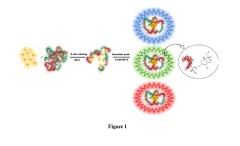
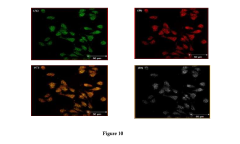
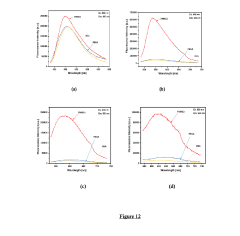
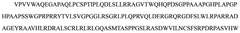Advances in Magnesium Nitride Nanostructures for R&D
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg3N2 Nanostructure R&D Background and Objectives
Magnesium nitride (Mg3N2) nanostructures have emerged as a promising material in the field of advanced materials research and development. The evolution of this technology can be traced back to the early 2000s when researchers began exploring the potential of magnesium-based compounds in various applications. The growing interest in Mg3N2 nanostructures stems from their unique properties, including high specific surface area, excellent thermal stability, and remarkable chemical reactivity.
The development of Mg3N2 nanostructures has been driven by the increasing demand for lightweight, high-performance materials in industries such as aerospace, automotive, and energy storage. As global efforts to reduce carbon emissions intensify, the need for innovative materials that can contribute to energy efficiency and sustainability has become more pressing. Mg3N2 nanostructures have shown potential in addressing these challenges, particularly in areas such as hydrogen storage, catalysis, and optoelectronic devices.
The technological trajectory of Mg3N2 nanostructures has been marked by significant advancements in synthesis methods and characterization techniques. Early research focused primarily on bulk Mg3N2, but the shift towards nanostructured forms has opened up new possibilities for tailoring material properties at the nanoscale. This transition has been facilitated by improvements in nanofabrication techniques, such as chemical vapor deposition, sol-gel methods, and electrospinning.
One of the key objectives in Mg3N2 nanostructure R&D is to achieve precise control over morphology, size, and composition. Researchers aim to develop reproducible synthesis methods that can yield nanostructures with specific shapes, such as nanoparticles, nanowires, and nanotubes. These diverse morphologies offer unique advantages in different applications, making the ability to control nanostructure formation a critical aspect of ongoing research.
Another important goal is to enhance the stability and performance of Mg3N2 nanostructures in various environmental conditions. This includes improving their resistance to oxidation and moisture, which are common challenges for magnesium-based materials. Researchers are exploring strategies such as surface functionalization and composite formation to address these issues and expand the potential applications of Mg3N2 nanostructures.
The field of Mg3N2 nanostructure R&D is also focused on understanding the fundamental mechanisms underlying their unique properties. This involves in-depth investigations into the electronic structure, surface chemistry, and quantum confinement effects of these nanomaterials. By gaining a deeper understanding of these aspects, researchers aim to unlock new functionalities and optimize the performance of Mg3N2 nanostructures in targeted applications.
The development of Mg3N2 nanostructures has been driven by the increasing demand for lightweight, high-performance materials in industries such as aerospace, automotive, and energy storage. As global efforts to reduce carbon emissions intensify, the need for innovative materials that can contribute to energy efficiency and sustainability has become more pressing. Mg3N2 nanostructures have shown potential in addressing these challenges, particularly in areas such as hydrogen storage, catalysis, and optoelectronic devices.
The technological trajectory of Mg3N2 nanostructures has been marked by significant advancements in synthesis methods and characterization techniques. Early research focused primarily on bulk Mg3N2, but the shift towards nanostructured forms has opened up new possibilities for tailoring material properties at the nanoscale. This transition has been facilitated by improvements in nanofabrication techniques, such as chemical vapor deposition, sol-gel methods, and electrospinning.
One of the key objectives in Mg3N2 nanostructure R&D is to achieve precise control over morphology, size, and composition. Researchers aim to develop reproducible synthesis methods that can yield nanostructures with specific shapes, such as nanoparticles, nanowires, and nanotubes. These diverse morphologies offer unique advantages in different applications, making the ability to control nanostructure formation a critical aspect of ongoing research.
