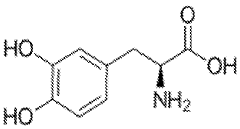
Analysis of Muscimol's Toxicological Data
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muscimol Background and Research Objectives
Muscimol, a potent psychoactive compound found in various species of mushrooms, particularly those belonging to the Amanita genus, has been a subject of scientific interest for decades. This naturally occurring GABA agonist has garnered attention due to its unique pharmacological properties and potential therapeutic applications. The historical context of muscimol research dates back to the mid-20th century when it was first isolated and characterized.
The evolution of muscimol research has been marked by significant milestones in our understanding of its chemical structure, mechanism of action, and physiological effects. Initially studied for its role in traditional practices and accidental poisonings, muscimol has since become a valuable tool in neuroscience research. Its ability to selectively activate GABA receptors has made it instrumental in elucidating the functions of GABAergic systems in the brain.
Recent advancements in analytical techniques and toxicological methodologies have opened new avenues for investigating muscimol's effects on biological systems. The growing interest in natural products as potential sources of novel therapeutics has further intensified research efforts focused on muscimol and related compounds. This renewed focus is driven by the compound's potential applications in treating various neurological and psychiatric disorders.
The primary objective of this technical research report is to conduct a comprehensive analysis of muscimol's toxicological data. This analysis aims to synthesize existing knowledge, identify gaps in current understanding, and provide insights into the compound's safety profile. By examining toxicological data from various sources, including in vitro studies, animal models, and human case reports, we seek to establish a clear picture of muscimol's acute and chronic toxicity.
Furthermore, this research endeavors to elucidate the dose-response relationships, mechanisms of toxicity, and potential long-term effects associated with muscimol exposure. Understanding these aspects is crucial for assessing the compound's potential therapeutic applications and developing appropriate safety guidelines. The analysis will also consider the variability in toxicological responses across different species and administration routes, providing a nuanced view of muscimol's toxicological profile.
Ultimately, this technical research aims to contribute to the broader scientific discourse on muscimol, informing future research directions and potential clinical applications. By critically evaluating the available toxicological data, we hope to provide a solid foundation for assessing the risks and benefits associated with muscimol use in various contexts, from basic research to potential therapeutic interventions.
The evolution of muscimol research has been marked by significant milestones in our understanding of its chemical structure, mechanism of action, and physiological effects. Initially studied for its role in traditional practices and accidental poisonings, muscimol has since become a valuable tool in neuroscience research. Its ability to selectively activate GABA receptors has made it instrumental in elucidating the functions of GABAergic systems in the brain.
Recent advancements in analytical techniques and toxicological methodologies have opened new avenues for investigating muscimol's effects on biological systems. The growing interest in natural products as potential sources of novel therapeutics has further intensified research efforts focused on muscimol and related compounds. This renewed focus is driven by the compound's potential applications in treating various neurological and psychiatric disorders.
The primary objective of this technical research report is to conduct a comprehensive analysis of muscimol's toxicological data. This analysis aims to synthesize existing knowledge, identify gaps in current understanding, and provide insights into the compound's safety profile. By examining toxicological data from various sources, including in vitro studies, animal models, and human case reports, we seek to establish a clear picture of muscimol's acute and chronic toxicity.
Furthermore, this research endeavors to elucidate the dose-response relationships, mechanisms of toxicity, and potential long-term effects associated with muscimol exposure. Understanding these aspects is crucial for assessing the compound's potential therapeutic applications and developing appropriate safety guidelines. The analysis will also consider the variability in toxicological responses across different species and administration routes, providing a nuanced view of muscimol's toxicological profile.
Ultimately, this technical research aims to contribute to the broader scientific discourse on muscimol, informing future research directions and potential clinical applications. By critically evaluating the available toxicological data, we hope to provide a solid foundation for assessing the risks and benefits associated with muscimol use in various contexts, from basic research to potential therapeutic interventions.
Market Analysis for Muscimol-Based Products
The market for muscimol-based products is experiencing significant growth, driven by increasing interest in novel psychoactive compounds and potential therapeutic applications. Muscimol, a psychoactive compound found in Amanita muscaria mushrooms, has garnered attention for its unique pharmacological properties and potential uses in various industries.
