How to Achieve Scalability in Magnesium Nitride Production?
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg3N2 Production Background and Objectives
Magnesium nitride (Mg3N2) production has gained significant attention in recent years due to its potential applications in various industries, including energy storage, catalysis, and advanced materials. The development of scalable production methods for Mg3N2 is crucial for meeting the growing demand and enabling its widespread use in commercial applications.
The evolution of Mg3N2 production techniques can be traced back to the early 20th century when small-scale laboratory synthesis methods were first developed. These early methods primarily involved direct nitridation of magnesium metal at high temperatures. However, these processes were limited in their scalability and often resulted in low yields and impure products.
As research in this field progressed, new synthesis routes were explored, including plasma-assisted methods, chemical vapor deposition, and solution-based approaches. These advancements aimed to improve the quality and yield of Mg3N2 while addressing the challenges associated with large-scale production.
The current technological landscape for Mg3N2 production is characterized by a diverse range of methods, each with its own advantages and limitations. Traditional solid-state reactions between magnesium metal and nitrogen gas remain widely used but face challenges in achieving uniform product quality and controlling particle size distribution. More recent innovations, such as mechanochemical synthesis and microwave-assisted methods, have shown promise in enhancing reaction kinetics and product purity.
The primary objective in the field of Mg3N2 production is to develop scalable, cost-effective, and environmentally friendly processes that can meet the increasing industrial demand. This involves overcoming several key challenges, including:
1. Improving reaction efficiency and yield to reduce production costs
2. Enhancing product purity and controlling particle morphology
3. Developing continuous production methods for large-scale manufacturing
4. Addressing safety concerns associated with handling reactive magnesium and nitrogen precursors
5. Optimizing energy consumption and reducing the environmental footprint of production processes
To achieve these objectives, researchers and industry professionals are exploring innovative approaches that combine advanced materials science, process engineering, and nanotechnology. These efforts aim to bridge the gap between laboratory-scale synthesis and industrial-scale production, paving the way for the widespread adoption of Mg3N2 in various applications.
The future of Mg3N2 production is likely to involve a combination of improved conventional methods and novel technologies. Emerging trends include the development of hybrid processes that integrate multiple synthesis techniques, the use of advanced reactor designs for better control over reaction parameters, and the exploration of alternative precursors and catalysts to enhance reaction kinetics and selectivity.
The evolution of Mg3N2 production techniques can be traced back to the early 20th century when small-scale laboratory synthesis methods were first developed. These early methods primarily involved direct nitridation of magnesium metal at high temperatures. However, these processes were limited in their scalability and often resulted in low yields and impure products.
As research in this field progressed, new synthesis routes were explored, including plasma-assisted methods, chemical vapor deposition, and solution-based approaches. These advancements aimed to improve the quality and yield of Mg3N2 while addressing the challenges associated with large-scale production.
The current technological landscape for Mg3N2 production is characterized by a diverse range of methods, each with its own advantages and limitations. Traditional solid-state reactions between magnesium metal and nitrogen gas remain widely used but face challenges in achieving uniform product quality and controlling particle size distribution. More recent innovations, such as mechanochemical synthesis and microwave-assisted methods, have shown promise in enhancing reaction kinetics and product purity.
The primary objective in the field of Mg3N2 production is to develop scalable, cost-effective, and environmentally friendly processes that can meet the increasing industrial demand. This involves overcoming several key challenges, including:
1. Improving reaction efficiency and yield to reduce production costs
2. Enhancing product purity and controlling particle morphology
3. Developing continuous production methods for large-scale manufacturing
4. Addressing safety concerns associated with handling reactive magnesium and nitrogen precursors
5. Optimizing energy consumption and reducing the environmental footprint of production processes
To achieve these objectives, researchers and industry professionals are exploring innovative approaches that combine advanced materials science, process engineering, and nanotechnology. These efforts aim to bridge the gap between laboratory-scale synthesis and industrial-scale production, paving the way for the widespread adoption of Mg3N2 in various applications.
