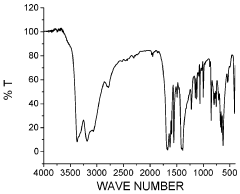
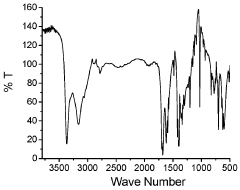
Discovering the Catalytic Properties of Magnesium Nitride
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg3N2 Catalysis Background
Magnesium nitride (Mg3N2) has emerged as a promising catalyst in recent years, attracting significant attention from researchers in the field of catalysis and materials science. The exploration of its catalytic properties represents a frontier in the development of sustainable and efficient catalytic systems. Historically, magnesium compounds have been widely used in various industrial processes, but the specific catalytic potential of Mg3N2 remained largely unexplored until the past decade.
The interest in Mg3N2 as a catalyst stems from its unique chemical and physical properties. As a binary nitride, it possesses a high nitrogen content and a relatively simple crystal structure, which contributes to its reactivity and potential for catalytic applications. The strong Mg-N bonds in the compound provide both stability and reactivity, making it an intriguing candidate for various catalytic reactions.
Early investigations into the catalytic properties of Mg3N2 focused primarily on its role in organic synthesis reactions. Researchers discovered its efficacy in promoting condensation reactions, particularly in the formation of nitrogen-containing heterocycles. This initial success sparked further interest in exploring its potential in other areas of catalysis.
The development of Mg3N2 as a catalyst has been driven by the increasing demand for more sustainable and environmentally friendly catalytic processes. Traditional catalysts often rely on precious metals or toxic compounds, whereas Mg3N2 offers a more abundant and less harmful alternative. This aligns with the growing emphasis on green chemistry and sustainable technologies in the chemical industry.
Recent advancements in synthesis methods and characterization techniques have significantly contributed to the understanding of Mg3N2's catalytic properties. Improved preparation methods have led to the production of high-purity Mg3N2 with controlled morphology and surface properties, enabling more precise studies of its catalytic behavior. Advanced spectroscopic and microscopic techniques have allowed researchers to probe the surface structure and active sites of Mg3N2 catalysts, providing crucial insights into their mechanism of action.
The catalytic applications of Mg3N2 have expanded beyond organic synthesis to include areas such as hydrogen production, CO2 conversion, and ammonia synthesis. Its ability to activate small molecules like H2 and N2 has opened up new possibilities in energy-related catalysis. Furthermore, the potential of Mg3N2 as a support material for other catalysts has been recognized, leading to the development of novel composite catalytic systems.
As research in this field progresses, the goal is to fully elucidate the catalytic properties of Mg3N2 and optimize its performance for various applications. This involves understanding the fundamental mechanisms of its catalytic activity, exploring its synergistic effects with other materials, and developing strategies to enhance its stability and recyclability. The ongoing investigation into Mg3N2 catalysis holds promise for advancing sustainable chemical processes and contributing to the development of next-generation catalytic technologies.
The interest in Mg3N2 as a catalyst stems from its unique chemical and physical properties. As a binary nitride, it possesses a high nitrogen content and a relatively simple crystal structure, which contributes to its reactivity and potential for catalytic applications. The strong Mg-N bonds in the compound provide both stability and reactivity, making it an intriguing candidate for various catalytic reactions.
Early investigations into the catalytic properties of Mg3N2 focused primarily on its role in organic synthesis reactions. Researchers discovered its efficacy in promoting condensation reactions, particularly in the formation of nitrogen-containing heterocycles. This initial success sparked further interest in exploring its potential in other areas of catalysis.
The development of Mg3N2 as a catalyst has been driven by the increasing demand for more sustainable and environmentally friendly catalytic processes. Traditional catalysts often rely on precious metals or toxic compounds, whereas Mg3N2 offers a more abundant and less harmful alternative. This aligns with the growing emphasis on green chemistry and sustainable technologies in the chemical industry.
