Advanced Spectroscopic Methods for Carbonyl Analysis
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbonyl Analysis Evolution and Objectives
Carbonyl analysis has undergone significant evolution since its inception in the early 20th century. Initially, simple chemical tests were used to detect the presence of carbonyl groups in organic compounds. As analytical techniques advanced, spectroscopic methods became increasingly important for carbonyl analysis, offering greater sensitivity, specificity, and quantitative capabilities.
The development of infrared (IR) spectroscopy in the 1940s marked a major milestone in carbonyl analysis. IR spectroscopy allowed for the identification of carbonyl groups based on their characteristic absorption bands, typically around 1700 cm^-1. This technique quickly became a standard tool for structural elucidation and quality control in various industries.
Nuclear Magnetic Resonance (NMR) spectroscopy, introduced in the 1950s, further revolutionized carbonyl analysis. 13C NMR, in particular, provided valuable information about the chemical environment of carbonyl carbons, enabling more detailed structural characterization of carbonyl-containing compounds.
The advent of mass spectrometry (MS) in the 1960s and its subsequent coupling with chromatographic techniques (GC-MS and LC-MS) opened new avenues for carbonyl analysis. These hyphenated techniques allowed for the separation and identification of complex mixtures containing carbonyl compounds, greatly expanding the scope of carbonyl analysis in environmental and biological samples.
In recent decades, the focus has shifted towards developing more sensitive and selective methods for carbonyl analysis. Advanced spectroscopic techniques such as two-dimensional NMR, time-resolved fluorescence spectroscopy, and surface-enhanced Raman spectroscopy (SERS) have emerged as powerful tools for studying carbonyl compounds in complex matrices and at low concentrations.
The primary objectives of current research in advanced spectroscopic methods for carbonyl analysis are multifaceted. Firstly, there is a drive to improve detection limits and quantification accuracy, particularly for trace-level analysis in environmental and biological samples. Secondly, researchers aim to develop non-destructive, real-time monitoring techniques for industrial processes and quality control applications.
Another key objective is to enhance the specificity of carbonyl analysis, enabling the differentiation between various types of carbonyl compounds (e.g., aldehydes, ketones, carboxylic acids) in complex mixtures. This is particularly important in fields such as metabolomics and food science, where detailed profiling of carbonyl compounds can provide valuable insights into biological processes and product quality.
Furthermore, there is a growing interest in developing portable and miniaturized spectroscopic devices for on-site carbonyl analysis. This trend is driven by the need for rapid, field-deployable analytical tools in environmental monitoring, food safety, and point-of-care diagnostics.
The development of infrared (IR) spectroscopy in the 1940s marked a major milestone in carbonyl analysis. IR spectroscopy allowed for the identification of carbonyl groups based on their characteristic absorption bands, typically around 1700 cm^-1. This technique quickly became a standard tool for structural elucidation and quality control in various industries.
Nuclear Magnetic Resonance (NMR) spectroscopy, introduced in the 1950s, further revolutionized carbonyl analysis. 13C NMR, in particular, provided valuable information about the chemical environment of carbonyl carbons, enabling more detailed structural characterization of carbonyl-containing compounds.
The advent of mass spectrometry (MS) in the 1960s and its subsequent coupling with chromatographic techniques (GC-MS and LC-MS) opened new avenues for carbonyl analysis. These hyphenated techniques allowed for the separation and identification of complex mixtures containing carbonyl compounds, greatly expanding the scope of carbonyl analysis in environmental and biological samples.
In recent decades, the focus has shifted towards developing more sensitive and selective methods for carbonyl analysis. Advanced spectroscopic techniques such as two-dimensional NMR, time-resolved fluorescence spectroscopy, and surface-enhanced Raman spectroscopy (SERS) have emerged as powerful tools for studying carbonyl compounds in complex matrices and at low concentrations.
The primary objectives of current research in advanced spectroscopic methods for carbonyl analysis are multifaceted. Firstly, there is a drive to improve detection limits and quantification accuracy, particularly for trace-level analysis in environmental and biological samples. Secondly, researchers aim to develop non-destructive, real-time monitoring techniques for industrial processes and quality control applications.
