How Magnesium Nitride Supports Next-Gen Battery Technologies?
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg3N2 in Battery Tech: Background and Objectives
Magnesium nitride (Mg3N2) has emerged as a promising material in the field of next-generation battery technologies, offering potential solutions to the growing demand for high-performance energy storage systems. The development of Mg3N2 in battery applications is rooted in the broader context of the energy transition and the need for more efficient, sustainable, and cost-effective energy storage solutions.
The evolution of battery technology has been driven by the increasing requirements for portable electronics, electric vehicles, and grid-scale energy storage. Traditional lithium-ion batteries, while widely adopted, face limitations in terms of energy density, safety, and resource availability. This has led researchers and industry experts to explore alternative materials and chemistries, with magnesium-based systems gaining significant attention.
Magnesium nitride's potential in battery technology stems from several key advantages. Firstly, magnesium is abundant in the Earth's crust, making it a more sustainable and economically viable option compared to lithium. Secondly, magnesium has a higher volumetric capacity than lithium, potentially enabling batteries with higher energy densities. Additionally, magnesium-based systems are generally considered safer due to their lower reactivity and reduced risk of dendrite formation.
The exploration of Mg3N2 in battery applications aims to leverage these advantages while addressing the challenges associated with magnesium-based energy storage. One of the primary objectives is to develop electrode materials and electrolytes that can effectively utilize the unique properties of magnesium nitride. Researchers are focusing on understanding the electrochemical behavior of Mg3N2, its interaction with other battery components, and its potential to enhance overall battery performance.
Another key goal is to improve the cycling stability and rate capability of magnesium-based batteries. Mg3N2 is being investigated for its potential to facilitate faster magnesium ion diffusion and to mitigate issues related to the formation of passivation layers on electrode surfaces. These advancements could lead to batteries with longer lifespans and faster charging capabilities.
Furthermore, the integration of Mg3N2 into battery systems aligns with the broader objective of developing more environmentally friendly energy storage solutions. By reducing reliance on scarce or toxic materials, magnesium nitride-based batteries could contribute to a more sustainable energy ecosystem.
As research in this field progresses, the ultimate aim is to create commercially viable battery technologies that can compete with or surpass the performance of current lithium-ion systems. This involves not only improving the fundamental electrochemistry but also addressing manufacturing challenges and ensuring scalability for mass production.
The evolution of battery technology has been driven by the increasing requirements for portable electronics, electric vehicles, and grid-scale energy storage. Traditional lithium-ion batteries, while widely adopted, face limitations in terms of energy density, safety, and resource availability. This has led researchers and industry experts to explore alternative materials and chemistries, with magnesium-based systems gaining significant attention.
Magnesium nitride's potential in battery technology stems from several key advantages. Firstly, magnesium is abundant in the Earth's crust, making it a more sustainable and economically viable option compared to lithium. Secondly, magnesium has a higher volumetric capacity than lithium, potentially enabling batteries with higher energy densities. Additionally, magnesium-based systems are generally considered safer due to their lower reactivity and reduced risk of dendrite formation.
The exploration of Mg3N2 in battery applications aims to leverage these advantages while addressing the challenges associated with magnesium-based energy storage. One of the primary objectives is to develop electrode materials and electrolytes that can effectively utilize the unique properties of magnesium nitride. Researchers are focusing on understanding the electrochemical behavior of Mg3N2, its interaction with other battery components, and its potential to enhance overall battery performance.
Another key goal is to improve the cycling stability and rate capability of magnesium-based batteries. Mg3N2 is being investigated for its potential to facilitate faster magnesium ion diffusion and to mitigate issues related to the formation of passivation layers on electrode surfaces. These advancements could lead to batteries with longer lifespans and faster charging capabilities.
Furthermore, the integration of Mg3N2 into battery systems aligns with the broader objective of developing more environmentally friendly energy storage solutions. By reducing reliance on scarce or toxic materials, magnesium nitride-based batteries could contribute to a more sustainable energy ecosystem.
As research in this field progresses, the ultimate aim is to create commercially viable battery technologies that can compete with or surpass the performance of current lithium-ion systems. This involves not only improving the fundamental electrochemistry but also addressing manufacturing challenges and ensuring scalability for mass production.
Market Demand for Advanced Energy Storage
The global energy storage market is experiencing unprecedented growth, driven by the increasing demand for advanced battery technologies. This surge is primarily fueled by the rapid expansion of electric vehicles (EVs), renewable energy integration, and the need for grid stabilization. As traditional lithium-ion batteries approach their theoretical limits, there is a growing market demand for next-generation energy storage solutions that can offer higher energy density, improved safety, and longer cycle life.
