Cathode Materials Comparison For Potassium-Ion Systems
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Potassium-Ion Battery Cathode Evolution and Objectives
Potassium-ion batteries (PIBs) have emerged as a promising alternative to lithium-ion batteries due to the abundance and low cost of potassium resources. The evolution of cathode materials for PIBs represents a critical aspect of their development trajectory. Initially, research focused on adapting lithium-ion battery cathode materials for potassium systems, with early investigations centered on layered transition metal oxides and Prussian blue analogs around 2004-2010.
The period from 2010 to 2015 marked significant advancements in understanding the fundamental intercalation chemistry of potassium ions in various host structures. Researchers identified key challenges including the large ionic radius of K+ (1.38 Å compared to 0.76 Å for Li+), which causes substantial structural strain during insertion/extraction processes, leading to capacity fading and reduced cycle life.
Between 2015 and 2020, research efforts intensified with the development of novel cathode materials specifically designed for potassium systems. These included polyanionic compounds, organic materials, and modified layered oxides with expanded interlayer spacing to accommodate the larger K+ ions. Concurrently, computational studies provided deeper insights into ion diffusion pathways and structural stability mechanisms.
Recent developments (2020-present) have focused on nanostructuring approaches and composite materials to enhance electrochemical performance. Researchers have explored various strategies including carbon coating, heteroatom doping, and defect engineering to improve electronic conductivity and structural stability during cycling.
The primary technical objectives for PIB cathode development include achieving high specific capacity (>200 mAh/g), excellent rate capability, and long cycle life (>1000 cycles with minimal capacity degradation). Additionally, researchers aim to develop cathodes that operate at higher voltages (>3.5V vs. K/K+) to increase energy density while maintaining structural stability.
Another critical objective is to minimize the use of critical elements such as cobalt and nickel, focusing instead on earth-abundant elements like iron, manganese, and vanadium. This aligns with sustainability goals and addresses supply chain concerns associated with conventional lithium-ion battery materials.
Understanding the fundamental structure-property relationships in potassium cathode materials remains a key research priority. This includes elucidating the mechanisms of K+ insertion/extraction, identifying rate-limiting steps in the electrochemical processes, and developing strategies to mitigate structural degradation during long-term cycling.
The technological trajectory suggests a convergence toward multi-component cathode systems that combine the advantages of different material classes. These hybrid approaches aim to overcome the inherent limitations of single-component cathodes and achieve performance metrics comparable to commercial lithium-ion systems while leveraging the cost advantages of potassium-based chemistry.
The period from 2010 to 2015 marked significant advancements in understanding the fundamental intercalation chemistry of potassium ions in various host structures. Researchers identified key challenges including the large ionic radius of K+ (1.38 Å compared to 0.76 Å for Li+), which causes substantial structural strain during insertion/extraction processes, leading to capacity fading and reduced cycle life.
Between 2015 and 2020, research efforts intensified with the development of novel cathode materials specifically designed for potassium systems. These included polyanionic compounds, organic materials, and modified layered oxides with expanded interlayer spacing to accommodate the larger K+ ions. Concurrently, computational studies provided deeper insights into ion diffusion pathways and structural stability mechanisms.
Recent developments (2020-present) have focused on nanostructuring approaches and composite materials to enhance electrochemical performance. Researchers have explored various strategies including carbon coating, heteroatom doping, and defect engineering to improve electronic conductivity and structural stability during cycling.
The primary technical objectives for PIB cathode development include achieving high specific capacity (>200 mAh/g), excellent rate capability, and long cycle life (>1000 cycles with minimal capacity degradation). Additionally, researchers aim to develop cathodes that operate at higher voltages (>3.5V vs. K/K+) to increase energy density while maintaining structural stability.
Another critical objective is to minimize the use of critical elements such as cobalt and nickel, focusing instead on earth-abundant elements like iron, manganese, and vanadium. This aligns with sustainability goals and addresses supply chain concerns associated with conventional lithium-ion battery materials.
Understanding the fundamental structure-property relationships in potassium cathode materials remains a key research priority. This includes elucidating the mechanisms of K+ insertion/extraction, identifying rate-limiting steps in the electrochemical processes, and developing strategies to mitigate structural degradation during long-term cycling.
The technological trajectory suggests a convergence toward multi-component cathode systems that combine the advantages of different material classes. These hybrid approaches aim to overcome the inherent limitations of single-component cathodes and achieve performance metrics comparable to commercial lithium-ion systems while leveraging the cost advantages of potassium-based chemistry.
Market Analysis for K-Ion Battery Applications
The potassium-ion battery market is experiencing significant growth potential as an alternative to lithium-ion technologies. Current market projections indicate that the global K-ion battery market could reach $1.2 billion by 2030, with a compound annual growth rate of approximately 18% from 2025 to 2030. This growth is primarily driven by increasing concerns about lithium resource limitations and price volatility in the lithium supply chain.
Key market segments for K-ion battery applications include grid energy storage, low-cost consumer electronics, and electric bicycles. The grid storage sector represents the largest potential market, with estimates suggesting it could account for 45% of K-ion battery demand by 2028. This is particularly relevant in regions with developing infrastructure where cost considerations outweigh energy density requirements.