Another important goal is to enhance the stability and performance of Mg3N2 nanostructures in various environmental conditions. This includes improving their resistance to oxidation and moisture, which are common challenges for magnesium-based materials. Researchers are exploring strategies such as surface functionalization and composite formation to address these issues and expand the potential applications of Mg3N2 nanostructures.
The field of Mg3N2 nanostructure R&D is also focused on understanding the fundamental mechanisms underlying their unique properties. This involves in-depth investigations into the electronic structure, surface chemistry, and quantum confinement effects of these nanomaterials. By gaining a deeper understanding of these aspects, researchers aim to unlock new functionalities and optimize the performance of Mg3N2 nanostructures in targeted applications.
Market Analysis for Mg3N2 Nanostructure Applications
The market for magnesium nitride (Mg3N2) nanostructures is experiencing significant growth, driven by their unique properties and diverse applications across multiple industries. These nanostructures exhibit exceptional characteristics, including high surface area, enhanced reactivity, and improved mechanical properties, making them attractive for various technological advancements.
In the energy sector, Mg3N2 nanostructures show promise in hydrogen storage applications. Their ability to efficiently store and release hydrogen makes them potential candidates for fuel cell technologies and clean energy solutions. This aligns with the global push towards sustainable energy sources and reduction of carbon emissions.
The electronics industry is another key market for Mg3N2 nanostructures. Their semiconducting properties and high electron mobility make them suitable for use in advanced electronic devices, such as high-performance transistors and sensors. As the demand for smaller, faster, and more efficient electronic components continues to grow, the market for Mg3N2 nanostructures in this sector is expected to expand.
In the field of catalysis, Mg3N2 nanostructures demonstrate excellent catalytic activity for various chemical reactions. This property is particularly valuable in the chemical and pharmaceutical industries, where efficient and selective catalysts are crucial for process optimization and cost reduction. The increasing focus on green chemistry and sustainable manufacturing processes further drives the demand for these nanostructures as catalysts.
The aerospace and automotive industries are also showing interest in Mg3N2 nanostructures due to their potential in lightweight structural materials. By incorporating these nanostructures into composite materials, manufacturers can develop stronger, lighter components that contribute to improved fuel efficiency and performance in vehicles and aircraft.
In the medical field, Mg3N2 nanostructures are being explored for drug delivery systems and biomedical imaging applications. Their biocompatibility and controllable degradation rates make them promising candidates for targeted drug release and tissue engineering scaffolds. As personalized medicine and advanced diagnostic techniques gain traction, the demand for these nanostructures in healthcare applications is expected to rise.
The global market for nanomaterials, including Mg3N2 nanostructures, is projected to grow steadily in the coming years. Factors such as increasing research and development activities, government funding for nanotechnology, and the expanding applications across various industries contribute to this growth. However, challenges such as high production costs and potential environmental and health concerns need to be addressed to fully realize the market potential of Mg3N2 nanostructures.
In the energy sector, Mg3N2 nanostructures show promise in hydrogen storage applications. Their ability to efficiently store and release hydrogen makes them potential candidates for fuel cell technologies and clean energy solutions. This aligns with the global push towards sustainable energy sources and reduction of carbon emissions.
The electronics industry is another key market for Mg3N2 nanostructures. Their semiconducting properties and high electron mobility make them suitable for use in advanced electronic devices, such as high-performance transistors and sensors. As the demand for smaller, faster, and more efficient electronic components continues to grow, the market for Mg3N2 nanostructures in this sector is expected to expand.
In the field of catalysis, Mg3N2 nanostructures demonstrate excellent catalytic activity for various chemical reactions. This property is particularly valuable in the chemical and pharmaceutical industries, where efficient and selective catalysts are crucial for process optimization and cost reduction. The increasing focus on green chemistry and sustainable manufacturing processes further drives the demand for these nanostructures as catalysts.