Another key objective is to enhance the specificity of carbonyl analysis, enabling the differentiation between various types of carbonyl compounds (e.g., aldehydes, ketones, carboxylic acids) in complex mixtures. This is particularly important in fields such as metabolomics and food science, where detailed profiling of carbonyl compounds can provide valuable insights into biological processes and product quality.
Furthermore, there is a growing interest in developing portable and miniaturized spectroscopic devices for on-site carbonyl analysis. This trend is driven by the need for rapid, field-deployable analytical tools in environmental monitoring, food safety, and point-of-care diagnostics.
Industrial Demand for Carbonyl Detection
The demand for advanced carbonyl detection methods in various industries has been steadily increasing due to the critical role carbonyl compounds play in numerous processes and products. Carbonyl groups, consisting of a carbon atom double-bonded to an oxygen atom, are present in a wide range of organic compounds, including aldehydes and ketones. These compounds are essential in pharmaceuticals, food and beverage production, environmental monitoring, and chemical manufacturing.
In the pharmaceutical industry, carbonyl detection is crucial for quality control and assurance of drug formulations. Many active pharmaceutical ingredients (APIs) contain carbonyl groups, and their precise quantification is vital for ensuring drug efficacy and safety. The industry requires highly sensitive and accurate methods to detect and analyze carbonyl compounds in complex matrices, driving the demand for advanced spectroscopic techniques.
The food and beverage sector also heavily relies on carbonyl detection for quality control and flavor analysis. Carbonyl compounds contribute significantly to the aroma and taste profiles of many food products. Advanced spectroscopic methods enable manufacturers to monitor and control the formation of desirable flavor compounds while minimizing the production of off-flavors or potentially harmful substances.
Environmental monitoring agencies and regulatory bodies have increased their focus on carbonyl compounds due to their potential health and environmental impacts. Atmospheric carbonyls, such as formaldehyde and acetaldehyde, are known air pollutants and can contribute to the formation of ground-level ozone. This has led to a growing demand for sensitive and reliable detection methods to monitor air quality and enforce emissions regulations.
The chemical manufacturing industry requires precise carbonyl detection for process control and product quality assurance. In polymer production, for instance, the presence and concentration of carbonyl groups can significantly affect the properties and performance of the final product. Advanced spectroscopic methods allow for real-time monitoring of reaction progress and product composition, enabling manufacturers to optimize their processes and maintain consistent product quality.
The increasing emphasis on sustainability and green chemistry has further driven the demand for advanced carbonyl detection methods. As industries strive to develop more environmentally friendly processes and products, there is a growing need for analytical techniques that can accurately quantify carbonyl compounds in complex mixtures, helping to identify and minimize potentially harmful byproducts.
In the pharmaceutical industry, carbonyl detection is crucial for quality control and assurance of drug formulations. Many active pharmaceutical ingredients (APIs) contain carbonyl groups, and their precise quantification is vital for ensuring drug efficacy and safety. The industry requires highly sensitive and accurate methods to detect and analyze carbonyl compounds in complex matrices, driving the demand for advanced spectroscopic techniques.
The food and beverage sector also heavily relies on carbonyl detection for quality control and flavor analysis. Carbonyl compounds contribute significantly to the aroma and taste profiles of many food products. Advanced spectroscopic methods enable manufacturers to monitor and control the formation of desirable flavor compounds while minimizing the production of off-flavors or potentially harmful substances.
Environmental monitoring agencies and regulatory bodies have increased their focus on carbonyl compounds due to their potential health and environmental impacts. Atmospheric carbonyls, such as formaldehyde and acetaldehyde, are known air pollutants and can contribute to the formation of ground-level ozone. This has led to a growing demand for sensitive and reliable detection methods to monitor air quality and enforce emissions regulations.
The chemical manufacturing industry requires precise carbonyl detection for process control and product quality assurance. In polymer production, for instance, the presence and concentration of carbonyl groups can significantly affect the properties and performance of the final product. Advanced spectroscopic methods allow for real-time monitoring of reaction progress and product composition, enabling manufacturers to optimize their processes and maintain consistent product quality.
The increasing emphasis on sustainability and green chemistry has further driven the demand for advanced carbonyl detection methods. As industries strive to develop more environmentally friendly processes and products, there is a growing need for analytical techniques that can accurately quantify carbonyl compounds in complex mixtures, helping to identify and minimize potentially harmful byproducts.