The EV sector, in particular, is a major driver of this demand. With projections indicating that EVs could account for up to 30% of global vehicle sales by 2030, automakers are actively seeking battery technologies that can extend driving range, reduce charging times, and lower costs. This has created a significant market opportunity for advanced battery chemistries, including those utilizing magnesium nitride.
Grid-scale energy storage is another key area driving demand for advanced battery technologies. As renewable energy sources like wind and solar continue to grow, the need for efficient, large-scale energy storage solutions becomes critical for grid stability and load balancing. The market for grid energy storage is expected to grow substantially in the coming years, with some estimates suggesting a compound annual growth rate of over 20% through 2025.
Consumer electronics represent another significant market segment demanding improved battery performance. With the proliferation of smartphones, laptops, and wearable devices, there is a constant push for batteries that can deliver longer usage times in smaller, lighter packages. This demand extends to emerging technologies such as augmented reality and virtual reality devices, which require high-performance energy storage solutions to become truly portable and practical.
The aerospace and defense sectors are also showing increased interest in advanced energy storage technologies. These industries require batteries with high energy density, extreme temperature tolerance, and enhanced safety features. As such, they represent a niche but high-value market for next-generation battery technologies.
In the context of these market demands, magnesium nitride-based battery technologies offer several potential advantages. These include the possibility of higher energy density, improved safety due to non-flammable electrolytes, and the abundance of magnesium as a resource compared to lithium. As research progresses, the market is closely watching developments in magnesium-based batteries as a potential solution to meet the growing demand for advanced energy storage across multiple sectors.
The EV sector, in particular, is a major driver of this demand. With projections indicating that EVs could account for up to 30% of global vehicle sales by 2030, automakers are actively seeking battery technologies that can extend driving range, reduce charging times, and lower costs. This has created a significant market opportunity for advanced battery chemistries, including those utilizing magnesium nitride.
Grid-scale energy storage is another key area driving demand for advanced battery technologies. As renewable energy sources like wind and solar continue to grow, the need for efficient, large-scale energy storage solutions becomes critical for grid stability and load balancing. The market for grid energy storage is expected to grow substantially in the coming years, with some estimates suggesting a compound annual growth rate of over 20% through 2025.
Consumer electronics represent another significant market segment demanding improved battery performance. With the proliferation of smartphones, laptops, and wearable devices, there is a constant push for batteries that can deliver longer usage times in smaller, lighter packages. This demand extends to emerging technologies such as augmented reality and virtual reality devices, which require high-performance energy storage solutions to become truly portable and practical.
The aerospace and defense sectors are also showing increased interest in advanced energy storage technologies. These industries require batteries with high energy density, extreme temperature tolerance, and enhanced safety features. As such, they represent a niche but high-value market for next-generation battery technologies.
In the context of these market demands, magnesium nitride-based battery technologies offer several potential advantages. These include the possibility of higher energy density, improved safety due to non-flammable electrolytes, and the abundance of magnesium as a resource compared to lithium. As research progresses, the market is closely watching developments in magnesium-based batteries as a potential solution to meet the growing demand for advanced energy storage across multiple sectors.
Mg3N2 in Batteries: Current State and Challenges
Magnesium nitride (Mg3N2) has emerged as a promising material for next-generation battery technologies, particularly in the realm of magnesium-based batteries. The current state of Mg3N2 in battery applications is characterized by significant potential but also substantial challenges that need to be addressed for widespread adoption.
One of the primary advantages of Mg3N2 in battery systems is its high theoretical capacity, which surpasses that of traditional lithium-ion batteries. This high capacity is attributed to the ability of Mg3N2 to store multiple electrons per formula unit, potentially leading to batteries with higher energy densities. Additionally, magnesium is more abundant and less expensive than lithium, making Mg3N2-based batteries an attractive option for large-scale energy storage applications.
However, the implementation of Mg3N2 in practical battery systems faces several critical challenges. One of the most significant hurdles is the slow kinetics of magnesium ion diffusion within the Mg3N2 structure. This sluggish ion movement results in poor rate capability and limited power output, which are crucial factors for high-performance batteries. Researchers are actively exploring ways to enhance the ionic conductivity of Mg3N2, including doping with other elements and nanostructuring the material.