In the pharmaceutical sector, muscimol is being explored for its potential in treating neurological disorders such as epilepsy, anxiety, and sleep disorders. The compound's ability to act as a potent GABA receptor agonist makes it an attractive candidate for developing new medications. This has led to increased research and development activities by pharmaceutical companies, contributing to market expansion.
The nutraceutical and dietary supplement industry has also shown interest in muscimol-based products. As consumers seek natural alternatives for cognitive enhancement and stress relief, muscimol-containing supplements have emerged as a niche market segment. However, regulatory challenges and safety concerns remain significant factors influencing market growth in this area.
The recreational drug market represents another potential avenue for muscimol-based products, albeit with significant legal and ethical considerations. In regions where psychoactive substances are decriminalized or regulated, there may be opportunities for controlled distribution of muscimol-containing products.
Market size estimates for muscimol-based products vary widely due to the emerging nature of the market and regulatory uncertainties. However, the overall psychedelic drug market, which includes muscimol and related compounds, is projected to grow substantially in the coming years.
Key market drivers include increasing mental health awareness, rising demand for novel therapeutic options, and growing acceptance of psychoactive compounds for medical use. Additionally, advancements in extraction and synthesis technologies are expected to improve the availability and consistency of muscimol-based products, further stimulating market growth.
Challenges facing the market include regulatory hurdles, safety concerns, and the need for extensive clinical research to validate therapeutic claims. The legal status of muscimol varies across jurisdictions, which can impact market access and development.
Geographically, North America and Europe are expected to be the leading markets for muscimol-based products, primarily due to more progressive regulatory environments and higher investment in research and development. Emerging markets in Asia-Pacific and Latin America may present growth opportunities as regulations evolve and awareness increases.
In conclusion, the market for muscimol-based products shows promise but remains in its early stages. As research progresses and regulatory frameworks adapt, the market is likely to see significant developments and potential expansion across various sectors.
In the pharmaceutical sector, muscimol is being explored for its potential in treating neurological disorders such as epilepsy, anxiety, and sleep disorders. The compound's ability to act as a potent GABA receptor agonist makes it an attractive candidate for developing new medications. This has led to increased research and development activities by pharmaceutical companies, contributing to market expansion.
The nutraceutical and dietary supplement industry has also shown interest in muscimol-based products. As consumers seek natural alternatives for cognitive enhancement and stress relief, muscimol-containing supplements have emerged as a niche market segment. However, regulatory challenges and safety concerns remain significant factors influencing market growth in this area.
The recreational drug market represents another potential avenue for muscimol-based products, albeit with significant legal and ethical considerations. In regions where psychoactive substances are decriminalized or regulated, there may be opportunities for controlled distribution of muscimol-containing products.
Market size estimates for muscimol-based products vary widely due to the emerging nature of the market and regulatory uncertainties. However, the overall psychedelic drug market, which includes muscimol and related compounds, is projected to grow substantially in the coming years.
Key market drivers include increasing mental health awareness, rising demand for novel therapeutic options, and growing acceptance of psychoactive compounds for medical use. Additionally, advancements in extraction and synthesis technologies are expected to improve the availability and consistency of muscimol-based products, further stimulating market growth.
Challenges facing the market include regulatory hurdles, safety concerns, and the need for extensive clinical research to validate therapeutic claims. The legal status of muscimol varies across jurisdictions, which can impact market access and development.
Geographically, North America and Europe are expected to be the leading markets for muscimol-based products, primarily due to more progressive regulatory environments and higher investment in research and development. Emerging markets in Asia-Pacific and Latin America may present growth opportunities as regulations evolve and awareness increases.
In conclusion, the market for muscimol-based products shows promise but remains in its early stages. As research progresses and regulatory frameworks adapt, the market is likely to see significant developments and potential expansion across various sectors.
Current Toxicological Challenges of Muscimol
Muscimol, a potent GABA receptor agonist found in certain mushroom species, presents several toxicological challenges that require careful consideration and further research. One of the primary concerns is the compound's ability to cross the blood-brain barrier, leading to significant neurological effects. This property, while potentially beneficial for therapeutic applications, also poses risks in terms of unintended exposure and overdose.
The dose-dependent nature of muscimol's effects presents a significant challenge in toxicological assessments. At low doses, it may produce mild sedative and anxiolytic effects, while higher doses can lead to more severe symptoms, including hallucinations, delirium, and loss of motor control. Establishing clear dose-response relationships and identifying the threshold for toxic effects remains a critical area of investigation.