The aerospace and automotive industries are also showing interest in Mg3N2 nanostructures due to their potential in lightweight structural materials. By incorporating these nanostructures into composite materials, manufacturers can develop stronger, lighter components that contribute to improved fuel efficiency and performance in vehicles and aircraft.
In the medical field, Mg3N2 nanostructures are being explored for drug delivery systems and biomedical imaging applications. Their biocompatibility and controllable degradation rates make them promising candidates for targeted drug release and tissue engineering scaffolds. As personalized medicine and advanced diagnostic techniques gain traction, the demand for these nanostructures in healthcare applications is expected to rise.
The global market for nanomaterials, including Mg3N2 nanostructures, is projected to grow steadily in the coming years. Factors such as increasing research and development activities, government funding for nanotechnology, and the expanding applications across various industries contribute to this growth. However, challenges such as high production costs and potential environmental and health concerns need to be addressed to fully realize the market potential of Mg3N2 nanostructures.
Current State and Challenges in Mg3N2 Nanostructure Synthesis
The current state of Mg3N2 nanostructure synthesis presents both significant advancements and persistent challenges. Recent years have witnessed remarkable progress in the development of various synthesis methods, enabling the production of magnesium nitride nanostructures with diverse morphologies and improved properties.
One of the primary synthesis routes for Mg3N2 nanostructures is the direct nitridation of magnesium metal or magnesium-containing precursors. This method has been refined to achieve better control over particle size and morphology. Researchers have successfully synthesized Mg3N2 nanoparticles, nanowires, and nanobelts using optimized nitridation conditions, including temperature, pressure, and nitrogen flow rates.
Solution-based methods have also emerged as promising approaches for Mg3N2 nanostructure synthesis. These techniques offer advantages in terms of scalability and the potential for producing uniform nanostructures. Sol-gel processes and solvothermal reactions have been employed to create Mg3N2 nanoparticles with controlled sizes and shapes.
Despite these advancements, several challenges persist in the synthesis of Mg3N2 nanostructures. One major obstacle is the high reactivity of magnesium nitride with moisture and oxygen, which can lead to rapid degradation of the nanostructures. This sensitivity necessitates stringent control over the synthesis environment and storage conditions, often requiring inert atmospheres or protective coatings.
Another significant challenge lies in achieving precise control over the size, shape, and composition of Mg3N2 nanostructures. While progress has been made, reproducibly synthesizing uniform nanostructures with desired characteristics remains difficult. This challenge is particularly pronounced when attempting to create complex nanoarchitectures or hierarchical structures.
The scalability of synthesis methods for Mg3N2 nanostructures also presents a hurdle for their widespread application. Many current techniques are limited to laboratory-scale production, and scaling up these processes while maintaining nanostructure quality and uniformity is an ongoing challenge.
Furthermore, the development of environmentally friendly and cost-effective synthesis routes for Mg3N2 nanostructures is an area that requires further attention. Some existing methods involve high-temperature processes or the use of hazardous precursors, which can limit their practical implementation on a larger scale.
In conclusion, while significant progress has been made in the synthesis of Mg3N2 nanostructures, addressing these challenges will be crucial for advancing their potential applications in various fields, including energy storage, catalysis, and optoelectronics. Future research efforts should focus on developing robust synthesis methods that offer precise control, scalability, and environmental sustainability to fully harness the potential of Mg3N2 nanostructures.
One of the primary synthesis routes for Mg3N2 nanostructures is the direct nitridation of magnesium metal or magnesium-containing precursors. This method has been refined to achieve better control over particle size and morphology. Researchers have successfully synthesized Mg3N2 nanoparticles, nanowires, and nanobelts using optimized nitridation conditions, including temperature, pressure, and nitrogen flow rates.
Solution-based methods have also emerged as promising approaches for Mg3N2 nanostructure synthesis. These techniques offer advantages in terms of scalability and the potential for producing uniform nanostructures. Sol-gel processes and solvothermal reactions have been employed to create Mg3N2 nanoparticles with controlled sizes and shapes.