Another challenge is the reactivity of Mg3N2 with common electrolytes. Many conventional electrolytes used in battery systems are incompatible with Mg3N2, leading to unwanted side reactions and degradation of the electrode material. This necessitates the development of new, stable electrolytes that can work effectively with Mg3N2 electrodes while maintaining long-term cycling stability.
The reversibility of the magnesium insertion and extraction process in Mg3N2 electrodes is also a concern. Unlike lithium-ion batteries, where the intercalation process is well-understood and optimized, the mechanisms of magnesium ion storage in Mg3N2 are still under investigation. Improving the reversibility of this process is crucial for achieving high cycle life and practical viability of Mg3N2-based batteries.
Furthermore, the volume changes associated with magnesium insertion and extraction in Mg3N2 can lead to mechanical stress and structural degradation of the electrode material over repeated cycling. This issue needs to be addressed to ensure long-term stability and performance of Mg3N2 electrodes.
Despite these challenges, ongoing research efforts are making steady progress in overcoming these obstacles. Advanced characterization techniques and computational studies are providing deeper insights into the behavior of Mg3N2 in battery environments, guiding the development of strategies to enhance its performance. As these challenges are addressed, Mg3N2 holds the potential to play a significant role in the next generation of high-energy-density, cost-effective battery technologies.
One of the primary advantages of Mg3N2 in battery systems is its high theoretical capacity, which surpasses that of traditional lithium-ion batteries. This high capacity is attributed to the ability of Mg3N2 to store multiple electrons per formula unit, potentially leading to batteries with higher energy densities. Additionally, magnesium is more abundant and less expensive than lithium, making Mg3N2-based batteries an attractive option for large-scale energy storage applications.
However, the implementation of Mg3N2 in practical battery systems faces several critical challenges. One of the most significant hurdles is the slow kinetics of magnesium ion diffusion within the Mg3N2 structure. This sluggish ion movement results in poor rate capability and limited power output, which are crucial factors for high-performance batteries. Researchers are actively exploring ways to enhance the ionic conductivity of Mg3N2, including doping with other elements and nanostructuring the material.
Another challenge is the reactivity of Mg3N2 with common electrolytes. Many conventional electrolytes used in battery systems are incompatible with Mg3N2, leading to unwanted side reactions and degradation of the electrode material. This necessitates the development of new, stable electrolytes that can work effectively with Mg3N2 electrodes while maintaining long-term cycling stability.
The reversibility of the magnesium insertion and extraction process in Mg3N2 electrodes is also a concern. Unlike lithium-ion batteries, where the intercalation process is well-understood and optimized, the mechanisms of magnesium ion storage in Mg3N2 are still under investigation. Improving the reversibility of this process is crucial for achieving high cycle life and practical viability of Mg3N2-based batteries.
Furthermore, the volume changes associated with magnesium insertion and extraction in Mg3N2 can lead to mechanical stress and structural degradation of the electrode material over repeated cycling. This issue needs to be addressed to ensure long-term stability and performance of Mg3N2 electrodes.
Despite these challenges, ongoing research efforts are making steady progress in overcoming these obstacles. Advanced characterization techniques and computational studies are providing deeper insights into the behavior of Mg3N2 in battery environments, guiding the development of strategies to enhance its performance. As these challenges are addressed, Mg3N2 holds the potential to play a significant role in the next generation of high-energy-density, cost-effective battery technologies.
Existing Mg3N2-Based Battery Solutions
01 Synthesis and production of magnesium nitride
Various methods for synthesizing and producing magnesium nitride are described. These include direct nitridation of magnesium metal, reaction of magnesium with ammonia, and plasma-assisted processes. The synthesis conditions, such as temperature, pressure, and reaction time, are optimized to improve yield and purity.- Synthesis and production methods of magnesium nitride: Various methods for synthesizing and producing magnesium nitride are described, including direct nitridation of magnesium metal, reaction of magnesium with ammonia, and plasma-assisted processes. These methods aim to improve yield, purity, and efficiency in the production of magnesium nitride.
- Applications of magnesium nitride in semiconductor devices: Magnesium nitride is utilized in the fabrication of semiconductor devices, particularly in the production of light-emitting diodes (LEDs) and other optoelectronic components. It serves as a buffer layer, electron-blocking layer, or as part of the active region in these devices.
- Use of magnesium nitride in energy storage and conversion: Magnesium nitride finds applications in energy storage and conversion technologies, including as a component in hydrogen storage materials, electrodes for batteries, and catalysts for various chemical reactions. Its properties contribute to improved performance in these energy-related applications.