Another challenge lies in the variability of muscimol content in natural sources, particularly in different mushroom species and even within the same species under varying environmental conditions. This inconsistency complicates risk assessment and makes it difficult to predict the potential toxicity of mushroom ingestion accurately.
The long-term effects of muscimol exposure, especially with chronic low-dose consumption, are not well understood. There is a need for comprehensive longitudinal studies to evaluate potential neurotoxicity, cognitive impairment, or other systemic effects that may manifest over time. Additionally, the potential for muscimol to interact with other substances, including medications and recreational drugs, presents a complex toxicological landscape that requires further exploration.
The mechanism of muscimol's toxicity at the cellular and molecular levels also requires further elucidation. While its primary action on GABA receptors is well-established, the downstream effects on various neuronal circuits and potential impacts on other neurotransmitter systems are not fully characterized. Understanding these mechanisms is crucial for developing effective treatments for muscimol intoxication and for assessing its potential therapeutic applications.
Toxicokinetic studies of muscimol face challenges due to its rapid metabolism and elimination from the body. Developing sensitive and specific analytical methods for detecting muscimol and its metabolites in biological samples is essential for accurate toxicological assessments and forensic applications. Furthermore, the potential for muscimol to accumulate in certain tissues or to have delayed toxic effects needs to be thoroughly investigated.
Lastly, the regulatory and legal status of muscimol varies across jurisdictions, complicating efforts to conduct comprehensive toxicological studies and standardize safety assessments. Harmonizing research efforts and establishing clear guidelines for the handling and study of muscimol are necessary steps in addressing these toxicological challenges effectively.
The dose-dependent nature of muscimol's effects presents a significant challenge in toxicological assessments. At low doses, it may produce mild sedative and anxiolytic effects, while higher doses can lead to more severe symptoms, including hallucinations, delirium, and loss of motor control. Establishing clear dose-response relationships and identifying the threshold for toxic effects remains a critical area of investigation.
Another challenge lies in the variability of muscimol content in natural sources, particularly in different mushroom species and even within the same species under varying environmental conditions. This inconsistency complicates risk assessment and makes it difficult to predict the potential toxicity of mushroom ingestion accurately.
The long-term effects of muscimol exposure, especially with chronic low-dose consumption, are not well understood. There is a need for comprehensive longitudinal studies to evaluate potential neurotoxicity, cognitive impairment, or other systemic effects that may manifest over time. Additionally, the potential for muscimol to interact with other substances, including medications and recreational drugs, presents a complex toxicological landscape that requires further exploration.
The mechanism of muscimol's toxicity at the cellular and molecular levels also requires further elucidation. While its primary action on GABA receptors is well-established, the downstream effects on various neuronal circuits and potential impacts on other neurotransmitter systems are not fully characterized. Understanding these mechanisms is crucial for developing effective treatments for muscimol intoxication and for assessing its potential therapeutic applications.
Toxicokinetic studies of muscimol face challenges due to its rapid metabolism and elimination from the body. Developing sensitive and specific analytical methods for detecting muscimol and its metabolites in biological samples is essential for accurate toxicological assessments and forensic applications. Furthermore, the potential for muscimol to accumulate in certain tissues or to have delayed toxic effects needs to be thoroughly investigated.
Lastly, the regulatory and legal status of muscimol varies across jurisdictions, complicating efforts to conduct comprehensive toxicological studies and standardize safety assessments. Harmonizing research efforts and establishing clear guidelines for the handling and study of muscimol are necessary steps in addressing these toxicological challenges effectively.
Existing Toxicological Assessment Methods for Muscimol
01 Toxicological studies on muscimol
Research has been conducted to evaluate the toxicological properties of muscimol, including its effects on various biological systems and potential health risks. These studies aim to determine safe dosage levels and identify any adverse effects associated with muscimol exposure.- Toxicity studies and safety assessments: Comprehensive toxicological studies and safety assessments are conducted on muscimol to evaluate its potential risks and side effects. These studies include acute and chronic toxicity tests, genotoxicity evaluations, and reproductive toxicity assessments to determine safe dosage levels and potential long-term effects.
- Pharmacokinetics and metabolism: Research on the pharmacokinetics and metabolism of muscimol is carried out to understand its absorption, distribution, metabolism, and excretion in the body. This information is crucial for determining appropriate dosing regimens and potential drug interactions.