Recent advancements in synthesis methods and characterization techniques have significantly contributed to the understanding of Mg3N2's catalytic properties. Improved preparation methods have led to the production of high-purity Mg3N2 with controlled morphology and surface properties, enabling more precise studies of its catalytic behavior. Advanced spectroscopic and microscopic techniques have allowed researchers to probe the surface structure and active sites of Mg3N2 catalysts, providing crucial insights into their mechanism of action.
The catalytic applications of Mg3N2 have expanded beyond organic synthesis to include areas such as hydrogen production, CO2 conversion, and ammonia synthesis. Its ability to activate small molecules like H2 and N2 has opened up new possibilities in energy-related catalysis. Furthermore, the potential of Mg3N2 as a support material for other catalysts has been recognized, leading to the development of novel composite catalytic systems.
As research in this field progresses, the goal is to fully elucidate the catalytic properties of Mg3N2 and optimize its performance for various applications. This involves understanding the fundamental mechanisms of its catalytic activity, exploring its synergistic effects with other materials, and developing strategies to enhance its stability and recyclability. The ongoing investigation into Mg3N2 catalysis holds promise for advancing sustainable chemical processes and contributing to the development of next-generation catalytic technologies.
Market Demand Analysis
The market demand for magnesium nitride (Mg3N2) and its catalytic properties has been steadily growing in recent years, driven by various industrial applications and research interests. The compound's unique characteristics, particularly its potential as a catalyst, have attracted attention from multiple sectors, including chemical manufacturing, energy production, and environmental remediation.
In the chemical industry, magnesium nitride has shown promise as a catalyst for various organic synthesis reactions. Its ability to facilitate carbon-carbon bond formation and nitrogen fixation processes has sparked interest among researchers and manufacturers alike. This potential application could lead to more efficient and environmentally friendly production methods for pharmaceuticals, agrochemicals, and fine chemicals.
The energy sector has also recognized the potential of magnesium nitride, particularly in the field of hydrogen production. As the world shifts towards cleaner energy sources, hydrogen fuel cells have gained traction as a viable alternative to fossil fuels. Magnesium nitride's catalytic properties could play a crucial role in improving the efficiency of hydrogen generation processes, potentially reducing costs and increasing the viability of hydrogen as a widespread energy carrier.
Environmental applications represent another significant area of market demand for magnesium nitride catalysts. The compound has shown potential in the treatment of wastewater and air pollution, particularly in the removal of nitrogen-containing pollutants. As environmental regulations become increasingly stringent worldwide, the demand for effective and sustainable pollution control technologies is expected to rise, creating opportunities for magnesium nitride-based solutions.
The electronics industry has also expressed interest in magnesium nitride, particularly for its potential use in semiconductor manufacturing. The compound's unique electronic properties and thermal stability make it a candidate for advanced electronic materials and devices, potentially opening up new avenues for innovation in this rapidly evolving sector.
While the market for magnesium nitride catalysts is still in its early stages, industry analysts project significant growth potential in the coming years. The compound's versatility and the ongoing research into its catalytic properties suggest that new applications and market opportunities may emerge as our understanding of the material deepens.
However, it is important to note that the widespread adoption of magnesium nitride-based catalysts faces some challenges. These include the need for further research to fully understand and optimize its catalytic properties, potential scalability issues in manufacturing processes, and competition from established catalytic materials. Overcoming these hurdles will be crucial in realizing the full market potential of magnesium nitride catalysts across various industries.
In the chemical industry, magnesium nitride has shown promise as a catalyst for various organic synthesis reactions. Its ability to facilitate carbon-carbon bond formation and nitrogen fixation processes has sparked interest among researchers and manufacturers alike. This potential application could lead to more efficient and environmentally friendly production methods for pharmaceuticals, agrochemicals, and fine chemicals.
The energy sector has also recognized the potential of magnesium nitride, particularly in the field of hydrogen production. As the world shifts towards cleaner energy sources, hydrogen fuel cells have gained traction as a viable alternative to fossil fuels. Magnesium nitride's catalytic properties could play a crucial role in improving the efficiency of hydrogen generation processes, potentially reducing costs and increasing the viability of hydrogen as a widespread energy carrier.