- Magnesium nitride as a precursor for other materials: Magnesium nitride serves as a precursor for the synthesis of other materials, such as magnesium-based alloys, ceramics, and composite materials. It is used in processes to create advanced materials with specific properties for various industrial applications.
- Surface treatment and coating applications of magnesium nitride: Magnesium nitride is employed in surface treatment and coating processes to enhance the properties of various materials. It is used to improve corrosion resistance, hardness, and wear resistance of metals and other substrates through techniques such as plasma nitriding and chemical vapor deposition.
02 Applications in semiconductor devices
Magnesium nitride is utilized in the fabrication of semiconductor devices, particularly in the production of light-emitting diodes (LEDs) and other optoelectronic components. It serves as a buffer layer, electron-blocking layer, or dopant in various semiconductor structures to enhance device performance.Expand Specific Solutions03 Use in energy storage and conversion
Magnesium nitride finds applications in energy storage and conversion technologies. It is investigated as a potential material for hydrogen storage, as an electrode material in batteries, and as a catalyst in various energy-related processes. Its properties are exploited to improve the efficiency and performance of these systems.Expand Specific Solutions04 Magnesium nitride in composite materials
The incorporation of magnesium nitride into composite materials is explored to enhance their properties. These composites find applications in areas such as thermal management, structural reinforcement, and electromagnetic shielding. The addition of magnesium nitride can improve mechanical strength, thermal conductivity, and other functional properties of the composites.Expand Specific Solutions05 Purification and characterization techniques
Various methods for purifying and characterizing magnesium nitride are developed. These include techniques for removing impurities, analyzing crystal structure, determining particle size distribution, and assessing chemical composition. Advanced analytical tools and processes are employed to ensure high-quality magnesium nitride for different applications.Expand Specific Solutions
Key Players in Mg3N2 Battery Research and Development
The magnesium nitride battery technology market is in its early development stage, with significant potential for growth as next-generation energy storage solutions gain traction. While the market size is currently limited, it is expected to expand rapidly as research progresses and commercial applications emerge. The technology's maturity is still evolving, with academic institutions like Chongqing University and Shanghai Jiao Tong University leading fundamental research. Major industry players such as Toyota Motor Corp., GS Yuasa International Ltd., and LG Energy Solution Ltd. are investing in R&D to advance the technology towards commercialization. Collaboration between academia and industry is driving innovation, with companies like Murata Manufacturing Co. Ltd. and Samsung Electronics Co., Ltd. exploring potential applications in consumer electronics and electric vehicles.
Toyota Motor Corp.
Technical Solution: Toyota Motor Corp. has been actively researching magnesium nitride (Mg3N2) as a potential material for next-generation battery technologies. Their approach involves using Mg3N2 as a conversion-type anode material in rechargeable magnesium batteries. The company has developed a novel synthesis method for Mg3N2 nanoparticles, which exhibit improved electrochemical performance compared to bulk Mg3N2 [1]. Toyota's research has shown that Mg3N2 anodes can achieve a high theoretical capacity of 1352 mAh/g, significantly higher than conventional graphite anodes used in lithium-ion batteries [2]. The company is also exploring the use of Mg3N2 in solid-state electrolytes to enhance the overall performance and safety of magnesium batteries [3].
Strengths: High theoretical capacity, potential for improved safety in solid-state electrolytes. Weaknesses: Challenges in controlling the conversion reaction, potential for capacity fading over multiple cycles.
GS Yuasa International Ltd.
Technical Solution: GS Yuasa International Ltd. is investigating the use of magnesium nitride in advanced battery systems, particularly focusing on its application in magnesium-sulfur batteries. The company's research involves incorporating Mg3N2 as a protective layer on magnesium metal anodes to mitigate the formation of passivation films and enhance the cycling stability of magnesium-based batteries [4]. GS Yuasa has developed a proprietary coating process that allows for uniform deposition of Mg3N2 on magnesium foils, resulting in improved interfacial properties and reduced dendrite formation [5]. Additionally, the company is exploring the potential of Mg3N2 as a catalyst for the sulfur cathode in Mg-S batteries, aiming to increase the utilization of active material and boost overall energy density [6].
Strengths: Enhanced cycling stability, improved interfacial properties. Weaknesses: Complexity of coating process, potential cost increase in battery production.