Consumer electronics manufacturers are showing increasing interest in K-ion technology for applications where cost efficiency is prioritized over maximum energy density. Market research indicates that approximately 30% of low-to-mid-range portable electronic devices could potentially utilize K-ion batteries by 2029, representing a substantial market opportunity.
The electric mobility sector, particularly electric bicycles and scooters in emerging markets, presents another significant application area. These markets are highly price-sensitive, making the lower-cost profile of K-ion batteries particularly attractive despite their somewhat lower energy density compared to lithium-ion alternatives.
Geographically, Asia-Pacific dominates the potential market landscape, with China leading research and development investments. European markets show growing interest driven by sustainability initiatives and diversification strategies for battery technologies. North American markets remain more focused on high-performance applications where lithium-ion technologies currently maintain advantages.
Market barriers include competition from established lithium-ion technology, which benefits from economies of scale and extensive manufacturing infrastructure. Additionally, the relatively lower energy density of current K-ion cathode materials presents challenges for applications where space and weight are critical factors.
Customer demand analysis reveals strong interest in sustainable battery technologies with reduced supply chain risks. Corporate sustainability initiatives and governmental policies promoting resource diversification are creating favorable market conditions for alternative battery chemistries like potassium-ion systems.
The cost advantage of potassium-based cathode materials represents the most significant market driver, with raw material costs approximately 60-80% lower than comparable lithium compounds. This economic advantage positions K-ion batteries favorably for price-sensitive applications and markets where total cost of ownership outweighs maximum performance considerations.
Key market segments for K-ion battery applications include grid energy storage, low-cost consumer electronics, and electric bicycles. The grid storage sector represents the largest potential market, with estimates suggesting it could account for 45% of K-ion battery demand by 2028. This is particularly relevant in regions with developing infrastructure where cost considerations outweigh energy density requirements.
Consumer electronics manufacturers are showing increasing interest in K-ion technology for applications where cost efficiency is prioritized over maximum energy density. Market research indicates that approximately 30% of low-to-mid-range portable electronic devices could potentially utilize K-ion batteries by 2029, representing a substantial market opportunity.
The electric mobility sector, particularly electric bicycles and scooters in emerging markets, presents another significant application area. These markets are highly price-sensitive, making the lower-cost profile of K-ion batteries particularly attractive despite their somewhat lower energy density compared to lithium-ion alternatives.
Geographically, Asia-Pacific dominates the potential market landscape, with China leading research and development investments. European markets show growing interest driven by sustainability initiatives and diversification strategies for battery technologies. North American markets remain more focused on high-performance applications where lithium-ion technologies currently maintain advantages.
Market barriers include competition from established lithium-ion technology, which benefits from economies of scale and extensive manufacturing infrastructure. Additionally, the relatively lower energy density of current K-ion cathode materials presents challenges for applications where space and weight are critical factors.
Customer demand analysis reveals strong interest in sustainable battery technologies with reduced supply chain risks. Corporate sustainability initiatives and governmental policies promoting resource diversification are creating favorable market conditions for alternative battery chemistries like potassium-ion systems.
The cost advantage of potassium-based cathode materials represents the most significant market driver, with raw material costs approximately 60-80% lower than comparable lithium compounds. This economic advantage positions K-ion batteries favorably for price-sensitive applications and markets where total cost of ownership outweighs maximum performance considerations.
Current Challenges in K-Ion Cathode Development
Despite significant advancements in potassium-ion battery research, cathode material development remains a critical bottleneck. The large ionic radius of K+ (1.38 Å) compared to Li+ (0.76 Å) and Na+ (1.02 Å) creates substantial challenges for intercalation chemistry, leading to severe structural instability during charge-discharge cycles. This fundamental issue manifests as rapid capacity fading and shortened battery lifespans, particularly evident in layered oxide materials that show promising initial capacity but poor cycling stability.
Volume expansion during K+ insertion/extraction represents another significant hurdle, with many cathode materials experiencing lattice parameter changes exceeding 10%. This expansion leads to mechanical stress, particle cracking, and eventual electrode pulverization, compromising both electronic and ionic conductivity pathways. The structural degradation accelerates with cycling, creating a compounding negative effect on battery performance.
Potassium's high reactivity presents additional challenges for electrolyte compatibility and interfacial stability. The cathode-electrolyte interface often suffers from parasitic reactions, leading to the formation of resistive surface layers that impede ion transport. These reactions consume active material and electrolyte components, contributing to capacity loss and impedance growth over time.
Energy density limitations persist across most K-ion cathode materials. Current state-of-the-art materials typically deliver specific capacities below 150 mAh/g and operating voltages around 3.5-4.0V, resulting in energy densities significantly lower than commercial lithium-ion systems. This gap must be narrowed for K-ion batteries to become commercially competitive beyond niche applications.
The synthesis of high-quality, phase-pure K-containing cathode materials presents unique difficulties due to potassium's high reactivity with moisture and CO2. Conventional synthesis routes often yield materials with potassium deficiency or unwanted impurity phases, necessitating specialized handling techniques and modified synthesis protocols that add complexity and cost to manufacturing processes.
Rate capability remains suboptimal in most K-ion cathodes due to sluggish K+ diffusion kinetics within host structures. The large ionic radius creates high migration barriers, limiting power density and practical application scenarios where rapid charging is required. This limitation is particularly pronounced at lower temperatures, where ion mobility is further reduced.