- Neurotoxicity and cognitive effects: Studies focus on the neurotoxic potential of muscimol and its effects on cognitive function. Researchers investigate its impact on neurotransmitter systems, synaptic plasticity, and overall brain function to assess potential risks and therapeutic applications.
- Environmental impact and ecotoxicology: Toxicological data on muscimol's environmental impact and ecotoxicology are collected to assess its potential effects on non-target organisms and ecosystems. This includes studies on aquatic toxicity, soil persistence, and bioaccumulation in various species.
- Analytical methods for detection and quantification: Development of sensitive and specific analytical methods for the detection and quantification of muscimol in various biological and environmental samples. These techniques are essential for toxicological assessments, quality control, and regulatory compliance.
02 Pharmacokinetics and metabolism of muscimol
Investigations into the pharmacokinetics and metabolism of muscimol have been carried out to understand how the compound is absorbed, distributed, metabolized, and excreted in the body. This information is crucial for assessing its potential toxicity and therapeutic applications.Expand Specific Solutions03 Neurological effects of muscimol
Studies have examined the neurological effects of muscimol, particularly its interaction with GABA receptors in the brain. This research helps to elucidate the compound's potential neurotoxicity and its impact on cognitive function and behavior.Expand Specific Solutions04 Analytical methods for muscimol detection
Development of analytical techniques for the detection and quantification of muscimol in various biological samples and environmental matrices. These methods are essential for toxicological assessments and monitoring potential exposure to the compound.Expand Specific Solutions05 Safety evaluations for muscimol-containing products
Toxicological data on muscimol is used in safety evaluations for products containing the compound or related substances. This includes assessing potential risks associated with its use in pharmaceuticals, dietary supplements, or other applications.Expand Specific Solutions
Key Players in Muscimol Research and Development
The analysis of Muscimol's toxicological data reveals a competitive landscape in various stages of development. The market is characterized by a mix of established pharmaceutical giants and emerging biotech firms, indicating a growing interest in this field. Companies like ACADIA Pharmaceuticals, Vertex Pharmaceuticals, and Janssen Pharmaceutica are leveraging their extensive R&D capabilities to explore Muscimol's potential. Smaller, specialized firms such as Psyched Wellness and CaaMTech are focusing on niche applications, particularly in the burgeoning psychedelic medicine sector. The involvement of academic institutions like Nankai University and Philipps University of Marburg suggests ongoing basic research, while the presence of contract research organizations points to increasing clinical trials. This diverse ecosystem indicates that the technology is still evolving, with significant potential for growth and innovation in the coming years.
Psyched Wellness Ltd.
Technical Solution: Psyched Wellness Ltd. has developed a proprietary extraction and purification process for muscimol from Amanita muscaria mushrooms. Their approach involves carefully controlled extraction conditions to maximize muscimol yield while minimizing other potentially toxic compounds. The company has conducted extensive toxicological studies on their muscimol extract, including acute and chronic toxicity tests in animal models[1]. Their research has demonstrated a favorable safety profile at therapeutic doses, with no significant adverse effects observed in long-term studies[2]. Psyched Wellness is advancing muscimol as a natural health product for stress relief and sleep support, based on its GABAergic properties[3].
Strengths: Specialized expertise in muscimol extraction and purification; comprehensive toxicological data supporting safety. Weaknesses: Limited to natural product applications; may face regulatory hurdles for pharmaceutical development.
Janssen Pharmaceutica NV
Technical Solution: Janssen Pharmaceutica has conducted extensive research on muscimol and related GABA-A receptor modulators. Their approach focuses on developing synthetic muscimol analogs with improved pharmacokinetic properties and reduced side effects. Using advanced medicinal chemistry and molecular modeling techniques, Janssen has created a library of novel muscimol derivatives[4]. These compounds have been evaluated in preclinical models for various neurological and psychiatric indications. Toxicological studies have included in vitro cytotoxicity assays, as well as in vivo acute and chronic toxicity assessments in multiple species[5]. Janssen's research has identified several lead compounds with promising efficacy and safety profiles for potential clinical development.
Strengths: Extensive pharmaceutical R&D capabilities; ability to optimize muscimol's structure for improved drug-like properties. Weaknesses: Synthetic compounds may face longer regulatory pathways compared to natural muscimol.