The future of Mg3N2 production is likely to involve a combination of improved conventional methods and novel technologies. Emerging trends include the development of hybrid processes that integrate multiple synthesis techniques, the use of advanced reactor designs for better control over reaction parameters, and the exploration of alternative precursors and catalysts to enhance reaction kinetics and selectivity.
Market Analysis for Mg3N2 Applications
The market for magnesium nitride (Mg3N2) applications is experiencing significant growth driven by its unique properties and versatile uses across various industries. The global demand for Mg3N2 is primarily fueled by its applications in advanced materials, energy storage, and chemical synthesis.
In the advanced materials sector, Mg3N2 is gaining traction as a precursor for the production of high-performance ceramics and composites. These materials find applications in aerospace, automotive, and electronics industries, where lightweight and durable components are crucial. The aerospace industry, in particular, is showing increased interest in Mg3N2-derived materials for their potential to reduce aircraft weight and improve fuel efficiency.
The energy storage market presents a promising avenue for Mg3N2 applications. Research into magnesium-based batteries as an alternative to lithium-ion technology has sparked interest in Mg3N2 as a potential electrode material. The abundance of magnesium and its theoretical energy density make it an attractive option for next-generation energy storage solutions. This market segment is expected to grow as the demand for more efficient and sustainable energy storage systems increases.
In the chemical industry, Mg3N2 serves as a valuable reagent for the synthesis of various organic compounds, particularly in the production of pharmaceuticals and agrochemicals. Its ability to act as a strong base and a source of ammonia makes it useful in numerous chemical reactions. The pharmaceutical sector's continuous growth and the increasing demand for novel agrochemicals are likely to drive the market for Mg3N2 in this application area.
The semiconductor industry is another potential growth market for Mg3N2. Its use in the production of gallium nitride (GaN) and other III-V compound semiconductors positions it as a key material in the development of advanced electronic devices, including high-power and high-frequency applications.
Geographically, Asia-Pacific is expected to be the fastest-growing market for Mg3N2 applications, driven by rapid industrialization, increasing investments in research and development, and the presence of major electronics and automotive manufacturers. North America and Europe are also significant markets, particularly in the advanced materials and energy storage sectors.
Despite the promising outlook, challenges remain in scaling up Mg3N2 production to meet growing demand. The current production methods are often energy-intensive and costly, which could potentially limit market growth. Addressing these scalability issues is crucial for unlocking the full market potential of Mg3N2 applications across various industries.
In the advanced materials sector, Mg3N2 is gaining traction as a precursor for the production of high-performance ceramics and composites. These materials find applications in aerospace, automotive, and electronics industries, where lightweight and durable components are crucial. The aerospace industry, in particular, is showing increased interest in Mg3N2-derived materials for their potential to reduce aircraft weight and improve fuel efficiency.
The energy storage market presents a promising avenue for Mg3N2 applications. Research into magnesium-based batteries as an alternative to lithium-ion technology has sparked interest in Mg3N2 as a potential electrode material. The abundance of magnesium and its theoretical energy density make it an attractive option for next-generation energy storage solutions. This market segment is expected to grow as the demand for more efficient and sustainable energy storage systems increases.
In the chemical industry, Mg3N2 serves as a valuable reagent for the synthesis of various organic compounds, particularly in the production of pharmaceuticals and agrochemicals. Its ability to act as a strong base and a source of ammonia makes it useful in numerous chemical reactions. The pharmaceutical sector's continuous growth and the increasing demand for novel agrochemicals are likely to drive the market for Mg3N2 in this application area.
The semiconductor industry is another potential growth market for Mg3N2. Its use in the production of gallium nitride (GaN) and other III-V compound semiconductors positions it as a key material in the development of advanced electronic devices, including high-power and high-frequency applications.
Geographically, Asia-Pacific is expected to be the fastest-growing market for Mg3N2 applications, driven by rapid industrialization, increasing investments in research and development, and the presence of major electronics and automotive manufacturers. North America and Europe are also significant markets, particularly in the advanced materials and energy storage sectors.
