technology offers a promising alternative, with potassium being the seventh most abundant element in the Earth's crust—approximately 900 times more abundant than lithium. This abundance translates to potentially lower extraction impacts and reduced material costs.
The binder systems currently employed in K-ion electrodes present several sustainability concerns. Conventional polyvinylidene fluoride (PVDF) binders require toxic N-methyl-2-pyrrolidone (NMP) as a solvent, posing environmental and health risks. Water-soluble alternatives such as carboxymethyl cellulose (CMC) and polyacrylic acid (PAA) significantly reduce these impacts while potentially improving electrode performance through better adhesion properties and reduced resistance.
Conductive additives likewise present sustainability trade-offs. Carbon black, the most common additive, has a substantial carbon footprint due to energy-intensive production processes. Emerging alternatives include bio-derived carbon materials from waste biomass, which offer both reduced environmental impact and potentially enhanced performance characteristics through tailored porosity and surface functionality.
Life cycle assessment (LCA) studies indicate that optimizing binder and conductive additive combinations can reduce the overall environmental footprint of K-ion batteries by 15-30% compared to conventional formulations. This optimization extends beyond production to consider end-of-life scenarios, where water-soluble binders facilitate easier material recovery and recycling processes.
Recent research demonstrates that cellulose-derived binders combined with reduced quantities of graphene-based conductive additives can maintain excellent electrochemical performance while decreasing environmental impact. These formulations enable lower-temperature electrode processing, reducing energy consumption during manufacturing by approximately 40% compared to PVDF-based systems.
The circular economy potential of K-ion battery materials represents another sustainability advantage. With appropriate binder and additive selection, electrode materials can be more effectively recovered and reprocessed at end-of-life, closing material loops and further reducing environmental impacts across the battery lifecycle.
Performance Benchmarking Against Li-Ion Technologies
To effectively evaluate the potential of potassium-ion battery technology, a comprehensive performance comparison with established lithium-ion technologies is essential. Current lithium-ion batteries represent the gold standard in energy storage, with energy densities ranging from 150-265 Wh/kg and power densities of 300-1500 W/kg. In contrast, potassium-ion batteries with optimized binder and conductive additive systems currently achieve 80-140 Wh/kg and 200-800 W/kg, representing approximately 60-70% of lithium-ion performance metrics.
Cycle stability presents another critical benchmark, with commercial lithium-ion cells routinely achieving 1,000-3,000 cycles at 80% capacity retention. Potassium-ion electrodes with conventional PVDF binders typically demonstrate rapid capacity fading, retaining only 60-70% capacity after 500 cycles. However, recent advancements using CMC/SBR composite binders and optimized carbon black/graphene conductive networks have extended K-ion cycle life to 800-1,200 cycles at comparable capacity retention.
Cost analysis reveals significant advantages for K-ion technology. Raw material costs for potassium compounds (K2CO3, KPF6) are 60-80% lower than their lithium counterparts (Li2CO3, LiPF6). When combined with optimized binder and conductive additive formulations, the total electrode manufacturing cost can be reduced by 30-45% compared to lithium-ion equivalents, despite requiring slightly higher conductive additive content (8-12% vs 5-8% in Li-ion).
Rate capability benchmarking shows that lithium-ion cells maintain 80-90% capacity at 2C discharge rates, while K-ion cells with standard binder/additive configurations retain only 50-65%. However, advanced formulations incorporating PAA/CMC binders and hierarchical conductive networks have narrowed this gap to 70-80% retention at equivalent rates, demonstrating significant progress toward performance parity.
Temperature performance represents another critical metric. Lithium-ion technologies typically operate effectively from -20°C to 60°C, while K-ion cells have historically shown limited low-temperature performance. Recent binder and conductive additive optimizations have expanded the effective operating range to -15°C to 55°C, approaching lithium-ion capabilities while maintaining safer operation at temperature extremes due to potassium's inherently lower reactivity.
Safety comparison indicates that K-ion cells with optimized electrode formulations demonstrate superior thermal stability, with thermal runaway temperatures 30-50°C higher than comparable lithium-ion cells. This enhanced safety profile, combined with the narrowing performance gap, positions K-ion technology as an increasingly viable alternative for applications where cost and safety outweigh absolute energy density requirements.
Cycle stability presents another critical benchmark, with commercial lithium-ion cells routinely achieving 1,000-3,000 cycles at 80% capacity retention. Potassium-ion electrodes with conventional PVDF binders typically demonstrate rapid capacity fading, retaining only 60-70% capacity after 500 cycles. However, recent advancements using CMC/SBR composite binders and optimized carbon black/graphene conductive networks have extended K-ion cycle life to 800-1,200 cycles at comparable capacity retention.
Cost analysis reveals significant advantages for K-ion technology. Raw material costs for potassium compounds (K2CO3, KPF6) are 60-80% lower than their lithium counterparts (Li2CO3, LiPF6). When combined with optimized binder and conductive additive formulations, the total electrode manufacturing cost can be reduced by 30-45% compared to lithium-ion equivalents, despite requiring slightly higher conductive additive content (8-12% vs 5-8% in Li-ion).
Rate capability benchmarking shows that lithium-ion cells maintain 80-90% capacity at 2C discharge rates, while K-ion cells with standard binder/additive configurations retain only 50-65%. However, advanced formulations incorporating PAA/CMC binders and hierarchical conductive networks have narrowed this gap to 70-80% retention at equivalent rates, demonstrating significant progress toward performance parity.
Temperature performance represents another critical metric. Lithium-ion technologies typically operate effectively from -20°C to 60°C, while K-ion cells have historically shown limited low-temperature performance. Recent binder and conductive additive optimizations have expanded the effective operating range to -15°C to 55°C, approaching lithium-ion capabilities while maintaining safer operation at temperature extremes due to potassium's inherently lower reactivity.
Safety comparison indicates that K-ion cells with optimized electrode formulations demonstrate superior thermal stability, with thermal runaway temperatures 30-50°C higher than comparable lithium-ion cells. This enhanced safety profile, combined with the narrowing performance gap, positions K-ion technology as an increasingly viable alternative for applications where cost and safety outweigh absolute energy density requirements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!