Sustainable and scalable material selection represents an emerging challenge as research moves toward commercialization. While certain high-performance cathodes incorporate critical elements like cobalt or vanadium, long-term viability demands transition toward earth-abundant, low-cost alternatives that maintain performance metrics while enabling large-scale production.
Volume expansion during K+ insertion/extraction represents another significant hurdle, with many cathode materials experiencing lattice parameter changes exceeding 10%. This expansion leads to mechanical stress, particle cracking, and eventual electrode pulverization, compromising both electronic and ionic conductivity pathways. The structural degradation accelerates with cycling, creating a compounding negative effect on battery performance.
Potassium's high reactivity presents additional challenges for electrolyte compatibility and interfacial stability. The cathode-electrolyte interface often suffers from parasitic reactions, leading to the formation of resistive surface layers that impede ion transport. These reactions consume active material and electrolyte components, contributing to capacity loss and impedance growth over time.
Energy density limitations persist across most K-ion cathode materials. Current state-of-the-art materials typically deliver specific capacities below 150 mAh/g and operating voltages around 3.5-4.0V, resulting in energy densities significantly lower than commercial lithium-ion systems. This gap must be narrowed for K-ion batteries to become commercially competitive beyond niche applications.
The synthesis of high-quality, phase-pure K-containing cathode materials presents unique difficulties due to potassium's high reactivity with moisture and CO2. Conventional synthesis routes often yield materials with potassium deficiency or unwanted impurity phases, necessitating specialized handling techniques and modified synthesis protocols that add complexity and cost to manufacturing processes.
Rate capability remains suboptimal in most K-ion cathodes due to sluggish K+ diffusion kinetics within host structures. The large ionic radius creates high migration barriers, limiting power density and practical application scenarios where rapid charging is required. This limitation is particularly pronounced at lower temperatures, where ion mobility is further reduced.
Sustainable and scalable material selection represents an emerging challenge as research moves toward commercialization. While certain high-performance cathodes incorporate critical elements like cobalt or vanadium, long-term viability demands transition toward earth-abundant, low-cost alternatives that maintain performance metrics while enabling large-scale production.
Comparative Analysis of Current Cathode Materials
01 Transition metal-based cathode materials
Transition metal compounds, particularly oxides, phosphates, and sulfides, serve as effective cathode materials for potassium-ion batteries. These materials provide stable crystal structures that can accommodate potassium ion insertion and extraction. The incorporation of transition metals such as manganese, iron, cobalt, and nickel enables high capacity and good cycling stability. These cathode materials typically operate through intercalation mechanisms, allowing for reversible potassium storage.- Transition metal-based cathode materials: Transition metal compounds, particularly oxides, phosphates, and sulfides, serve as promising cathode materials for potassium-ion batteries due to their stable crystal structures and ability to intercalate potassium ions. These materials offer high theoretical capacities and good cycling stability. Various transition metals such as manganese, iron, cobalt, and nickel are used in different compositions to optimize electrochemical performance, voltage profiles, and rate capabilities for potassium-ion systems.
- Prussian blue analogs for potassium storage: Prussian blue analogs (PBAs) represent an important class of cathode materials for potassium-ion batteries due to their open framework structure that facilitates rapid potassium-ion diffusion. These materials feature a cubic structure with large interstitial sites that can accommodate potassium ions without significant structural strain. PBAs offer advantages including low cost, environmental friendliness, and excellent cycling stability, making them attractive for large-scale energy storage applications.
- Carbon-based cathode materials: Carbon-based materials, including graphene, reduced graphene oxide, carbon nanotubes, and other nanostructured carbons, serve as effective cathode materials for potassium-ion batteries. These materials provide large interlayer spacing that can accommodate potassium ions, which are larger than lithium ions. The high electrical conductivity, structural stability, and large surface area of carbon-based materials contribute to improved electrochemical performance, including enhanced rate capability and cycling stability.
- Composite and doped cathode materials: Composite and doped cathode materials combine multiple components to enhance the overall performance of potassium-ion batteries. These materials typically involve doping conventional cathode materials with various elements or creating composites with conductive additives. Such modifications improve electronic conductivity, structural stability, and potassium-ion diffusion kinetics. Common approaches include nitrogen doping, metal doping, and creating carbon-coated composites, all aimed at addressing the challenges associated with the large size of potassium ions.
- Organic cathode materials: Organic compounds represent an emerging class of cathode materials for potassium-ion batteries, offering advantages such as structural diversity, sustainability, and potential for high capacity. These materials typically contain conjugated carbonyl groups or other redox-active moieties that can reversibly store potassium ions. Organic cathodes can be derived from renewable resources and designed with specific molecular structures to optimize electrochemical properties, making them environmentally friendly alternatives to conventional inorganic cathode materials.