Environmental applications represent another significant area of market demand for magnesium nitride catalysts. The compound has shown potential in the treatment of wastewater and air pollution, particularly in the removal of nitrogen-containing pollutants. As environmental regulations become increasingly stringent worldwide, the demand for effective and sustainable pollution control technologies is expected to rise, creating opportunities for magnesium nitride-based solutions.
The electronics industry has also expressed interest in magnesium nitride, particularly for its potential use in semiconductor manufacturing. The compound's unique electronic properties and thermal stability make it a candidate for advanced electronic materials and devices, potentially opening up new avenues for innovation in this rapidly evolving sector.
While the market for magnesium nitride catalysts is still in its early stages, industry analysts project significant growth potential in the coming years. The compound's versatility and the ongoing research into its catalytic properties suggest that new applications and market opportunities may emerge as our understanding of the material deepens.
However, it is important to note that the widespread adoption of magnesium nitride-based catalysts faces some challenges. These include the need for further research to fully understand and optimize its catalytic properties, potential scalability issues in manufacturing processes, and competition from established catalytic materials. Overcoming these hurdles will be crucial in realizing the full market potential of magnesium nitride catalysts across various industries.
Current State and Challenges
The current state of research on the catalytic properties of magnesium nitride (Mg3N2) reveals a promising yet challenging landscape. Recent studies have demonstrated the potential of Mg3N2 as a catalyst in various chemical reactions, particularly in the synthesis of ammonia and the conversion of methane to higher hydrocarbons. However, the full extent of its catalytic capabilities remains largely unexplored, presenting both opportunities and obstacles for researchers and industry professionals.
One of the primary challenges in studying Mg3N2 as a catalyst is its high reactivity with water and air, which necessitates careful handling and storage procedures. This reactivity also complicates in-situ characterization techniques, limiting the depth of understanding of its catalytic mechanisms. Researchers are actively developing novel methods to overcome these limitations, including the use of protective coatings and advanced spectroscopic techniques under controlled atmospheres.
The synthesis of high-purity Mg3N2 with specific surface properties and morphologies is another significant challenge. Current production methods often result in materials with varying degrees of purity and inconsistent catalytic performance. Efforts are underway to develop more precise synthesis routes, including sol-gel methods and controlled nitridation processes, to produce Mg3N2 with tailored properties for specific catalytic applications.
In terms of catalytic activity, Mg3N2 has shown promise in several key areas. Its ability to activate molecular nitrogen under mild conditions has attracted attention for potential use in ammonia synthesis, a process of immense industrial importance. Additionally, recent studies have highlighted its effectiveness in C-H bond activation, opening avenues for methane conversion and other hydrocarbon transformations. However, the stability of Mg3N2 under reaction conditions and its long-term catalytic performance remain areas of concern that require further investigation.
The geographical distribution of Mg3N2 research is primarily concentrated in countries with advanced materials science and catalysis programs, including the United States, China, Japan, and several European nations. This distribution reflects the complex nature of the research, requiring sophisticated analytical tools and multidisciplinary expertise. Collaboration between academic institutions and industrial partners is increasingly common, aiming to bridge the gap between fundamental research and practical applications.
Despite the challenges, the potential applications of Mg3N2 in catalysis continue to drive research forward. The development of more efficient and sustainable chemical processes is a key motivator, particularly in the context of green chemistry and renewable energy technologies. As such, overcoming the current limitations in Mg3N2 catalysis could have far-reaching implications for various industries, from fertilizer production to petrochemicals and beyond.
One of the primary challenges in studying Mg3N2 as a catalyst is its high reactivity with water and air, which necessitates careful handling and storage procedures. This reactivity also complicates in-situ characterization techniques, limiting the depth of understanding of its catalytic mechanisms. Researchers are actively developing novel methods to overcome these limitations, including the use of protective coatings and advanced spectroscopic techniques under controlled atmospheres.