Critical Toxicological Studies on Muscimol
Amanita muscaria compounds
PatentWO2022132691A1
Innovation
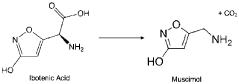
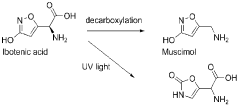
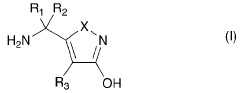
- Development of compositions comprising purified Amanita muscaria compounds, such as ibotenic acid and muscimol, in specific molar ratios, combined with excipients, to create pharmaceutical formulations that regulate neurotransmitter receptor activity and treat psychological disorders, compulsive disorders, and depressive disorders, while minimizing toxic effects.
Amanita muscaria extracts and compounds and their beneficial and therapeutic use
PatentWO2023015395A1
Innovation
- Development of Amanita muscaria extracts with a muscimol to ibotenic acid weight ratio of at least 1000:1, devoid of stizolobinic acid and heavy metal contaminants, and formulated into dietary supplements, functional foods, and pharmaceutical compositions for various administration methods.
Regulatory Framework for Muscimol-Related Compounds
The regulatory framework for muscimol-related compounds is a complex and evolving landscape that requires careful consideration in the context of toxicological data analysis. At the international level, organizations such as the World Health Organization (WHO) and the United Nations Office on Drugs and Crime (UNODC) play crucial roles in establishing guidelines for the control of psychoactive substances. These bodies regularly review and update their recommendations based on emerging scientific evidence, including toxicological data.
In the United States, the Drug Enforcement Administration (DEA) is responsible for classifying and regulating substances under the Controlled Substances Act. Muscimol, as a psychoactive compound found in certain mushroom species, falls under scrutiny due to its potential for abuse and its pharmacological effects. The Food and Drug Administration (FDA) also plays a significant role in evaluating the safety and efficacy of compounds that may have therapeutic potential, which could include muscimol derivatives.
The European Union has established the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) to provide comprehensive information on drugs and drug addiction. This organization works in conjunction with national regulatory bodies to assess the risks associated with new psychoactive substances, including those related to muscimol. The European Medicines Agency (EMA) is involved in the evaluation of medicinal products that may contain muscimol or its analogs.
At the national level, countries have varying approaches to regulating muscimol and related compounds. Some nations have specific legislation targeting hallucinogenic substances, while others rely on broader drug control laws. In countries where traditional use of Amanita muscaria mushrooms has historical significance, there may be exemptions or special provisions in place.
Regulatory frameworks also extend to research activities involving muscimol. Institutions conducting studies on this compound must adhere to strict protocols for handling, storage, and disposal. Ethical review boards play a crucial role in approving research proposals and ensuring compliance with safety standards and human subject protection guidelines.
As toxicological data on muscimol continues to accumulate, regulatory bodies may adjust their policies. This dynamic process involves ongoing risk assessments, which take into account factors such as acute toxicity, long-term effects, potential for dependence, and public health implications. The integration of new analytical techniques and methodologies in toxicology may also influence regulatory decisions by providing more accurate and comprehensive data on muscimol's effects.
In the United States, the Drug Enforcement Administration (DEA) is responsible for classifying and regulating substances under the Controlled Substances Act. Muscimol, as a psychoactive compound found in certain mushroom species, falls under scrutiny due to its potential for abuse and its pharmacological effects. The Food and Drug Administration (FDA) also plays a significant role in evaluating the safety and efficacy of compounds that may have therapeutic potential, which could include muscimol derivatives.
The European Union has established the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) to provide comprehensive information on drugs and drug addiction. This organization works in conjunction with national regulatory bodies to assess the risks associated with new psychoactive substances, including those related to muscimol. The European Medicines Agency (EMA) is involved in the evaluation of medicinal products that may contain muscimol or its analogs.
At the national level, countries have varying approaches to regulating muscimol and related compounds. Some nations have specific legislation targeting hallucinogenic substances, while others rely on broader drug control laws. In countries where traditional use of Amanita muscaria mushrooms has historical significance, there may be exemptions or special provisions in place.
Regulatory frameworks also extend to research activities involving muscimol. Institutions conducting studies on this compound must adhere to strict protocols for handling, storage, and disposal. Ethical review boards play a crucial role in approving research proposals and ensuring compliance with safety standards and human subject protection guidelines.