02 Prussian blue analogs for potassium-ion cathodes
Prussian blue analogs (PBAs) represent a promising class of cathode materials for potassium-ion batteries due to their open framework structure with large interstitial sites. These materials, typically composed of transition metal hexacyanoferrates, offer fast ion diffusion pathways and structural stability during potassium insertion/extraction. Their synthesis can be tailored to optimize electrochemical performance through control of composition, particle size, and morphology, resulting in improved cycling stability and rate capability.Expand Specific Solutions03 Carbon-based composite cathode materials
Carbon-based composite materials serve as effective cathode components for potassium-ion systems. These composites typically combine carbon materials (such as graphene, carbon nanotubes, or amorphous carbon) with active materials to enhance conductivity and structural stability. The carbon component provides conductive networks and buffers volume changes during cycling, while also potentially offering additional potassium storage sites. These composite structures demonstrate improved rate capability and cycling performance compared to non-composite alternatives.Expand Specific Solutions04 Organic cathode materials for potassium-ion batteries
Organic compounds represent a sustainable alternative to inorganic cathode materials for potassium-ion batteries. These materials, including conjugated carbonyl compounds, conductive polymers, and organosulfur compounds, offer advantages such as structural diversity, sustainability, and potentially high theoretical capacities. The electrochemical reactions typically involve redox processes of functional groups that can reversibly bind with potassium ions. Modifications to molecular structure can be made to optimize voltage, capacity, and cycling stability.Expand Specific Solutions05 Layered oxide cathode materials
Layered oxide materials represent an important class of cathode materials for potassium-ion batteries. These materials typically have a structure where potassium ions can be inserted and extracted between oxide layers. Various compositions, including potassium transition metal oxides with elements like manganese, cobalt, nickel, and iron, have been developed to optimize electrochemical performance. Strategies such as doping, surface modification, and controlled synthesis have been employed to address challenges like structural instability and capacity fading during cycling.Expand Specific Solutions
Leading Research Groups and Industrial Players
The potassium-ion battery cathode materials market is in an early growth phase, characterized by increasing research activity but limited commercialization. Market size remains modest compared to lithium-ion technologies, though projections indicate significant expansion potential due to potassium's abundance and cost advantages. Technologically, the field is still developing, with varying levels of maturity across different cathode chemistries. Leading players include established battery material manufacturers like Shenzhen Zhenhua New Materials and BTR Nano Tech focusing on commercial development, while research institutions such as SIAT, USTB, and South China University of Technology drive fundamental innovation. Major battery producers including CATL (Ningde Amperex) and Panasonic are increasingly investing in potassium-ion technology as a complementary solution to lithium-ion systems, suggesting growing industry confidence in this emerging technology.
Ningde Amperex Technology Ltd.
Technical Solution: Ningde Amperex Technology Ltd. (CATL) has developed advanced Prussian Blue Analogs (PBAs) as cathode materials for potassium-ion batteries (PIBs). Their technology focuses on addressing the large ionic radius of K+ (1.38 Å) which causes structural instability during cycling. CATL's approach involves carbon coating of PBA particles and precise control of Fe/Mn ratios in the crystal structure to enhance stability. They've achieved cathodes with capacities of 140 mAh/g and cycling stability over 1000 cycles with 80% capacity retention. Additionally, CATL has developed layered transition metal oxides with expanded interlayer spacing specifically engineered for K-ion intercalation, using dopants like titanium and niobium to stabilize the structure during potassium insertion/extraction. Their manufacturing process includes low-temperature synthesis methods that reduce production costs by approximately 15% compared to conventional lithium-ion cathode materials.
Strengths: Superior cycling stability compared to competitors, cost-effective production methods, and established large-scale manufacturing capabilities. Their materials show excellent rate capability suitable for fast-charging applications. Weaknesses: Energy density remains lower than lithium-ion counterparts, and the technology still faces challenges with voltage fade during extended cycling.
Panasonic Energy Co. Ltd.
Technical Solution: Panasonic Energy has pioneered a dual-phase cathode material system for potassium-ion batteries combining layered and spinel structures. Their proprietary KxMnO2 cathode materials feature carefully engineered oxygen frameworks that accommodate the large K+ ions while minimizing structural distortion during cycling. The company employs a gradient concentration design where the material surface is manganese-rich for stability while the core contains nickel and cobalt to boost capacity, achieving 130-150 mAh/g. Panasonic's synthesis involves a specialized co-precipitation method followed by controlled calcination under specific oxygen partial pressures to create optimal crystal structures with reduced lattice strain. Their cathodes incorporate conductive carbon networks integrated during synthesis rather than simple post-mixing, creating robust electronic pathways throughout the material. Recent developments include fluorine-substituted variants where some oxygen sites are replaced with fluorine to strengthen the structure, resulting in cathodes that maintain 85% capacity after 500 cycles at elevated temperatures.
Strengths: Excellent thermal stability making their materials suitable for a wide operating temperature range; sophisticated manufacturing processes that ensure consistent quality at scale; strong integration with existing battery production lines. Weaknesses: Higher production costs compared to some competitors; materials require specialized electrolytes to achieve optimal performance; rate capability at low temperatures remains challenging.