Current Spectroscopic Techniques and Limitations
Spectroscopic techniques have long been the cornerstone of carbonyl analysis, offering valuable insights into molecular structures and chemical compositions. Currently, several methods dominate the field, each with its own strengths and limitations.
Infrared (IR) spectroscopy remains a widely used technique for carbonyl analysis. It provides rapid, non-destructive measurements and is particularly sensitive to the C=O stretching vibration, typically observed around 1700 cm^-1. However, IR spectroscopy often struggles with complex mixtures, as overlapping bands can obscure specific carbonyl signals. Additionally, quantitative analysis can be challenging due to variations in sample preparation and instrument response.
Nuclear Magnetic Resonance (NMR) spectroscopy, especially 13C NMR, offers high-resolution structural information for carbonyl compounds. It can distinguish between different types of carbonyls and provide detailed information about their chemical environment. Nevertheless, NMR suffers from low sensitivity, often requiring relatively large sample quantities and long acquisition times. This limitation can be particularly problematic when analyzing trace amounts of carbonyl compounds in complex matrices.
Raman spectroscopy has gained popularity for its ability to analyze aqueous samples and provide complementary information to IR spectroscopy. It is particularly useful for studying carbonyl groups in biological systems. However, Raman spectroscopy can be hindered by fluorescence interference and often requires higher concentrations compared to IR spectroscopy.
Mass spectrometry (MS), especially when coupled with chromatographic techniques, offers high sensitivity and specificity for carbonyl analysis. It can detect and quantify trace amounts of carbonyl compounds in complex mixtures. However, MS typically requires sample ionization, which can lead to fragmentation of labile carbonyl compounds. Additionally, the need for sample preparation and chromatographic separation can increase analysis time and complexity.
UV-Visible spectroscopy, while less specific than other techniques, can be useful for analyzing conjugated carbonyl systems. It offers high sensitivity and is well-suited for quantitative analysis. However, its application is limited to compounds with suitable chromophores, and it provides little structural information beyond the presence of conjugated systems.
Despite these advanced techniques, several limitations persist across the field. Many methods struggle with the analysis of complex mixtures, particularly in biological or environmental samples. Quantitative analysis remains challenging, often requiring careful calibration and consideration of matrix effects. Additionally, the detection and characterization of short-lived or reactive carbonyl species continue to pose significant challenges.
Furthermore, most current techniques provide limited spatial resolution, making it difficult to study carbonyl distributions in heterogeneous samples or living systems. There is also a growing need for real-time, in situ analysis capabilities to monitor dynamic processes involving carbonyl compounds.
Infrared (IR) spectroscopy remains a widely used technique for carbonyl analysis. It provides rapid, non-destructive measurements and is particularly sensitive to the C=O stretching vibration, typically observed around 1700 cm^-1. However, IR spectroscopy often struggles with complex mixtures, as overlapping bands can obscure specific carbonyl signals. Additionally, quantitative analysis can be challenging due to variations in sample preparation and instrument response.
Nuclear Magnetic Resonance (NMR) spectroscopy, especially 13C NMR, offers high-resolution structural information for carbonyl compounds. It can distinguish between different types of carbonyls and provide detailed information about their chemical environment. Nevertheless, NMR suffers from low sensitivity, often requiring relatively large sample quantities and long acquisition times. This limitation can be particularly problematic when analyzing trace amounts of carbonyl compounds in complex matrices.
Raman spectroscopy has gained popularity for its ability to analyze aqueous samples and provide complementary information to IR spectroscopy. It is particularly useful for studying carbonyl groups in biological systems. However, Raman spectroscopy can be hindered by fluorescence interference and often requires higher concentrations compared to IR spectroscopy.
Mass spectrometry (MS), especially when coupled with chromatographic techniques, offers high sensitivity and specificity for carbonyl analysis. It can detect and quantify trace amounts of carbonyl compounds in complex mixtures. However, MS typically requires sample ionization, which can lead to fragmentation of labile carbonyl compounds. Additionally, the need for sample preparation and chromatographic separation can increase analysis time and complexity.