The synthesis of high-purity Mg3N2 with specific surface properties and morphologies is another significant challenge. Current production methods often result in materials with varying degrees of purity and inconsistent catalytic performance. Efforts are underway to develop more precise synthesis routes, including sol-gel methods and controlled nitridation processes, to produce Mg3N2 with tailored properties for specific catalytic applications.
In terms of catalytic activity, Mg3N2 has shown promise in several key areas. Its ability to activate molecular nitrogen under mild conditions has attracted attention for potential use in ammonia synthesis, a process of immense industrial importance. Additionally, recent studies have highlighted its effectiveness in C-H bond activation, opening avenues for methane conversion and other hydrocarbon transformations. However, the stability of Mg3N2 under reaction conditions and its long-term catalytic performance remain areas of concern that require further investigation.
The geographical distribution of Mg3N2 research is primarily concentrated in countries with advanced materials science and catalysis programs, including the United States, China, Japan, and several European nations. This distribution reflects the complex nature of the research, requiring sophisticated analytical tools and multidisciplinary expertise. Collaboration between academic institutions and industrial partners is increasingly common, aiming to bridge the gap between fundamental research and practical applications.
Despite the challenges, the potential applications of Mg3N2 in catalysis continue to drive research forward. The development of more efficient and sustainable chemical processes is a key motivator, particularly in the context of green chemistry and renewable energy technologies. As such, overcoming the current limitations in Mg3N2 catalysis could have far-reaching implications for various industries, from fertilizer production to petrochemicals and beyond.
Existing Catalytic Solutions
01 Catalytic properties in ammonia synthesis
Magnesium nitride exhibits catalytic properties in the synthesis of ammonia. It can be used as a catalyst or catalyst support in ammonia production processes, potentially improving efficiency and yield. The material's unique structure and chemical properties make it suitable for this application in the chemical industry.- Catalytic properties in ammonia synthesis: Magnesium nitride exhibits catalytic properties in the synthesis of ammonia. It can be used as a catalyst or catalyst support in ammonia production processes, potentially improving efficiency and yield. The material's unique structure and chemical properties make it suitable for this application in the chemical industry.
- Photocatalytic applications: Magnesium nitride demonstrates photocatalytic properties, making it useful in various environmental and energy applications. It can be employed in photocatalytic water splitting, degradation of organic pollutants, and other light-driven chemical reactions. The material's band gap and electronic structure contribute to its photocatalytic activity.
- Catalytic properties in organic synthesis: Magnesium nitride shows catalytic activity in various organic synthesis reactions. It can be used as a heterogeneous catalyst for carbon-carbon bond formation, hydrogenation, and other transformations. The material's surface properties and Lewis acidity contribute to its effectiveness in organic chemistry applications.
- Electrocatalytic properties: Magnesium nitride exhibits electrocatalytic properties, making it suitable for applications in electrochemical systems. It can be used as an electrocatalyst or electrode material in fuel cells, electrolyzers, and other electrochemical devices. The material's electronic structure and surface properties contribute to its electrocatalytic activity.
- Catalytic properties in gas sensing: Magnesium nitride demonstrates catalytic properties that can be utilized in gas sensing applications. It can be employed as a sensing material or catalyst in gas sensors for detecting various gases and volatile organic compounds. The material's surface reactivity and electronic properties contribute to its effectiveness in gas sensing technologies.