Key Patents and Scientific Breakthroughs
Iron-based cathode material for sodium-ion battery, preparation method thereof, and corresponding sodium-ion full battery
PatentActiveUS20210202946A1
Innovation
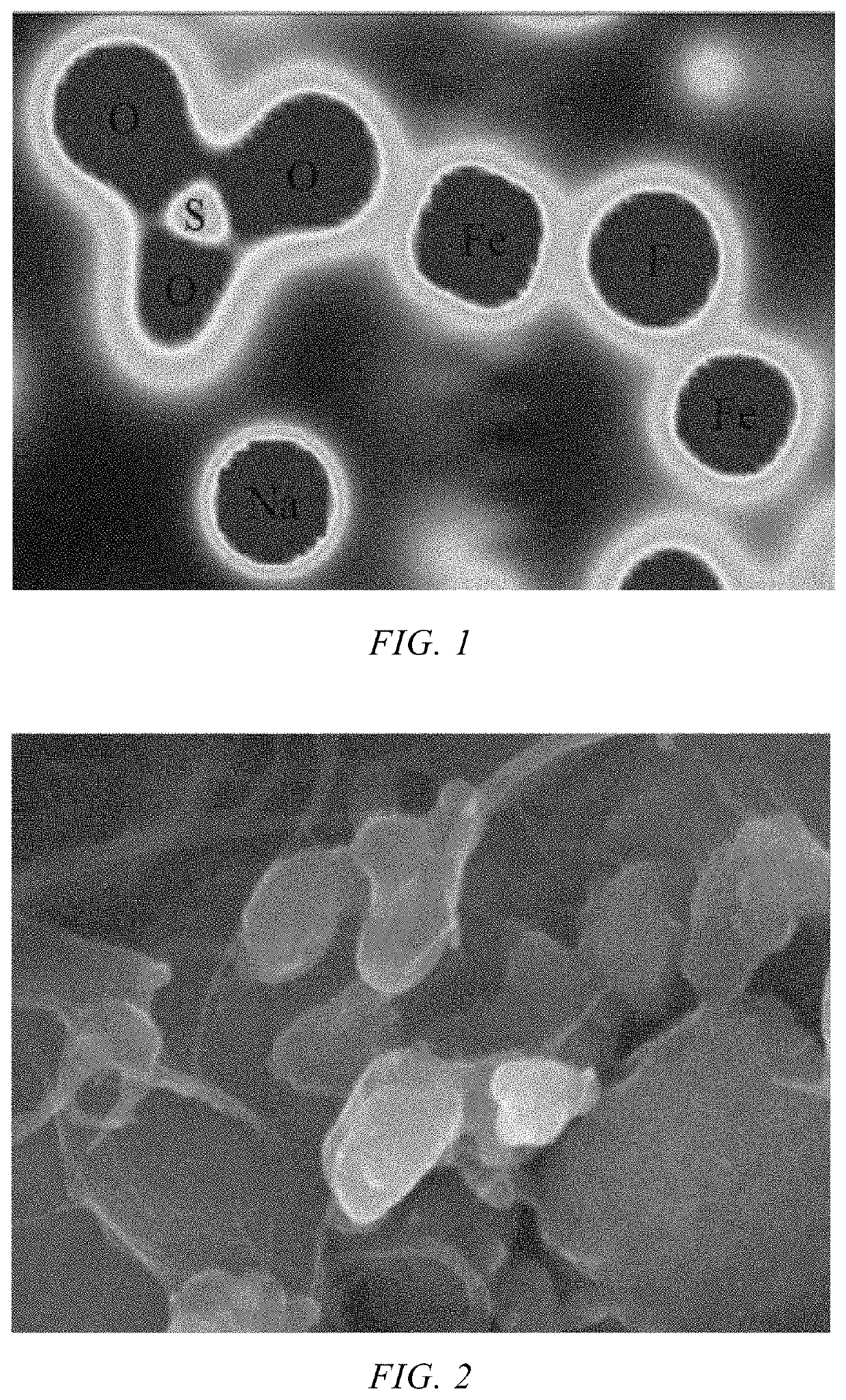
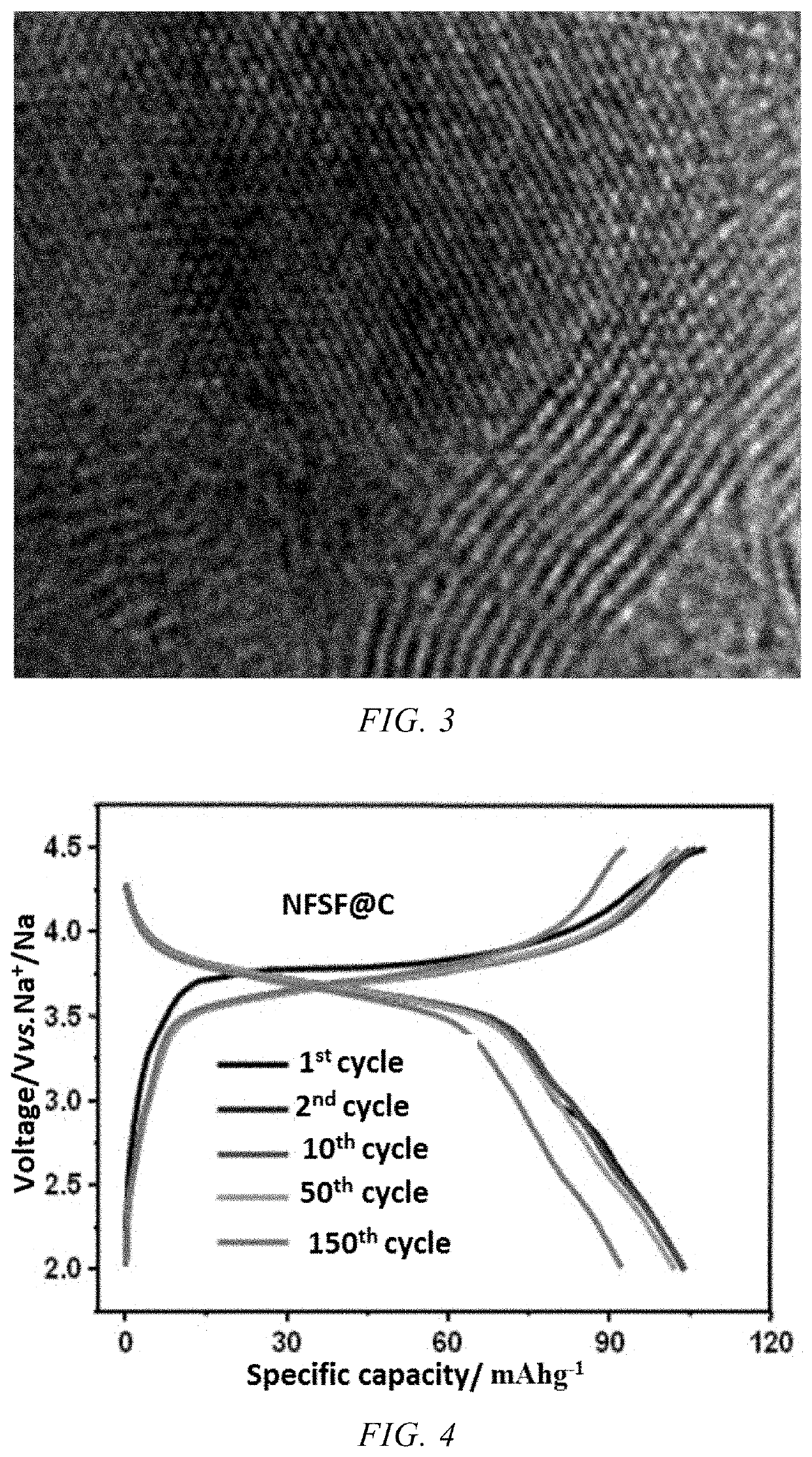
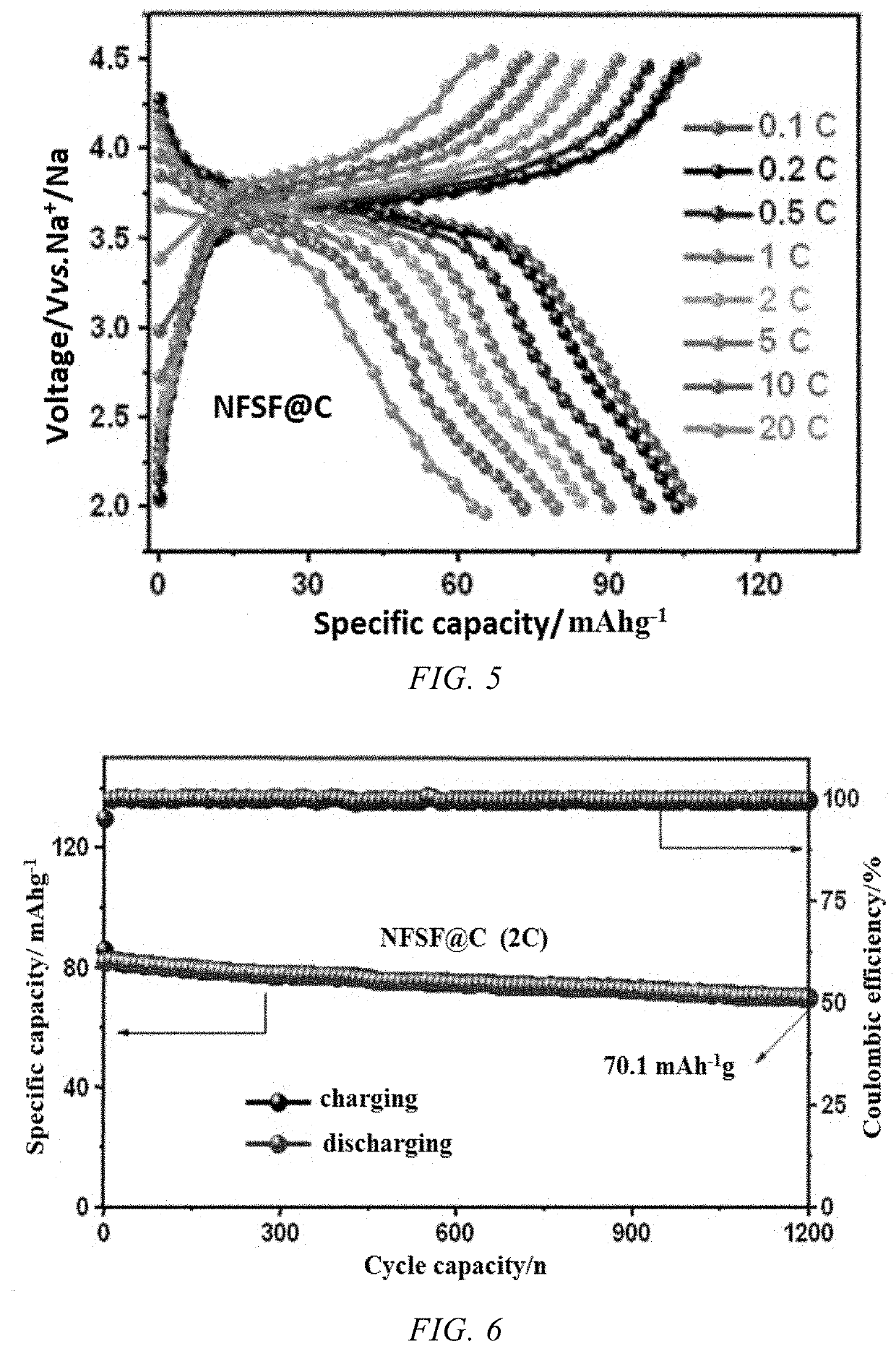
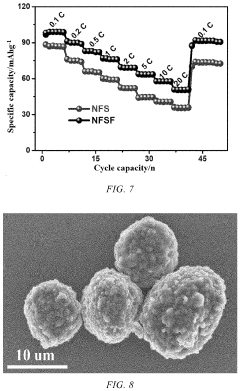
- A Na3Fe2(SO4)3F/C composite material is developed, where a carbon-based material is embedded into the bulk structure of the Na3Fe2(SO4)3F cathode material, with a carbon content between 1-10%, using a method involving ball milling and low-temperature sintering to enhance conductivity and stability.