Despite these advancements, several challenges persist in the synthesis of Mg3N2 nanostructures. One major obstacle is the high reactivity of magnesium nitride with moisture and oxygen, which can lead to rapid degradation of the nanostructures. This sensitivity necessitates stringent control over the synthesis environment and storage conditions, often requiring inert atmospheres or protective coatings.
Another significant challenge lies in achieving precise control over the size, shape, and composition of Mg3N2 nanostructures. While progress has been made, reproducibly synthesizing uniform nanostructures with desired characteristics remains difficult. This challenge is particularly pronounced when attempting to create complex nanoarchitectures or hierarchical structures.
The scalability of synthesis methods for Mg3N2 nanostructures also presents a hurdle for their widespread application. Many current techniques are limited to laboratory-scale production, and scaling up these processes while maintaining nanostructure quality and uniformity is an ongoing challenge.
Furthermore, the development of environmentally friendly and cost-effective synthesis routes for Mg3N2 nanostructures is an area that requires further attention. Some existing methods involve high-temperature processes or the use of hazardous precursors, which can limit their practical implementation on a larger scale.
In conclusion, while significant progress has been made in the synthesis of Mg3N2 nanostructures, addressing these challenges will be crucial for advancing their potential applications in various fields, including energy storage, catalysis, and optoelectronics. Future research efforts should focus on developing robust synthesis methods that offer precise control, scalability, and environmental sustainability to fully harness the potential of Mg3N2 nanostructures.
Current Mg3N2 Nanostructure Synthesis Methods
01 Synthesis methods for magnesium nitride nanostructures
Various techniques are employed to synthesize magnesium nitride nanostructures, including chemical vapor deposition, sol-gel methods, and plasma-enhanced processes. These methods allow for precise control over the size, shape, and composition of the nanostructures, enabling tailored properties for specific applications.- Synthesis methods for magnesium nitride nanostructures: Various methods are employed to synthesize magnesium nitride nanostructures, including chemical vapor deposition, thermal decomposition, and plasma-enhanced techniques. These processes allow for the controlled growth of nanostructures with specific morphologies and properties, such as nanowires, nanoparticles, and thin films.
- Applications in optoelectronic devices: Magnesium nitride nanostructures show promise in optoelectronic applications due to their unique electronic and optical properties. They can be used in the fabrication of light-emitting diodes (LEDs), photodetectors, and other semiconductor devices, offering potential improvements in efficiency and performance.
- Doping and composition control: Researchers are exploring methods to control the composition and doping of magnesium nitride nanostructures. This includes incorporating other elements or compounds to modify their electronic, optical, or magnetic properties, enabling the tailoring of nanostructures for specific applications.
- Characterization techniques: Advanced characterization techniques are employed to analyze the structure, composition, and properties of magnesium nitride nanostructures. These include electron microscopy, X-ray diffraction, spectroscopy, and electrical measurements, providing crucial insights into their physical and chemical characteristics.
- Energy storage and conversion applications: Magnesium nitride nanostructures show potential in energy-related applications, such as hydrogen storage, catalysis, and battery technologies. Their high surface area and unique properties make them attractive candidates for improving the efficiency and performance of various energy storage and conversion devices.
02 Applications in optoelectronic devices
Magnesium nitride nanostructures show promise in optoelectronic applications due to their unique electronic and optical properties. They can be used in the fabrication of light-emitting diodes, photodetectors, and other semiconductor devices, offering improved performance and efficiency.Expand Specific Solutions03 Use in energy storage and conversion
Magnesium nitride nanostructures have potential applications in energy storage and conversion technologies. They can be utilized in batteries, fuel cells, and hydrogen storage systems, offering enhanced capacity, cycling stability, and energy efficiency compared to conventional materials.Expand Specific Solutions04 Catalytic properties and applications
The high surface area and unique chemical properties of magnesium nitride nanostructures make them effective catalysts for various chemical reactions. They can be used in organic synthesis, environmental remediation, and industrial processes, offering improved reaction rates and selectivity.Expand Specific Solutions05 Characterization and analysis techniques
Advanced characterization methods are employed to analyze the structure, composition, and properties of magnesium nitride nanostructures. These techniques include electron microscopy, X-ray diffraction, spectroscopic methods, and computational modeling, enabling a deeper understanding of their fundamental properties and behavior.Expand Specific Solutions
Key Players in Mg3N2 Nanostructure Research and Development
The field of magnesium nitride nanostructures for R&D is in an early stage of development, with significant potential for growth. The market size is currently modest but expanding as researchers explore applications in areas such as energy storage, catalysis, and electronics. The technology's maturity is still evolving, with academic institutions leading much of the research. Key players include Indian Institute of Technology Madras, Nanjing Tech University, and Peking University, focusing on fundamental studies and potential applications. Industry involvement is limited but growing, with companies like Grimat Engineering Institute Co., Ltd. and Beijing Haoyun New Material Co., Ltd. beginning to explore commercial possibilities in this emerging field.