UV-Visible spectroscopy, while less specific than other techniques, can be useful for analyzing conjugated carbonyl systems. It offers high sensitivity and is well-suited for quantitative analysis. However, its application is limited to compounds with suitable chromophores, and it provides little structural information beyond the presence of conjugated systems.
Despite these advanced techniques, several limitations persist across the field. Many methods struggle with the analysis of complex mixtures, particularly in biological or environmental samples. Quantitative analysis remains challenging, often requiring careful calibration and consideration of matrix effects. Additionally, the detection and characterization of short-lived or reactive carbonyl species continue to pose significant challenges.
Furthermore, most current techniques provide limited spatial resolution, making it difficult to study carbonyl distributions in heterogeneous samples or living systems. There is also a growing need for real-time, in situ analysis capabilities to monitor dynamic processes involving carbonyl compounds.
State-of-the-Art Carbonyl Analysis Solutions
01 Infrared spectroscopy for carbonyl analysis
Infrared spectroscopy is a widely used method for carbonyl analysis. It can detect and quantify carbonyl compounds based on their characteristic absorption bands in the infrared region. This technique is particularly useful for identifying different types of carbonyl groups and their structural environments.- Infrared spectroscopy for carbonyl analysis: Infrared spectroscopy is a widely used method for carbonyl analysis. It can detect and quantify carbonyl compounds based on their characteristic absorption bands in the infrared region. This technique is particularly useful for identifying different types of carbonyl groups and their structural environments.
- Raman spectroscopy for carbonyl detection: Raman spectroscopy offers a complementary approach to infrared spectroscopy for carbonyl analysis. It can provide detailed information about the vibrational modes of carbonyl groups, allowing for the identification and characterization of various carbonyl-containing compounds in complex mixtures.
- Near-infrared spectroscopy for carbonyl quantification: Near-infrared spectroscopy is employed for the quantitative analysis of carbonyl compounds. This method is particularly useful for rapid, non-destructive measurements in industrial settings, allowing for real-time monitoring of carbonyl content in various products and processes.
- UV-Visible spectroscopy for carbonyl analysis: UV-Visible spectroscopy is utilized for the analysis of carbonyl compounds, especially those with conjugated systems. This technique can provide information about the electronic transitions in carbonyl-containing molecules, allowing for both qualitative and quantitative analysis in various applications.
- Hyperspectral imaging for spatial carbonyl analysis: Hyperspectral imaging combines spectroscopic methods with imaging techniques to provide spatial information about carbonyl distribution. This approach allows for the visualization of carbonyl compounds across a sample surface, offering insights into the heterogeneity and localization of carbonyl groups in complex materials.
02 Raman spectroscopy for carbonyl detection
Raman spectroscopy is another powerful tool for carbonyl analysis. It provides complementary information to infrared spectroscopy and can be particularly useful for aqueous samples or in situations where infrared absorption is problematic. Raman spectroscopy can detect subtle changes in carbonyl structures and is often used in conjunction with other spectroscopic methods.Expand Specific Solutions03 UV-Visible spectroscopy for carbonyl compounds
UV-Visible spectroscopy is employed for analyzing carbonyl compounds, especially those with conjugated systems. This method is particularly useful for quantitative analysis of carbonyl-containing molecules in solution and can provide information about electronic transitions associated with carbonyl groups.Expand Specific Solutions04 Mass spectrometry for carbonyl identification
Mass spectrometry is a powerful technique for identifying and characterizing carbonyl compounds. It can provide detailed structural information, including the molecular mass and fragmentation patterns of carbonyl-containing molecules. This method is often combined with chromatographic techniques for complex mixture analysis.Expand Specific Solutions05 Advanced data analysis for spectroscopic carbonyl detection
Advanced data analysis techniques, including chemometrics and machine learning algorithms, are increasingly used to enhance the interpretation of spectroscopic data for carbonyl analysis. These methods can improve the sensitivity and specificity of carbonyl detection, especially in complex matrices or when dealing with overlapping spectral features.Expand Specific Solutions
Key Players in Spectroscopic Instrumentation
The field of advanced spectroscopic methods for carbonyl analysis is in a mature stage of development, with a diverse range of established players and ongoing research. The market size is substantial, driven by applications in various industries such as petrochemicals, pharmaceuticals, and materials science. Key players like ExxonMobil Chemical Patents, BASF Corp., and Canon, Inc. have made significant contributions to the technology's advancement. Academic institutions, including Zhejiang Sci-Tech University and Hunan University, are also actively involved in research. The technology's maturity is evident in the sophisticated instruments and techniques available, with companies like Waters Technology Corp. and Janssen Pharmaceutica NV pushing the boundaries of sensitivity and accuracy in carbonyl analysis.