Cathode material and preparation method thereof and sodium ion battery
PatentPendingUS20240322164A1
Innovation
- A cathode material is developed by doping sodium iron manganese titanium silicate with titanium and manganese, and coating it with carbon, resulting in improved electron transfer pathways and enhanced capacity, with a molecular formula of NaqFexMny(TiO2)z(SiO4)m, where specific ratios of q, x, y, z, and m are optimized, and a carbon layer thickness of 2-3 nm.
Sustainability and Resource Considerations
The sustainability profile of potassium-ion battery systems represents a significant advantage over lithium-ion technologies, primarily due to the greater abundance of potassium resources globally. Potassium is approximately 1000 times more abundant in the Earth's crust than lithium, with concentrations of approximately 2.1% versus 0.002% for lithium. This fundamental resource advantage translates to lower extraction costs and reduced geopolitical supply risks, as potassium resources are more evenly distributed worldwide compared to lithium deposits concentrated in the "Lithium Triangle" of South America and other limited regions.
When evaluating cathode materials for potassium-ion systems, sustainability considerations must extend beyond raw material abundance to include energy requirements for synthesis, environmental impact of extraction processes, and end-of-life recyclability. Manganese-based cathodes (such as K0.3MnO2) offer particular advantages in this regard, as manganese is the 12th most abundant element in the Earth's crust and has established recycling infrastructure from existing battery applications.
Prussian Blue Analogues (PBAs) represent another promising cathode material class from a sustainability perspective. Their synthesis typically occurs at relatively low temperatures (often below 100°C) compared to other cathode materials requiring high-temperature calcination processes exceeding 700°C. This reduced energy requirement for manufacturing translates to lower carbon footprints during production phases.
The water stability of certain potassium cathode materials also enables aqueous processing routes, eliminating the need for toxic organic solvents commonly used in lithium-ion battery manufacturing. This not only reduces environmental hazards during production but also simplifies recycling processes at end-of-life. However, challenges remain in developing efficient recycling protocols specifically designed for potassium-ion systems, as current battery recycling infrastructure is predominantly optimized for lithium-ion chemistries.
Life cycle assessment (LCA) studies comparing potassium and lithium systems remain limited, but preliminary analyses suggest potential reductions of 25-40% in global warming potential for K-ion batteries utilizing earth-abundant cathode materials compared to conventional NMC-based Li-ion batteries. These sustainability advantages must be balanced against current performance limitations, particularly regarding energy density, which may require more material per kWh of storage capacity.
The transition metal content in various potassium cathode materials also presents different sustainability profiles. Vanadium-based compounds, while offering excellent electrochemical performance, face resource constraints similar to cobalt, whereas iron-based materials present fewer supply chain concerns but typically deliver lower energy densities.
When evaluating cathode materials for potassium-ion systems, sustainability considerations must extend beyond raw material abundance to include energy requirements for synthesis, environmental impact of extraction processes, and end-of-life recyclability. Manganese-based cathodes (such as K0.3MnO2) offer particular advantages in this regard, as manganese is the 12th most abundant element in the Earth's crust and has established recycling infrastructure from existing battery applications.
Prussian Blue Analogues (PBAs) represent another promising cathode material class from a sustainability perspective. Their synthesis typically occurs at relatively low temperatures (often below 100°C) compared to other cathode materials requiring high-temperature calcination processes exceeding 700°C. This reduced energy requirement for manufacturing translates to lower carbon footprints during production phases.
The water stability of certain potassium cathode materials also enables aqueous processing routes, eliminating the need for toxic organic solvents commonly used in lithium-ion battery manufacturing. This not only reduces environmental hazards during production but also simplifies recycling processes at end-of-life. However, challenges remain in developing efficient recycling protocols specifically designed for potassium-ion systems, as current battery recycling infrastructure is predominantly optimized for lithium-ion chemistries.