Despite the promising outlook, challenges remain in scaling up Mg3N2 production to meet growing demand. The current production methods are often energy-intensive and costly, which could potentially limit market growth. Addressing these scalability issues is crucial for unlocking the full market potential of Mg3N2 applications across various industries.
Current Challenges in Mg3N2 Synthesis
The synthesis of magnesium nitride (Mg3N2) faces several significant challenges that hinder its large-scale production. One of the primary obstacles is the high reactivity of magnesium with oxygen and moisture, which necessitates stringent control over the reaction environment. This sensitivity to air and water makes handling and storage of both raw materials and the final product particularly demanding, requiring specialized equipment and facilities.
Another major challenge lies in the high temperatures required for the direct reaction between magnesium and nitrogen. Conventional synthesis methods often involve temperatures exceeding 700°C, which not only increases energy consumption but also poses safety risks and equipment wear. The high-temperature process also limits the choice of reactor materials and design, further complicating scalability efforts.
The reaction kinetics of Mg3N2 formation present additional hurdles. The nitridation process is typically slow and incomplete, resulting in low yields and the presence of unreacted magnesium. This inefficiency necessitates extended reaction times or multiple processing steps, which are not conducive to large-scale production. Moreover, the formation of a nitride layer on the surface of magnesium particles can impede further reaction, leading to incomplete conversion.
Controlling the particle size and morphology of the synthesized Mg3N2 is another critical challenge. The high-temperature synthesis often results in sintering and agglomeration of particles, which can negatively impact the material's properties and subsequent applications. Achieving uniform particle size distribution and desired morphology on a large scale remains a significant technical barrier.
The purity of the final product is also a concern in Mg3N2 synthesis. Impurities, particularly oxygen-containing species, can significantly affect the material's properties and performance. Ensuring high purity on an industrial scale requires sophisticated purification techniques and stringent quality control measures, adding complexity and cost to the production process.
Lastly, the lack of efficient and scalable production methods poses a significant challenge to the widespread adoption of Mg3N2. Current laboratory-scale synthesis techniques often do not translate well to industrial-scale production, necessitating the development of novel approaches that can maintain product quality while increasing output. This gap between lab-scale success and industrial viability represents a major hurdle in the commercialization of Mg3N2-based technologies.
Another major challenge lies in the high temperatures required for the direct reaction between magnesium and nitrogen. Conventional synthesis methods often involve temperatures exceeding 700°C, which not only increases energy consumption but also poses safety risks and equipment wear. The high-temperature process also limits the choice of reactor materials and design, further complicating scalability efforts.
The reaction kinetics of Mg3N2 formation present additional hurdles. The nitridation process is typically slow and incomplete, resulting in low yields and the presence of unreacted magnesium. This inefficiency necessitates extended reaction times or multiple processing steps, which are not conducive to large-scale production. Moreover, the formation of a nitride layer on the surface of magnesium particles can impede further reaction, leading to incomplete conversion.
Controlling the particle size and morphology of the synthesized Mg3N2 is another critical challenge. The high-temperature synthesis often results in sintering and agglomeration of particles, which can negatively impact the material's properties and subsequent applications. Achieving uniform particle size distribution and desired morphology on a large scale remains a significant technical barrier.
The purity of the final product is also a concern in Mg3N2 synthesis. Impurities, particularly oxygen-containing species, can significantly affect the material's properties and performance. Ensuring high purity on an industrial scale requires sophisticated purification techniques and stringent quality control measures, adding complexity and cost to the production process.
Lastly, the lack of efficient and scalable production methods poses a significant challenge to the widespread adoption of Mg3N2. Current laboratory-scale synthesis techniques often do not translate well to industrial-scale production, necessitating the development of novel approaches that can maintain product quality while increasing output. This gap between lab-scale success and industrial viability represents a major hurdle in the commercialization of Mg3N2-based technologies.