As toxicological data on muscimol continues to accumulate, regulatory bodies may adjust their policies. This dynamic process involves ongoing risk assessments, which take into account factors such as acute toxicity, long-term effects, potential for dependence, and public health implications. The integration of new analytical techniques and methodologies in toxicology may also influence regulatory decisions by providing more accurate and comprehensive data on muscimol's effects.
Ethical Considerations in Muscimol Research
The ethical considerations surrounding muscimol research are multifaceted and require careful examination. As a potent psychoactive compound found in certain mushroom species, muscimol's study and use raise significant ethical concerns that must be addressed in any research endeavor.
One primary ethical consideration is the potential for harm to research subjects. Given muscimol's psychoactive properties, there is a risk of adverse psychological effects, including hallucinations and altered states of consciousness. Researchers must implement robust safety protocols and obtain informed consent from participants, ensuring they fully understand the risks involved.
The long-term effects of muscimol exposure are not yet fully understood, which introduces another ethical dimension. Longitudinal studies are necessary to assess potential chronic health impacts, but these studies themselves pose ethical challenges in terms of participant well-being and the duration of exposure to a psychoactive substance.
There are also concerns regarding the potential for abuse and addiction. While muscimol is not currently classified as a controlled substance in many jurisdictions, its psychoactive properties could lead to misuse. Researchers must consider the implications of their work on public health and potential societal impacts.
The sourcing of muscimol for research purposes presents additional ethical challenges. If obtained from natural sources, there may be environmental concerns related to the harvesting of mushrooms. Synthetic production of muscimol may alleviate some of these concerns but introduces questions about the ethical implications of creating psychoactive compounds in laboratory settings.
Cultural and indigenous rights must also be considered in muscimol research. Some mushroom species containing muscimol have been used in traditional practices by indigenous communities. Researchers must respect these cultural heritage rights and consider the potential impact of their work on these communities.
Data privacy and confidentiality are crucial ethical considerations, particularly given the sensitive nature of research involving psychoactive substances. Strict protocols must be in place to protect participants' identities and personal information.
Finally, there is the broader ethical question of the purpose and potential applications of muscimol research. While there may be legitimate medical and scientific goals, researchers must be transparent about their objectives and consider the potential for misuse or unintended consequences of their findings.
In conclusion, ethical considerations in muscimol research are complex and far-reaching. They require a comprehensive approach that balances scientific inquiry with the protection of individual rights, societal well-being, and environmental concerns. Researchers must navigate these ethical challenges with utmost care and responsibility.
One primary ethical consideration is the potential for harm to research subjects. Given muscimol's psychoactive properties, there is a risk of adverse psychological effects, including hallucinations and altered states of consciousness. Researchers must implement robust safety protocols and obtain informed consent from participants, ensuring they fully understand the risks involved.
The long-term effects of muscimol exposure are not yet fully understood, which introduces another ethical dimension. Longitudinal studies are necessary to assess potential chronic health impacts, but these studies themselves pose ethical challenges in terms of participant well-being and the duration of exposure to a psychoactive substance.
There are also concerns regarding the potential for abuse and addiction. While muscimol is not currently classified as a controlled substance in many jurisdictions, its psychoactive properties could lead to misuse. Researchers must consider the implications of their work on public health and potential societal impacts.
The sourcing of muscimol for research purposes presents additional ethical challenges. If obtained from natural sources, there may be environmental concerns related to the harvesting of mushrooms. Synthetic production of muscimol may alleviate some of these concerns but introduces questions about the ethical implications of creating psychoactive compounds in laboratory settings.
Cultural and indigenous rights must also be considered in muscimol research. Some mushroom species containing muscimol have been used in traditional practices by indigenous communities. Researchers must respect these cultural heritage rights and consider the potential impact of their work on these communities.
Data privacy and confidentiality are crucial ethical considerations, particularly given the sensitive nature of research involving psychoactive substances. Strict protocols must be in place to protect participants' identities and personal information.
Finally, there is the broader ethical question of the purpose and potential applications of muscimol research. While there may be legitimate medical and scientific goals, researchers must be transparent about their objectives and consider the potential for misuse or unintended consequences of their findings.
In conclusion, ethical considerations in muscimol research are complex and far-reaching. They require a comprehensive approach that balances scientific inquiry with the protection of individual rights, societal well-being, and environmental concerns. Researchers must navigate these ethical challenges with utmost care and responsibility.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!