ExxonMobil Chemical Patents, Inc.
Technical Solution: ExxonMobil has developed proprietary spectroscopic methods for carbonyl analysis in petroleum products and lubricants. Their approach combines near-infrared (NIR) and mid-infrared (MIR) spectroscopy with advanced chemometric modeling for rapid, in-line monitoring of carbonyl formation during oxidation processes[2]. ExxonMobil's method utilizes fiber-optic probes for remote sensing capabilities, allowing real-time analysis in harsh industrial environments[4]. They have also implemented multivariate curve resolution (MCR) algorithms to deconvolute complex spectral data, enabling the simultaneous quantification of multiple carbonyl species in fuel and lubricant formulations[6].
Strengths: Real-time monitoring capabilities, robust performance in industrial settings. Weaknesses: May be limited to specific applications in the petroleum and lubricant industries.
BASF Corp.
Technical Solution: BASF has developed advanced spectroscopic methods for carbonyl analysis, focusing on Fourier Transform Infrared (FTIR) spectroscopy and Raman spectroscopy. Their approach combines these techniques with chemometric analysis for enhanced sensitivity and specificity. BASF's method utilizes attenuated total reflection (ATR) FTIR for rapid, non-destructive analysis of liquid and solid samples[1]. They have also implemented surface-enhanced Raman spectroscopy (SERS) for trace-level detection of carbonyl compounds in complex matrices[3]. BASF's integrated spectroscopic platform incorporates machine learning algorithms for automated peak identification and quantification, significantly improving the accuracy and speed of carbonyl analysis in various industrial applications[5].
Strengths: High sensitivity, non-destructive analysis, versatility across sample types. Weaknesses: Potential interference from complex matrices, requires specialized equipment and expertise.
Innovative Spectroscopic Approaches for Carbonyls
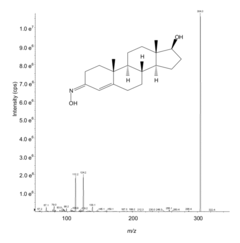
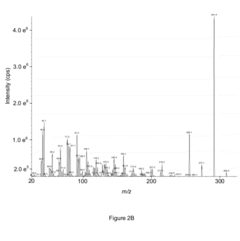
Enhanced Sensitivity for Analysis of Carbonyl Containing Compounds Using Mass Spectrometry
PatentActiveUS20120126107A1
Innovation
- Derivatizing carbonyl group-containing compounds with O-substituted hydroxylamines to produce oximes, which enhances ionization efficiency and fragmentation, thereby increasing the sensitivity of detection in mass spectrometry, as demonstrated by the use of O-tert-butyl hydroxylamine for DHT, resulting in significant improvements in ion signal and chromatographic separation.
Nickel carbonyl analyzer and nickel carbonyl analyzing method
PatentInactiveJP2018169209A
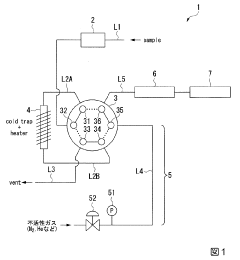
Innovation
- A nickel carbonyl analyzer comprising a collection device, carrier gas supply, decomposition device with a metal catalyst, and carbon monoxide quantification device, allowing for concentration, decomposition, and quantification of nickel carbonyl without specialized equipment, using temperature control to manage equilibrium conditions.
Environmental Impact of Carbonyl Analysis Methods
The environmental impact of carbonyl analysis methods is a critical consideration in the development and application of advanced spectroscopic techniques. Traditional methods for carbonyl analysis often involve the use of hazardous chemicals and generate significant amounts of waste, posing potential risks to the environment. However, advanced spectroscopic methods offer promising alternatives that can significantly reduce these environmental concerns.