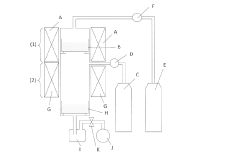
Existing Scalable Mg3N2 Production Methods
01 Synthesis methods for magnesium nitride
Various methods for synthesizing magnesium nitride are explored to improve scalability. These include direct nitridation of magnesium, plasma-enhanced chemical vapor deposition, and solution-based approaches. The choice of synthesis method can significantly impact the scalability and quality of the produced magnesium nitride.- Synthesis methods for magnesium nitride: Various methods for synthesizing magnesium nitride are explored to improve scalability. These include direct nitridation of magnesium metal, plasma-assisted processes, and chemical vapor deposition techniques. The choice of synthesis method can significantly impact the quality and yield of magnesium nitride produced at scale.
- Reactor design for large-scale production: Specialized reactor designs are developed to facilitate the scalable production of magnesium nitride. These reactors consider factors such as temperature control, gas flow dynamics, and uniform nitridation to ensure consistent product quality in larger batches. Innovations in reactor technology aim to overcome challenges associated with scaling up magnesium nitride production.
- Precursor material preparation and handling: The preparation and handling of precursor materials play a crucial role in the scalability of magnesium nitride production. Techniques for purifying and processing magnesium metal or other precursors are developed to ensure consistent quality and reactivity. Efficient handling systems are designed to manage larger quantities of raw materials in industrial settings.
- Process optimization and control: Advanced process control strategies are implemented to optimize the production of magnesium nitride at scale. This includes the use of sensors, real-time monitoring systems, and predictive models to maintain ideal reaction conditions. Automated control systems help manage complex parameters such as temperature, pressure, and gas composition to enhance yield and quality.
- Post-synthesis treatment and quality control: Methods for post-synthesis treatment and quality control of magnesium nitride are developed to ensure product consistency at larger scales. This includes purification techniques, particle size control, and surface modification processes. Advanced characterization methods are employed to verify the purity and properties of the final product, ensuring it meets the required specifications for various applications.
02 Particle size control and uniformity
Controlling the particle size and achieving uniformity in magnesium nitride production is crucial for scalability. Techniques such as ball milling, spray pyrolysis, and microwave-assisted synthesis are employed to produce uniform, nano-sized particles. This enhances the material's properties and facilitates large-scale production.Expand Specific Solutions03 Reactor design and process optimization
Optimizing reactor design and process parameters is essential for scaling up magnesium nitride production. This includes considerations such as temperature control, gas flow rates, and residence time. Advanced reactor designs, such as fluidized bed reactors or continuous flow systems, can significantly improve production efficiency and scalability.Expand Specific Solutions04 Precursor selection and preparation
The choice and preparation of precursors play a crucial role in the scalability of magnesium nitride production. Various precursors, including magnesium metal, magnesium salts, and organometallic compounds, are investigated. Proper selection and preparation of precursors can enhance reaction kinetics, yield, and purity of the final product.Expand Specific Solutions05 Purification and quality control
Developing efficient purification methods and implementing robust quality control measures are essential for scaling up magnesium nitride production. Techniques such as chemical etching, thermal treatment, and advanced characterization methods are employed to ensure high-purity products suitable for various applications.Expand Specific Solutions
Key Players in Mg3N2 Manufacturing
The production of magnesium nitride is currently in a nascent stage of development, with the market size still relatively small but showing potential for growth. The technology is in its early phases of maturity, with ongoing research and development efforts focused on improving scalability and efficiency. Key players in this field include Sumitomo Chemical Co., Ltd., which has a strong presence in advanced materials, and SLT-Technologies GmbH & Co. KG, specializing in automation and engineering services. Academic institutions like the University of Science & Technology of China and Shanghai Jiao Tong University are also contributing to research advancements. As the technology evolves, collaboration between industry leaders and research institutions will be crucial for overcoming scalability challenges and driving market expansion.
Sumitomo Chemical Co., Ltd.