Nanjing Tech University
Technical Solution: Nanjing Tech University has made significant advances in magnesium nitride nanostructures for R&D. They have developed a novel synthesis method using plasma-enhanced chemical vapor deposition (PECVD) to create high-quality Mg3N2 nanostructures with controlled morphology and composition[1]. This technique allows for the production of nanowires, nanobelts, and nanosheets with tunable dimensions and properties. The university has also explored the use of these nanostructures in various applications, including hydrogen storage, catalysis, and optoelectronic devices[3]. Their research has demonstrated enhanced surface area and reactivity of Mg3N2 nanostructures compared to bulk materials, leading to improved performance in energy storage and conversion applications[5].
Strengths: Advanced synthesis techniques, precise control over nanostructure morphology, and demonstrated applications in energy-related fields. Weaknesses: Potential scalability issues for large-scale production and long-term stability of nanostructures in ambient conditions.
Nanjing University
Technical Solution: Nanjing University has made substantial progress in the field of magnesium nitride nanostructures for R&D. Their research team has developed a unique electrochemical synthesis method to produce Mg3N2 nanoparticles with high purity and controlled size distribution[2]. This approach offers advantages in terms of low-temperature processing and environmentally friendly production. The university has also investigated the optical and electronic properties of these nanostructures, revealing their potential for use in light-emitting diodes and photodetectors[4]. Additionally, they have explored the use of magnesium nitride nanostructures as catalysts for nitrogen fixation and ammonia synthesis, demonstrating improved efficiency compared to traditional catalysts[6].
Strengths: Innovative synthesis methods, in-depth characterization of nanostructure properties, and exploration of novel applications. Weaknesses: Limited research on large-scale production and integration of nanostructures into practical devices.
Core Innovations in Mg3N2 Nanostructure Engineering
White light emitting metallic magnesium nanoclusters and a method of production thereof
PatentPendingIN202311013937A
Innovation
- A one-pot facile synthesis method using magnesium chloride or acetate, ascorbic acid, and bovine serum albumin (BSA) to produce ultra-small, fluorescent magnesium nanoclusters with white light emission spectra from 400 nm to 620 nm, achieving high quantum yield and excellent water solubility.
Bifunctional fusion protein platform used for drug research and development
PatentWO2020077991A1
Innovation
- Using a bifunctional fusion protein platform, the first receptor fragment, IgG1Fc fragment and second receptor fragment are combined to form a dimer structure, and a stable complex is formed through the disulfide bond of the IgG1Fc fragment to prolong the drug's retention in the plasma. half-life, and improve the stability and biological activity of the molecule through specific binding.
Environmental Impact of Mg3N2 Nanostructure Production
The production of magnesium nitride (Mg3N2) nanostructures has significant environmental implications that warrant careful consideration. The synthesis process typically involves high-temperature reactions and the use of various precursors, which can lead to energy-intensive operations and potential emissions of harmful byproducts.
One of the primary environmental concerns is the energy consumption associated with the production of Mg3N2 nanostructures. The high temperatures required for synthesis, often exceeding 600°C, contribute to increased greenhouse gas emissions if non-renewable energy sources are used. This energy-intensive process underscores the need for more efficient production methods and the integration of renewable energy sources to mitigate the carbon footprint.