Core Innovations in Mg3N2 Battery Chemistry
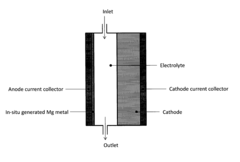
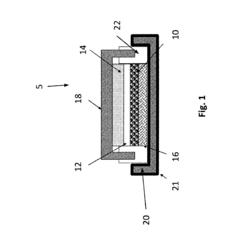
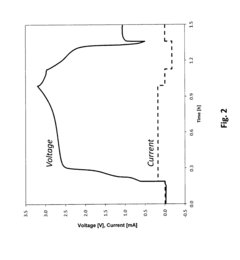
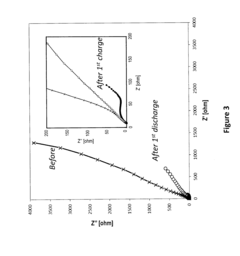
In-situ magnesium-metal generated rechargeable magnesium battery
PatentActiveUS20160156063A1
Innovation
- A method involving the electrochemical deposition of magnesium metal onto an uncoated current collector within a non-aqueous solvent-based electrolyte system, eliminating the need for pre-formed magnesium metal and reducing interfacial impedance by generating magnesium in-situ during the charging process.
Environmental Impact of Mg3N2 Battery Production
The environmental impact of Mg3N2 battery production is a critical consideration as the technology advances towards commercial viability. The manufacturing process of magnesium nitride-based batteries involves several stages that can potentially affect the environment, both positively and negatively.
One of the primary environmental benefits of Mg3N2 battery production is the reduced reliance on rare earth elements and heavy metals commonly used in traditional lithium-ion batteries. This shift can lead to a decrease in environmentally damaging mining activities associated with these materials. Additionally, magnesium is more abundant and widely distributed geographically, potentially reducing the environmental footprint of resource extraction and transportation.
However, the production of Mg3N2 itself requires significant energy input, particularly in the synthesis of magnesium nitride from its constituent elements. This energy-intensive process may contribute to increased carbon emissions if not powered by renewable energy sources. The reaction between magnesium and nitrogen typically occurs at high temperatures, necessitating substantial thermal energy and potentially resulting in heat pollution if not properly managed.
Water usage is another environmental concern in Mg3N2 battery production. The synthesis and purification processes may require substantial amounts of water, which could strain local water resources in areas of production. Proper water management and recycling systems are crucial to mitigate this impact.
Chemical waste generation is an additional environmental consideration. The production of Mg3N2 and its integration into battery systems may involve the use of various solvents and reagents. Proper disposal and treatment of these chemical wastes are essential to prevent soil and water contamination.
On the positive side, Mg3N2 batteries have the potential for improved recyclability compared to some existing battery technologies. The simpler chemistry and absence of toxic heavy metals could make the recycling process more straightforward and environmentally friendly. This could lead to a more circular economy in battery production and reduce the overall environmental impact of the battery lifecycle.
The scalability of Mg3N2 battery production also has environmental implications. As production scales up to meet potential demand, there will be opportunities to implement more efficient and environmentally friendly manufacturing processes. This could include the integration of renewable energy sources, closed-loop water systems, and advanced waste management techniques.
In conclusion, while Mg3N2 battery production presents some environmental challenges, particularly in energy consumption and resource use, it also offers significant potential benefits in terms of reduced reliance on scarce materials and improved recyclability. The net environmental impact will largely depend on the specific production methods employed and the degree to which sustainable practices are integrated into the manufacturing process.
One of the primary environmental benefits of Mg3N2 battery production is the reduced reliance on rare earth elements and heavy metals commonly used in traditional lithium-ion batteries. This shift can lead to a decrease in environmentally damaging mining activities associated with these materials. Additionally, magnesium is more abundant and widely distributed geographically, potentially reducing the environmental footprint of resource extraction and transportation.
However, the production of Mg3N2 itself requires significant energy input, particularly in the synthesis of magnesium nitride from its constituent elements. This energy-intensive process may contribute to increased carbon emissions if not powered by renewable energy sources. The reaction between magnesium and nitrogen typically occurs at high temperatures, necessitating substantial thermal energy and potentially resulting in heat pollution if not properly managed.
Water usage is another environmental concern in Mg3N2 battery production. The synthesis and purification processes may require substantial amounts of water, which could strain local water resources in areas of production. Proper water management and recycling systems are crucial to mitigate this impact.
Chemical waste generation is an additional environmental consideration. The production of Mg3N2 and its integration into battery systems may involve the use of various solvents and reagents. Proper disposal and treatment of these chemical wastes are essential to prevent soil and water contamination.