Technical Solution: Sumitomo Chemical has developed a scalable production process for magnesium nitride using a continuous flow reactor system. This method involves the reaction of magnesium vapor with nitrogen gas at high temperatures (800-1000°C) in a controlled atmosphere. The process utilizes a series of interconnected reaction chambers, allowing for continuous production and easy scale-up[1]. The company has also implemented advanced process control systems to maintain consistent product quality across different production scales[2]. Additionally, Sumitomo has invested in developing novel catalysts that enhance the reaction efficiency and reduce energy consumption in large-scale production[3].
Strengths: Continuous production capability, advanced process control, and energy-efficient catalysts. Weaknesses: High initial investment costs and potential complexity in maintaining optimal reaction conditions at larger scales.
Hengyang Kaixin Special Materials Technology Co., Ltd.
Technical Solution: Hengyang Kaixin has pioneered a scalable magnesium nitride production method using a modified plasma-enhanced chemical vapor deposition (PECVD) technique. This approach involves the reaction of magnesium precursors with nitrogen plasma in a specially designed reactor. The company has developed a modular reactor design that allows for easy capacity expansion by adding parallel processing units[4]. They have also implemented in-situ monitoring systems using spectroscopic techniques to ensure consistent product quality across different production scales[5]. Furthermore, Hengyang Kaixin has developed a proprietary post-processing technique that enhances the purity and stability of the produced magnesium nitride, making it suitable for various high-tech applications[6].
Strengths: Modular scalability, advanced in-situ monitoring, and high-purity product output. Weaknesses: Potentially high energy consumption due to plasma generation and specialized equipment requirements.
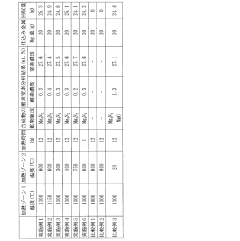
Innovative Approaches in Mg3N2 Synthesis
A method for upscalable precipitation synthesis of battery materials with tunable particle size distribution
PatentPendingEP3487813A1
Innovation
- A method for upscalable precipitation synthesis involving multiple stirred tank reactors, where seed formation is separated in time and space from particle growth, allowing for tunable particle size distribution without the need for particle size separation or selection, using a specific reaction scheme and adjusting pH levels to control agglomeration and growth.
Production method of magnesium nitride powder
PatentActiveJP2018131366A
Innovation
- A method involving a two-zone reaction apparatus with controlled vaporization and precipitation of metallic magnesium, using zones heated to specific temperature ranges in argon and mixed nitrogen-argon atmospheres to produce magnesium nitride powder continuously, ensuring the magnesium vapor does not liquefy and is recovered as a high-purity powder.
Environmental Impact of Mg3N2 Production
The production of magnesium nitride (Mg3N2) has significant environmental implications that must be carefully considered as the industry scales up. The primary environmental concerns stem from the energy-intensive nature of the production process and the potential for emissions and waste generation.
The conventional method of producing Mg3N2 involves the direct nitridation of magnesium metal at high temperatures, typically around 700-800°C. This process requires substantial energy input, contributing to increased greenhouse gas emissions if the energy source is not renewable. As production scales up, the carbon footprint associated with energy consumption becomes a critical environmental factor.
Nitrogen gas is a key reactant in Mg3N2 production. While nitrogen is abundant in the atmosphere, its separation and purification for industrial use require additional energy and resources. The large-scale production of Mg3N2 would necessitate a corresponding increase in nitrogen gas production, potentially leading to indirect environmental impacts through increased energy consumption and emissions from gas separation plants.
The handling and storage of Mg3N2 present environmental challenges due to its reactivity with water. When exposed to moisture, Mg3N2 hydrolyzes to form magnesium hydroxide and ammonia. This reaction can lead to the release of ammonia gas, which is a respiratory irritant and can contribute to air pollution if not properly controlled. As production scales up, the risk of accidental releases and their environmental consequences increases proportionally.
Waste management is another significant environmental consideration in Mg3N2 production. The process may generate by-products and unreacted materials that require proper disposal or recycling. Scaling up production would amplify these waste streams, necessitating robust waste management strategies to minimize environmental impact and resource depletion.