The use of chemical precursors in the synthesis of Mg3N2 nanostructures also raises environmental concerns. Depending on the specific production method, these precursors may include magnesium metal, ammonia, or nitrogen gas. The handling and disposal of these materials require strict safety protocols to prevent environmental contamination. Ammonia, in particular, is known for its potential to contribute to air pollution and eutrophication if released into the environment.
Waste management is another critical aspect of the environmental impact of Mg3N2 nanostructure production. The synthesis process may generate byproducts and unreacted materials that require proper disposal or recycling. Improper handling of these wastes can lead to soil and water pollution, potentially affecting local ecosystems and human health.
The production of nanostructures also raises concerns about the release of nanoparticles into the environment. While Mg3N2 nanostructures have many beneficial applications, their small size and unique properties may pose risks if they enter ecosystems. The potential for bioaccumulation and long-term environmental effects of these nanoparticles is an area that requires further research and monitoring.
Water usage in the production process is another environmental factor to consider. Depending on the synthesis method, significant amounts of water may be required for cooling, cleaning, or as a reaction medium. Efficient water management and recycling systems are essential to minimize the impact on local water resources.
To address these environmental concerns, researchers and manufacturers are exploring more sustainable production methods for Mg3N2 nanostructures. These include the development of low-temperature synthesis techniques, the use of environmentally friendly precursors, and the implementation of closed-loop production systems to minimize waste and emissions. Additionally, life cycle assessments are being conducted to better understand and quantify the environmental impacts throughout the entire production and use cycle of Mg3N2 nanostructures.
One of the primary environmental concerns is the energy consumption associated with the production of Mg3N2 nanostructures. The high temperatures required for synthesis, often exceeding 600°C, contribute to increased greenhouse gas emissions if non-renewable energy sources are used. This energy-intensive process underscores the need for more efficient production methods and the integration of renewable energy sources to mitigate the carbon footprint.
The use of chemical precursors in the synthesis of Mg3N2 nanostructures also raises environmental concerns. Depending on the specific production method, these precursors may include magnesium metal, ammonia, or nitrogen gas. The handling and disposal of these materials require strict safety protocols to prevent environmental contamination. Ammonia, in particular, is known for its potential to contribute to air pollution and eutrophication if released into the environment.
Waste management is another critical aspect of the environmental impact of Mg3N2 nanostructure production. The synthesis process may generate byproducts and unreacted materials that require proper disposal or recycling. Improper handling of these wastes can lead to soil and water pollution, potentially affecting local ecosystems and human health.
The production of nanostructures also raises concerns about the release of nanoparticles into the environment. While Mg3N2 nanostructures have many beneficial applications, their small size and unique properties may pose risks if they enter ecosystems. The potential for bioaccumulation and long-term environmental effects of these nanoparticles is an area that requires further research and monitoring.
Water usage in the production process is another environmental factor to consider. Depending on the synthesis method, significant amounts of water may be required for cooling, cleaning, or as a reaction medium. Efficient water management and recycling systems are essential to minimize the impact on local water resources.
To address these environmental concerns, researchers and manufacturers are exploring more sustainable production methods for Mg3N2 nanostructures. These include the development of low-temperature synthesis techniques, the use of environmentally friendly precursors, and the implementation of closed-loop production systems to minimize waste and emissions. Additionally, life cycle assessments are being conducted to better understand and quantify the environmental impacts throughout the entire production and use cycle of Mg3N2 nanostructures.
Safety Considerations in Mg3N2 Nanostructure Handling
The handling of magnesium nitride (Mg3N2) nanostructures requires careful consideration of safety protocols due to their unique properties and potential hazards. These nanostructures are highly reactive with water and moisture, which can lead to the rapid release of ammonia gas. This reactivity necessitates stringent controls on environmental conditions during storage, handling, and experimentation.
Researchers and laboratory personnel must be equipped with appropriate personal protective equipment (PPE) when working with Mg3N2 nanostructures. This includes, but is not limited to, chemical-resistant gloves, safety goggles, and lab coats. In some cases, respiratory protection may be necessary, especially when dealing with powdered forms of the nanostructures that could become airborne.