On the positive side, Mg3N2 batteries have the potential for improved recyclability compared to some existing battery technologies. The simpler chemistry and absence of toxic heavy metals could make the recycling process more straightforward and environmentally friendly. This could lead to a more circular economy in battery production and reduce the overall environmental impact of the battery lifecycle.
The scalability of Mg3N2 battery production also has environmental implications. As production scales up to meet potential demand, there will be opportunities to implement more efficient and environmentally friendly manufacturing processes. This could include the integration of renewable energy sources, closed-loop water systems, and advanced waste management techniques.
In conclusion, while Mg3N2 battery production presents some environmental challenges, particularly in energy consumption and resource use, it also offers significant potential benefits in terms of reduced reliance on scarce materials and improved recyclability. The net environmental impact will largely depend on the specific production methods employed and the degree to which sustainable practices are integrated into the manufacturing process.
Safety Considerations for Mg3N2-Based Batteries
Safety considerations are paramount in the development and implementation of Mg3N2-based batteries. The reactive nature of magnesium nitride necessitates careful handling and storage protocols to prevent unintended reactions with moisture or air. When exposed to water, Mg3N2 can rapidly decompose, releasing ammonia gas and potentially causing pressure build-up within the battery structure.
To mitigate these risks, researchers are exploring various encapsulation techniques and protective coatings for Mg3N2 particles. These methods aim to isolate the reactive material from environmental factors while maintaining its electrochemical properties. Advanced packaging materials and designs are being investigated to ensure the long-term stability and safety of Mg3N2-based battery systems.
Thermal management is another critical aspect of safety for these next-generation batteries. Mg3N2 reactions can be exothermic, potentially leading to thermal runaway if not properly controlled. Innovative cooling systems and heat-dissipation strategies are being developed to maintain safe operating temperatures under various charging and discharging conditions.
Electrical safety is also a key consideration, particularly given the high energy density potential of Mg3N2-based batteries. Researchers are working on advanced battery management systems (BMS) that can monitor and control voltage, current, and temperature in real-time. These systems are designed to prevent overcharging, over-discharging, and short circuits, which could compromise battery integrity and user safety.
The potential for gas evolution during battery operation or in case of damage is being addressed through the development of robust venting mechanisms. These safety features are designed to release pressure in a controlled manner, preventing catastrophic failure and minimizing the risk of explosion or fire.
As Mg3N2-based batteries move closer to commercialization, standardization of safety protocols and testing procedures becomes increasingly important. Regulatory bodies and industry stakeholders are collaborating to establish comprehensive safety standards that address the unique characteristics of these novel battery technologies.
Environmental and health considerations are also being evaluated, including the potential impacts of Mg3N2 production, use, and disposal. Researchers are exploring recycling methods and end-of-life strategies to ensure the sustainable and safe management of these battery materials throughout their lifecycle.
To mitigate these risks, researchers are exploring various encapsulation techniques and protective coatings for Mg3N2 particles. These methods aim to isolate the reactive material from environmental factors while maintaining its electrochemical properties. Advanced packaging materials and designs are being investigated to ensure the long-term stability and safety of Mg3N2-based battery systems.
Thermal management is another critical aspect of safety for these next-generation batteries. Mg3N2 reactions can be exothermic, potentially leading to thermal runaway if not properly controlled. Innovative cooling systems and heat-dissipation strategies are being developed to maintain safe operating temperatures under various charging and discharging conditions.
Electrical safety is also a key consideration, particularly given the high energy density potential of Mg3N2-based batteries. Researchers are working on advanced battery management systems (BMS) that can monitor and control voltage, current, and temperature in real-time. These systems are designed to prevent overcharging, over-discharging, and short circuits, which could compromise battery integrity and user safety.
The potential for gas evolution during battery operation or in case of damage is being addressed through the development of robust venting mechanisms. These safety features are designed to release pressure in a controlled manner, preventing catastrophic failure and minimizing the risk of explosion or fire.
As Mg3N2-based batteries move closer to commercialization, standardization of safety protocols and testing procedures becomes increasingly important. Regulatory bodies and industry stakeholders are collaborating to establish comprehensive safety standards that address the unique characteristics of these novel battery technologies.
Environmental and health considerations are also being evaluated, including the potential impacts of Mg3N2 production, use, and disposal. Researchers are exploring recycling methods and end-of-life strategies to ensure the sustainable and safe management of these battery materials throughout their lifecycle.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!