Water usage and potential contamination are additional environmental concerns. While the production process itself may not be water-intensive, cooling systems and cleaning operations associated with large-scale manufacturing could significantly increase water consumption. Moreover, any water that comes into contact with Mg3N2 or its precursors must be carefully treated to prevent contamination of local water sources.
To mitigate these environmental impacts, several strategies can be employed as Mg3N2 production scales up. Implementing energy-efficient technologies and transitioning to renewable energy sources can significantly reduce the carbon footprint of the production process. Closed-loop systems for nitrogen recycling and advanced emission control technologies can minimize atmospheric releases. Additionally, developing improved methods for handling and storing Mg3N2 to prevent hydrolysis and ammonia release is crucial for environmental protection.
The conventional method of producing Mg3N2 involves the direct nitridation of magnesium metal at high temperatures, typically around 700-800°C. This process requires substantial energy input, contributing to increased greenhouse gas emissions if the energy source is not renewable. As production scales up, the carbon footprint associated with energy consumption becomes a critical environmental factor.
Nitrogen gas is a key reactant in Mg3N2 production. While nitrogen is abundant in the atmosphere, its separation and purification for industrial use require additional energy and resources. The large-scale production of Mg3N2 would necessitate a corresponding increase in nitrogen gas production, potentially leading to indirect environmental impacts through increased energy consumption and emissions from gas separation plants.
The handling and storage of Mg3N2 present environmental challenges due to its reactivity with water. When exposed to moisture, Mg3N2 hydrolyzes to form magnesium hydroxide and ammonia. This reaction can lead to the release of ammonia gas, which is a respiratory irritant and can contribute to air pollution if not properly controlled. As production scales up, the risk of accidental releases and their environmental consequences increases proportionally.
Waste management is another significant environmental consideration in Mg3N2 production. The process may generate by-products and unreacted materials that require proper disposal or recycling. Scaling up production would amplify these waste streams, necessitating robust waste management strategies to minimize environmental impact and resource depletion.
Water usage and potential contamination are additional environmental concerns. While the production process itself may not be water-intensive, cooling systems and cleaning operations associated with large-scale manufacturing could significantly increase water consumption. Moreover, any water that comes into contact with Mg3N2 or its precursors must be carefully treated to prevent contamination of local water sources.
To mitigate these environmental impacts, several strategies can be employed as Mg3N2 production scales up. Implementing energy-efficient technologies and transitioning to renewable energy sources can significantly reduce the carbon footprint of the production process. Closed-loop systems for nitrogen recycling and advanced emission control technologies can minimize atmospheric releases. Additionally, developing improved methods for handling and storing Mg3N2 to prevent hydrolysis and ammonia release is crucial for environmental protection.
Economic Feasibility of Large-scale Mg3N2 Synthesis
The economic feasibility of large-scale Mg3N2 synthesis is a critical factor in determining the scalability of magnesium nitride production. Current production methods for Mg3N2 are primarily limited to laboratory-scale processes, which present significant challenges when considering industrial-scale manufacturing. The primary economic considerations for scaling up production include raw material costs, energy requirements, equipment investments, and potential market demand.
Raw material costs play a crucial role in the economic viability of Mg3N2 synthesis. Magnesium, the primary precursor, is relatively abundant but requires energy-intensive extraction processes. The cost of high-purity nitrogen gas, another essential reactant, must also be factored into the overall production expenses. Developing efficient recycling methods for unreacted materials could significantly reduce raw material costs and improve economic feasibility.
Energy consumption is a major concern in large-scale Mg3N2 production. The synthesis process typically requires high temperatures, often exceeding 700°C, which translates to substantial energy inputs. Implementing energy-efficient reactor designs and exploring alternative heating methods, such as microwave-assisted synthesis, could potentially reduce energy costs and improve the economic viability of large-scale production.