The storage of Mg3N2 nanostructures demands particular attention. They should be kept in sealed, moisture-proof containers, preferably under an inert atmosphere such as argon or nitrogen. Desiccants may be used to further control humidity levels within storage units. It is crucial to maintain these materials away from water sources and to clearly label containers with appropriate hazard warnings.
When conducting experiments or manipulations involving Mg3N2 nanostructures, work should be performed in a controlled environment, such as a glove box or a fume hood with adequate ventilation. This helps to minimize exposure risks and contain any potential release of ammonia gas. Additionally, proper grounding equipment should be used to prevent the accumulation of static electricity, which could potentially ignite flammable gases.
Disposal of Mg3N2 nanostructures and related waste materials requires specific procedures. These materials should not be discarded in regular waste streams but should be treated as hazardous waste. Controlled decomposition or neutralization processes may be necessary before disposal, and all local regulations regarding hazardous waste management must be strictly followed.
Emergency response protocols should be established and clearly communicated to all personnel working with or around Mg3N2 nanostructures. This includes procedures for dealing with spills, accidental exposures, and the release of ammonia gas. Eyewash stations, safety showers, and appropriate fire suppression equipment should be readily accessible in areas where these materials are handled.
Training is a critical component of safety management for Mg3N2 nanostructure handling. All personnel involved in research or production activities should receive comprehensive instruction on the properties of these materials, proper handling techniques, and emergency procedures. Regular refresher courses and safety audits can help ensure ongoing compliance with safety protocols.
Lastly, it is important to consider the potential environmental impact of Mg3N2 nanostructures. Measures should be taken to prevent their release into the environment, as they could potentially affect aquatic ecosystems due to their reactivity with water. Research facilities should implement robust containment and filtration systems to mitigate any risk of environmental contamination.
Researchers and laboratory personnel must be equipped with appropriate personal protective equipment (PPE) when working with Mg3N2 nanostructures. This includes, but is not limited to, chemical-resistant gloves, safety goggles, and lab coats. In some cases, respiratory protection may be necessary, especially when dealing with powdered forms of the nanostructures that could become airborne.
The storage of Mg3N2 nanostructures demands particular attention. They should be kept in sealed, moisture-proof containers, preferably under an inert atmosphere such as argon or nitrogen. Desiccants may be used to further control humidity levels within storage units. It is crucial to maintain these materials away from water sources and to clearly label containers with appropriate hazard warnings.
When conducting experiments or manipulations involving Mg3N2 nanostructures, work should be performed in a controlled environment, such as a glove box or a fume hood with adequate ventilation. This helps to minimize exposure risks and contain any potential release of ammonia gas. Additionally, proper grounding equipment should be used to prevent the accumulation of static electricity, which could potentially ignite flammable gases.
Disposal of Mg3N2 nanostructures and related waste materials requires specific procedures. These materials should not be discarded in regular waste streams but should be treated as hazardous waste. Controlled decomposition or neutralization processes may be necessary before disposal, and all local regulations regarding hazardous waste management must be strictly followed.
Emergency response protocols should be established and clearly communicated to all personnel working with or around Mg3N2 nanostructures. This includes procedures for dealing with spills, accidental exposures, and the release of ammonia gas. Eyewash stations, safety showers, and appropriate fire suppression equipment should be readily accessible in areas where these materials are handled.
Training is a critical component of safety management for Mg3N2 nanostructure handling. All personnel involved in research or production activities should receive comprehensive instruction on the properties of these materials, proper handling techniques, and emergency procedures. Regular refresher courses and safety audits can help ensure ongoing compliance with safety protocols.
Lastly, it is important to consider the potential environmental impact of Mg3N2 nanostructures. Measures should be taken to prevent their release into the environment, as they could potentially affect aquatic ecosystems due to their reactivity with water. Research facilities should implement robust containment and filtration systems to mitigate any risk of environmental contamination.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!