Fourier-transform infrared (FTIR) spectroscopy, for instance, is a non-destructive technique that requires minimal sample preparation and produces no chemical waste. This method allows for rapid and accurate analysis of carbonyl compounds without the need for extensive sample processing or the use of harmful reagents. Similarly, Raman spectroscopy offers another environmentally friendly approach, providing detailed molecular information without the need for sample preparation or the generation of chemical waste.
Near-infrared (NIR) spectroscopy is another advanced method that has gained popularity due to its low environmental impact. NIR spectroscopy can be performed on solid, liquid, or gaseous samples without the need for extensive sample preparation or the use of solvents. This technique not only reduces waste generation but also minimizes energy consumption compared to traditional analytical methods.
The development of portable and handheld spectroscopic devices has further enhanced the environmental benefits of these advanced methods. These compact instruments allow for on-site analysis, reducing the need for sample transportation and storage, which in turn decreases the carbon footprint associated with analytical processes. Additionally, the ability to perform real-time measurements enables more efficient monitoring of environmental pollutants and industrial emissions, contributing to improved environmental management practices.
Advanced data analysis techniques, such as chemometrics and machine learning algorithms, have also played a crucial role in enhancing the environmental sustainability of carbonyl analysis. These computational methods enable more accurate and sensitive detection of carbonyl compounds from complex spectral data, reducing the need for multiple analyses or large sample volumes. This increased efficiency translates to lower energy consumption and reduced waste generation in analytical laboratories.
Furthermore, the integration of advanced spectroscopic methods with online monitoring systems has revolutionized process control in various industries. This integration allows for continuous, real-time analysis of carbonyl compounds in industrial processes, enabling more efficient resource utilization and minimizing the production of waste and byproducts. By optimizing production processes, these advanced analytical techniques contribute to overall environmental sustainability in industrial settings.
In conclusion, advanced spectroscopic methods for carbonyl analysis offer significant environmental benefits compared to traditional analytical techniques. These methods not only reduce the use of hazardous chemicals and waste generation but also contribute to more efficient and sustainable analytical practices across various industries and research fields.
Fourier-transform infrared (FTIR) spectroscopy, for instance, is a non-destructive technique that requires minimal sample preparation and produces no chemical waste. This method allows for rapid and accurate analysis of carbonyl compounds without the need for extensive sample processing or the use of harmful reagents. Similarly, Raman spectroscopy offers another environmentally friendly approach, providing detailed molecular information without the need for sample preparation or the generation of chemical waste.
Near-infrared (NIR) spectroscopy is another advanced method that has gained popularity due to its low environmental impact. NIR spectroscopy can be performed on solid, liquid, or gaseous samples without the need for extensive sample preparation or the use of solvents. This technique not only reduces waste generation but also minimizes energy consumption compared to traditional analytical methods.
The development of portable and handheld spectroscopic devices has further enhanced the environmental benefits of these advanced methods. These compact instruments allow for on-site analysis, reducing the need for sample transportation and storage, which in turn decreases the carbon footprint associated with analytical processes. Additionally, the ability to perform real-time measurements enables more efficient monitoring of environmental pollutants and industrial emissions, contributing to improved environmental management practices.
Advanced data analysis techniques, such as chemometrics and machine learning algorithms, have also played a crucial role in enhancing the environmental sustainability of carbonyl analysis. These computational methods enable more accurate and sensitive detection of carbonyl compounds from complex spectral data, reducing the need for multiple analyses or large sample volumes. This increased efficiency translates to lower energy consumption and reduced waste generation in analytical laboratories.
Furthermore, the integration of advanced spectroscopic methods with online monitoring systems has revolutionized process control in various industries. This integration allows for continuous, real-time analysis of carbonyl compounds in industrial processes, enabling more efficient resource utilization and minimizing the production of waste and byproducts. By optimizing production processes, these advanced analytical techniques contribute to overall environmental sustainability in industrial settings.
In conclusion, advanced spectroscopic methods for carbonyl analysis offer significant environmental benefits compared to traditional analytical techniques. These methods not only reduce the use of hazardous chemicals and waste generation but also contribute to more efficient and sustainable analytical practices across various industries and research fields.