Life cycle assessment (LCA) studies comparing potassium and lithium systems remain limited, but preliminary analyses suggest potential reductions of 25-40% in global warming potential for K-ion batteries utilizing earth-abundant cathode materials compared to conventional NMC-based Li-ion batteries. These sustainability advantages must be balanced against current performance limitations, particularly regarding energy density, which may require more material per kWh of storage capacity.
The transition metal content in various potassium cathode materials also presents different sustainability profiles. Vanadium-based compounds, while offering excellent electrochemical performance, face resource constraints similar to cobalt, whereas iron-based materials present fewer supply chain concerns but typically deliver lower energy densities.
Performance Benchmarking Against Li-Ion Systems
When comparing potassium-ion battery (KIB) cathode materials with their lithium-ion counterparts, several key performance metrics reveal both challenges and opportunities. Lithium-ion batteries (LIBs) currently dominate the market with energy densities ranging from 150-260 Wh/kg at the cell level, while KIBs typically achieve 70-140 Wh/kg. This performance gap stems primarily from the larger ionic radius of K+ (1.38 Å) compared to Li+ (0.76 Å), resulting in greater structural strain during intercalation processes.
Cycle stability presents another significant contrast, with commercial LIBs routinely achieving 1,000+ cycles at 80% capacity retention, while most KIB cathode materials struggle to maintain performance beyond 500 cycles. The larger K+ ion causes more pronounced structural changes during cycling, accelerating capacity fade. However, recent advances in Prussian blue analogues have demonstrated promising improvements, with some formulations approaching 800 cycles.
Rate capability metrics show LIBs typically retain 80-90% capacity at 2C rates, whereas KIB cathodes generally maintain only 60-75% under similar conditions. The diffusion kinetics of K+ ions through cathode materials are inherently slower due to stronger electrostatic interactions with host structures, though this gap narrows in open-framework materials specifically designed for potassium systems.
From an economic perspective, KIBs offer compelling advantages. Raw material costs for potassium compounds are approximately 80-90% lower than their lithium equivalents, with potassium resources being about 1,000 times more abundant in the Earth's crust. This translates to potential cell-level cost reductions of 25-40% compared to LIBs, depending on cathode chemistry selection.
Safety comparisons reveal mixed results. While KIBs operate at slightly lower voltages (reducing oxidative electrolyte decomposition risks), they typically exhibit higher reactivity with conventional electrolytes. Thermal runaway onset temperatures for KIBs with layered oxide cathodes occur approximately 20-30°C lower than comparable LIB materials, though phosphate-based KIB cathodes demonstrate thermal stability comparable to LiFePO₄.
Environmental impact assessments indicate KIBs may offer a 15-30% reduction in carbon footprint during manufacturing compared to LIBs, primarily due to less energy-intensive material processing requirements. Additionally, the greater abundance and geographical distribution of potassium resources reduce supply chain vulnerabilities and associated environmental impacts from long-distance transportation.
While KIBs currently lag behind LIBs in energy density and cycle life, their economic and sustainability advantages position them as promising alternatives for stationary storage applications where cost and resource considerations outweigh strict volumetric constraints. The performance gap continues to narrow as research intensifies on potassium-specific cathode architectures optimized for the unique characteristics of K+ ions.
Cycle stability presents another significant contrast, with commercial LIBs routinely achieving 1,000+ cycles at 80% capacity retention, while most KIB cathode materials struggle to maintain performance beyond 500 cycles. The larger K+ ion causes more pronounced structural changes during cycling, accelerating capacity fade. However, recent advances in Prussian blue analogues have demonstrated promising improvements, with some formulations approaching 800 cycles.
Rate capability metrics show LIBs typically retain 80-90% capacity at 2C rates, whereas KIB cathodes generally maintain only 60-75% under similar conditions. The diffusion kinetics of K+ ions through cathode materials are inherently slower due to stronger electrostatic interactions with host structures, though this gap narrows in open-framework materials specifically designed for potassium systems.
From an economic perspective, KIBs offer compelling advantages. Raw material costs for potassium compounds are approximately 80-90% lower than their lithium equivalents, with potassium resources being about 1,000 times more abundant in the Earth's crust. This translates to potential cell-level cost reductions of 25-40% compared to LIBs, depending on cathode chemistry selection.
Safety comparisons reveal mixed results. While KIBs operate at slightly lower voltages (reducing oxidative electrolyte decomposition risks), they typically exhibit higher reactivity with conventional electrolytes. Thermal runaway onset temperatures for KIBs with layered oxide cathodes occur approximately 20-30°C lower than comparable LIB materials, though phosphate-based KIB cathodes demonstrate thermal stability comparable to LiFePO₄.
Environmental impact assessments indicate KIBs may offer a 15-30% reduction in carbon footprint during manufacturing compared to LIBs, primarily due to less energy-intensive material processing requirements. Additionally, the greater abundance and geographical distribution of potassium resources reduce supply chain vulnerabilities and associated environmental impacts from long-distance transportation.
While KIBs currently lag behind LIBs in energy density and cycle life, their economic and sustainability advantages position them as promising alternatives for stationary storage applications where cost and resource considerations outweigh strict volumetric constraints. The performance gap continues to narrow as research intensifies on potassium-specific cathode architectures optimized for the unique characteristics of K+ ions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!