Capital investments in specialized equipment represent a significant upfront cost for scaling up Mg3N2 synthesis. This includes high-temperature reactors, gas handling systems, and purification equipment. The development of continuous flow reactors, as opposed to batch processes, could improve production efficiency and reduce equipment costs per unit of product. Additionally, investing in automation and process control systems may lead to long-term cost savings through improved yield and reduced labor requirements.
Market demand for Mg3N2 and its derivatives is a crucial factor in determining the economic feasibility of large-scale production. While current applications are limited, emerging technologies in areas such as hydrogen storage, catalysis, and advanced materials may drive increased demand. Conducting thorough market research and identifying potential high-value applications could justify the investment in scaling up production capabilities.
Process optimization and yield improvement are essential for enhancing the economic viability of large-scale Mg3N2 synthesis. This includes developing more efficient reaction pathways, optimizing reaction conditions, and improving product purification methods. Implementing in-situ monitoring and real-time process control could lead to higher yields and more consistent product quality, thereby improving the overall economics of production.
Considering environmental regulations and sustainability is crucial when assessing the economic feasibility of large-scale Mg3N2 production. Implementing pollution control measures and ensuring compliance with environmental standards may incur additional costs but are necessary for long-term viability. Exploring green chemistry principles and developing environmentally friendly synthesis routes could potentially lead to cost savings and improved public perception.
In conclusion, while challenges exist, the economic feasibility of large-scale Mg3N2 synthesis shows promise. By addressing key areas such as raw material costs, energy efficiency, equipment optimization, and market development, it is possible to improve the economic viability of scaled-up production. Continued research and development efforts, coupled with strategic investments in process technology, will be crucial in realizing the full potential of industrial-scale magnesium nitride production.
Raw material costs play a crucial role in the economic viability of Mg3N2 synthesis. Magnesium, the primary precursor, is relatively abundant but requires energy-intensive extraction processes. The cost of high-purity nitrogen gas, another essential reactant, must also be factored into the overall production expenses. Developing efficient recycling methods for unreacted materials could significantly reduce raw material costs and improve economic feasibility.
Energy consumption is a major concern in large-scale Mg3N2 production. The synthesis process typically requires high temperatures, often exceeding 700°C, which translates to substantial energy inputs. Implementing energy-efficient reactor designs and exploring alternative heating methods, such as microwave-assisted synthesis, could potentially reduce energy costs and improve the economic viability of large-scale production.
Capital investments in specialized equipment represent a significant upfront cost for scaling up Mg3N2 synthesis. This includes high-temperature reactors, gas handling systems, and purification equipment. The development of continuous flow reactors, as opposed to batch processes, could improve production efficiency and reduce equipment costs per unit of product. Additionally, investing in automation and process control systems may lead to long-term cost savings through improved yield and reduced labor requirements.
Market demand for Mg3N2 and its derivatives is a crucial factor in determining the economic feasibility of large-scale production. While current applications are limited, emerging technologies in areas such as hydrogen storage, catalysis, and advanced materials may drive increased demand. Conducting thorough market research and identifying potential high-value applications could justify the investment in scaling up production capabilities.
Process optimization and yield improvement are essential for enhancing the economic viability of large-scale Mg3N2 synthesis. This includes developing more efficient reaction pathways, optimizing reaction conditions, and improving product purification methods. Implementing in-situ monitoring and real-time process control could lead to higher yields and more consistent product quality, thereby improving the overall economics of production.
Considering environmental regulations and sustainability is crucial when assessing the economic feasibility of large-scale Mg3N2 production. Implementing pollution control measures and ensuring compliance with environmental standards may incur additional costs but are necessary for long-term viability. Exploring green chemistry principles and developing environmentally friendly synthesis routes could potentially lead to cost savings and improved public perception.
In conclusion, while challenges exist, the economic feasibility of large-scale Mg3N2 synthesis shows promise. By addressing key areas such as raw material costs, energy efficiency, equipment optimization, and market development, it is possible to improve the economic viability of scaled-up production. Continued research and development efforts, coupled with strategic investments in process technology, will be crucial in realizing the full potential of industrial-scale magnesium nitride production.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!