02 Application in semiconductor manufacturing
Magnesium nitride shows catalytic properties useful in semiconductor manufacturing processes. It can be employed in the production of nitride-based semiconductors, potentially catalyzing reactions or serving as a precursor material. This application highlights its importance in the electronics and optoelectronics industries.Expand Specific Solutions03 Hydrogen storage and generation
The catalytic properties of magnesium nitride are utilized in hydrogen storage and generation systems. It can facilitate the release of hydrogen from various compounds or act as a catalyst in hydrogen production processes. This application is particularly relevant for clean energy technologies and fuel cell development.Expand Specific Solutions04 Photocatalytic applications
Magnesium nitride exhibits photocatalytic properties that can be harnessed for various applications. It may be used in photocatalytic water splitting, air purification, or degradation of organic pollutants. These properties make it a potential material for environmental remediation and clean energy production.Expand Specific Solutions05 Catalyst for organic synthesis reactions
The catalytic properties of magnesium nitride are explored in organic synthesis reactions. It can catalyze various transformations, potentially offering advantages such as improved selectivity, yield, or reaction conditions. This application demonstrates its potential in the field of organic chemistry and pharmaceutical synthesis.Expand Specific Solutions
Key Industry Players
The catalytic properties of magnesium nitride represent an emerging field of research with significant potential for industrial applications. The competitive landscape is characterized by early-stage development, with major players from the petrochemical and chemical industries showing interest. Companies like China Petroleum & Chemical Corp., SABIC, and ExxonMobil Chemical Patents are likely at the forefront of research efforts. The market size is currently limited but expected to grow as applications in areas such as hydrogen production and ammonia synthesis become more established. While the technology is still in its infancy, rapid advancements are anticipated due to the involvement of well-resourced industry leaders and research institutions.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has been exploring the catalytic properties of magnesium nitride for various applications in the petrochemical industry. Their research focuses on using Mg3N2 as a catalyst support for hydrodesulfurization (HDS) reactions. The company has developed a novel synthesis method for preparing high-surface-area Mg3N2 with enhanced thermal stability and mechanical strength[1]. This material shows promising results in removing sulfur compounds from petroleum feedstocks, potentially improving fuel quality and reducing environmental impact. Sinopec's approach involves doping the Mg3N2 support with transition metals to create bifunctional catalysts that exhibit both acidic and basic sites, enhancing overall catalytic performance[3].
Strengths: Improved catalyst support with high surface area and stability. Enhanced sulfur removal efficiency. Potential for cleaner fuel production. Weaknesses: May require specialized handling due to air and moisture sensitivity of Mg3N2. Possible higher production costs compared to traditional catalysts.
SINOPEC Beijing Research Institute of Chemical Industry
Technical Solution: The SINOPEC Beijing Research Institute of Chemical Industry has been at the forefront of investigating magnesium nitride's catalytic properties, particularly in the context of ammonia synthesis. Their research has focused on developing a novel Mg3N2-based catalyst system that operates at lower temperatures and pressures compared to traditional iron-based catalysts[2]. The institute has successfully synthesized nanostructured Mg3N2 with controlled morphology and high surface area, which exhibits enhanced catalytic activity for N2 dissociation – a crucial step in ammonia production. Their approach involves doping the Mg3N2 catalyst with small amounts of transition metals to create active sites for hydrogen activation, resulting in a bifunctional catalyst system[4]. Preliminary results show a significant reduction in the activation energy for ammonia synthesis, potentially leading to more energy-efficient industrial processes.
Strengths: Lower energy requirements for ammonia synthesis. Potential for more sustainable fertilizer production. Innovative nanostructured catalyst design. Weaknesses: Scalability challenges for industrial implementation. Potential sensitivity to catalyst poisoning.
Core Mg3N2 Innovations
Improvements in or relating to processes for the manufacture of magnesium or alloys thereof
PatentInactiveGB465623A
Innovation
- A process involving the formation of magnesium nitride by passing nitrogen through a powdered magnesium compound at high temperature in a moisture-free atmosphere, followed by conversion to magnesium sulphide using sulphuretted hydrogen, and subsequent reduction with calcium carbide in an inert gas environment to obtain high-purity metallic magnesium.
Preparation of catalyst and efficient synthesis of nicotinamide therefrom
PatentPendingIN202211050957A
Innovation
- A novel process employing microwave irradiation and surface-active agents to prepare nano-rod manganese dioxide catalysts, which enhances surface properties and facilitates the efficient synthesis of nicotinamide with high yield and short reaction times, using only water as a solvent and allowing for catalyst recycling.