Regulatory Framework for Spectroscopic Analysis
The regulatory framework for spectroscopic analysis in carbonyl compound detection plays a crucial role in ensuring the reliability, accuracy, and standardization of analytical methods across various industries. Regulatory bodies such as the Food and Drug Administration (FDA), Environmental Protection Agency (EPA), and International Organization for Standardization (ISO) have established guidelines and standards for the use of advanced spectroscopic techniques in carbonyl analysis.
These regulations typically cover aspects such as method validation, instrument calibration, sample preparation, data analysis, and reporting. For instance, the FDA's Good Laboratory Practice (GLP) regulations provide a framework for conducting non-clinical laboratory studies, including those involving spectroscopic analysis of carbonyls. These guidelines ensure the quality and integrity of test data submitted to regulatory agencies.
In the pharmaceutical industry, the International Conference on Harmonisation (ICH) has developed guidelines for the validation of analytical procedures, including spectroscopic methods used in carbonyl analysis. These guidelines outline the characteristics that need to be considered during method validation, such as specificity, linearity, range, accuracy, precision, and robustness.
Environmental agencies like the EPA have established specific methods for the analysis of carbonyls in air and water samples using spectroscopic techniques. For example, EPA Method TO-11A outlines the procedure for determining formaldehyde and other carbonyl compounds in ambient air using active sampling on 2,4-dinitrophenylhydrazine (DNPH) cartridges followed by high-performance liquid chromatography (HPLC) analysis.
The ISO has developed several standards related to spectroscopic analysis, including ISO/IEC 17025, which specifies the general requirements for the competence of testing and calibration laboratories. This standard is widely used for accrediting laboratories that perform spectroscopic analysis of carbonyls.
In the food industry, regulatory bodies have set limits for carbonyl compounds in various food products and have specified analytical methods for their determination. For instance, the European Union has established maximum levels for certain carbonyls in food additives and has recommended spectroscopic methods for their quantification.
Compliance with these regulatory frameworks is essential for laboratories and industries utilizing advanced spectroscopic methods for carbonyl analysis. It ensures the comparability of results across different laboratories, facilitates international trade, and provides a basis for regulatory decision-making. As new spectroscopic techniques emerge and existing methods are refined, regulatory bodies continue to update their guidelines to keep pace with technological advancements and ensure the highest standards of analytical quality and safety.
These regulations typically cover aspects such as method validation, instrument calibration, sample preparation, data analysis, and reporting. For instance, the FDA's Good Laboratory Practice (GLP) regulations provide a framework for conducting non-clinical laboratory studies, including those involving spectroscopic analysis of carbonyls. These guidelines ensure the quality and integrity of test data submitted to regulatory agencies.
In the pharmaceutical industry, the International Conference on Harmonisation (ICH) has developed guidelines for the validation of analytical procedures, including spectroscopic methods used in carbonyl analysis. These guidelines outline the characteristics that need to be considered during method validation, such as specificity, linearity, range, accuracy, precision, and robustness.
Environmental agencies like the EPA have established specific methods for the analysis of carbonyls in air and water samples using spectroscopic techniques. For example, EPA Method TO-11A outlines the procedure for determining formaldehyde and other carbonyl compounds in ambient air using active sampling on 2,4-dinitrophenylhydrazine (DNPH) cartridges followed by high-performance liquid chromatography (HPLC) analysis.
The ISO has developed several standards related to spectroscopic analysis, including ISO/IEC 17025, which specifies the general requirements for the competence of testing and calibration laboratories. This standard is widely used for accrediting laboratories that perform spectroscopic analysis of carbonyls.
In the food industry, regulatory bodies have set limits for carbonyl compounds in various food products and have specified analytical methods for their determination. For instance, the European Union has established maximum levels for certain carbonyls in food additives and has recommended spectroscopic methods for their quantification.
Compliance with these regulatory frameworks is essential for laboratories and industries utilizing advanced spectroscopic methods for carbonyl analysis. It ensures the comparability of results across different laboratories, facilitates international trade, and provides a basis for regulatory decision-making. As new spectroscopic techniques emerge and existing methods are refined, regulatory bodies continue to update their guidelines to keep pace with technological advancements and ensure the highest standards of analytical quality and safety.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!