Environmental Impact
The environmental impact of magnesium nitride's catalytic properties is a crucial aspect to consider in its development and application. As a catalyst, magnesium nitride has the potential to significantly reduce energy consumption and improve efficiency in various chemical processes. This could lead to a decrease in overall carbon emissions and contribute to more sustainable industrial practices.
However, the production and use of magnesium nitride also present environmental challenges. The synthesis of magnesium nitride typically involves high-temperature processes, which can be energy-intensive and contribute to greenhouse gas emissions. Additionally, the raw materials required for its production, particularly magnesium, may have their own environmental footprint associated with mining and processing.
In terms of waste management, the disposal or recycling of spent magnesium nitride catalysts must be carefully considered. Improper handling could lead to the release of nitrogen compounds into the environment, potentially contributing to soil and water pollution. On the other hand, effective recycling methods could mitigate these risks and promote a more circular economy approach.
The use of magnesium nitride as a catalyst in ammonia synthesis offers significant environmental benefits. Traditional ammonia production methods, such as the Haber-Bosch process, are notoriously energy-intensive and contribute substantially to global CO2 emissions. Magnesium nitride's potential to enable ammonia synthesis under milder conditions could dramatically reduce the carbon footprint of this essential industrial process.
Furthermore, magnesium nitride's catalytic properties may find applications in environmental remediation. Research has shown promise in using magnesium nitride-based materials for the degradation of organic pollutants in water treatment processes. This could contribute to more effective and environmentally friendly water purification technologies.
The long-term environmental impact of widespread magnesium nitride use in catalytic applications remains an area requiring further study. While its potential to enable more efficient chemical processes is clear, the full lifecycle assessment of magnesium nitride catalysts, from production to disposal or recycling, needs comprehensive evaluation. This includes considering factors such as resource depletion, ecosystem impacts, and potential for environmental accumulation.
As research into magnesium nitride's catalytic properties progresses, it is essential to prioritize the development of environmentally benign synthesis methods and explore green chemistry principles in its application. This approach will help maximize the positive environmental impact of magnesium nitride while minimizing potential negative consequences, ensuring a balanced and sustainable integration of this promising catalyst into industrial processes.
However, the production and use of magnesium nitride also present environmental challenges. The synthesis of magnesium nitride typically involves high-temperature processes, which can be energy-intensive and contribute to greenhouse gas emissions. Additionally, the raw materials required for its production, particularly magnesium, may have their own environmental footprint associated with mining and processing.
In terms of waste management, the disposal or recycling of spent magnesium nitride catalysts must be carefully considered. Improper handling could lead to the release of nitrogen compounds into the environment, potentially contributing to soil and water pollution. On the other hand, effective recycling methods could mitigate these risks and promote a more circular economy approach.
The use of magnesium nitride as a catalyst in ammonia synthesis offers significant environmental benefits. Traditional ammonia production methods, such as the Haber-Bosch process, are notoriously energy-intensive and contribute substantially to global CO2 emissions. Magnesium nitride's potential to enable ammonia synthesis under milder conditions could dramatically reduce the carbon footprint of this essential industrial process.
Furthermore, magnesium nitride's catalytic properties may find applications in environmental remediation. Research has shown promise in using magnesium nitride-based materials for the degradation of organic pollutants in water treatment processes. This could contribute to more effective and environmentally friendly water purification technologies.
The long-term environmental impact of widespread magnesium nitride use in catalytic applications remains an area requiring further study. While its potential to enable more efficient chemical processes is clear, the full lifecycle assessment of magnesium nitride catalysts, from production to disposal or recycling, needs comprehensive evaluation. This includes considering factors such as resource depletion, ecosystem impacts, and potential for environmental accumulation.
As research into magnesium nitride's catalytic properties progresses, it is essential to prioritize the development of environmentally benign synthesis methods and explore green chemistry principles in its application. This approach will help maximize the positive environmental impact of magnesium nitride while minimizing potential negative consequences, ensuring a balanced and sustainable integration of this promising catalyst into industrial processes.
Scalability and Production
The scalability and production of magnesium nitride (Mg3N2) as a catalyst present both challenges and opportunities in the field of industrial chemistry. The synthesis of Mg3N2 typically involves the direct nitridation of magnesium metal at high temperatures, a process that can be energy-intensive and potentially costly at large scales. However, recent advancements in production techniques have shown promise for improving efficiency and reducing costs.
One of the key factors affecting scalability is the control of particle size and morphology during Mg3N2 synthesis. Smaller, more uniform particles generally exhibit enhanced catalytic activity due to their increased surface area. Researchers have explored various methods to achieve this, including ball milling, solution-based synthesis, and plasma-assisted techniques. These approaches have demonstrated potential for producing Mg3N2 with tailored properties suitable for specific catalytic applications.
The production of Mg3N2 on an industrial scale requires careful consideration of reactor design and process parameters. Continuous flow reactors have shown potential for large-scale synthesis, offering better control over reaction conditions and product consistency compared to batch processes. Additionally, the use of microwave-assisted synthesis has been investigated as a means to reduce energy consumption and processing time.
Another critical aspect of Mg3N2 production is the handling and storage of the final product. Mg3N2 is highly sensitive to moisture and air, readily hydrolyzing to form magnesium hydroxide and ammonia. This reactivity necessitates stringent handling protocols and specialized packaging to maintain catalyst integrity during storage and transportation. The development of stabilization techniques, such as surface passivation or encapsulation, could significantly enhance the practicality of Mg3N2 as a commercial catalyst.
The economic viability of large-scale Mg3N2 production is closely tied to the availability and cost of raw materials. Magnesium metal, the primary precursor, is relatively abundant but can be subject to price fluctuations. Diversifying magnesium sources and exploring recycling options for spent catalysts could help mitigate supply chain risks and improve overall process sustainability.
As research into the catalytic properties of Mg3N2 progresses, there is growing interest in developing more efficient and cost-effective production methods. Collaborative efforts between academia and industry are crucial for translating laboratory-scale discoveries into commercially viable processes. The optimization of synthesis conditions, exploration of novel precursors, and integration of advanced process control systems are areas of ongoing investigation that could significantly impact the scalability and production of Mg3N2 catalysts.
One of the key factors affecting scalability is the control of particle size and morphology during Mg3N2 synthesis. Smaller, more uniform particles generally exhibit enhanced catalytic activity due to their increased surface area. Researchers have explored various methods to achieve this, including ball milling, solution-based synthesis, and plasma-assisted techniques. These approaches have demonstrated potential for producing Mg3N2 with tailored properties suitable for specific catalytic applications.
The production of Mg3N2 on an industrial scale requires careful consideration of reactor design and process parameters. Continuous flow reactors have shown potential for large-scale synthesis, offering better control over reaction conditions and product consistency compared to batch processes. Additionally, the use of microwave-assisted synthesis has been investigated as a means to reduce energy consumption and processing time.
Another critical aspect of Mg3N2 production is the handling and storage of the final product. Mg3N2 is highly sensitive to moisture and air, readily hydrolyzing to form magnesium hydroxide and ammonia. This reactivity necessitates stringent handling protocols and specialized packaging to maintain catalyst integrity during storage and transportation. The development of stabilization techniques, such as surface passivation or encapsulation, could significantly enhance the practicality of Mg3N2 as a commercial catalyst.
The economic viability of large-scale Mg3N2 production is closely tied to the availability and cost of raw materials. Magnesium metal, the primary precursor, is relatively abundant but can be subject to price fluctuations. Diversifying magnesium sources and exploring recycling options for spent catalysts could help mitigate supply chain risks and improve overall process sustainability.
As research into the catalytic properties of Mg3N2 progresses, there is growing interest in developing more efficient and cost-effective production methods. Collaborative efforts between academia and industry are crucial for translating laboratory-scale discoveries into commercially viable processes. The optimization of synthesis conditions, exploration of novel precursors, and integration of advanced process control systems are areas of ongoing investigation that could significantly impact the scalability and production of Mg3N2 catalysts.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!