Analysis of Sulphanilic Acid Interactions with Heavy Metal Ions in Aqueous Solutions
JUL 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulphanilic Acid-Metal Ion Interaction Background
Sulphanilic acid, an aromatic compound with both amino and sulfonic acid groups, has garnered significant attention in environmental and analytical chemistry due to its unique interactions with heavy metal ions in aqueous solutions. This interaction forms the basis for various applications in water treatment, environmental monitoring, and industrial processes.
The study of sulphanilic acid's interactions with heavy metal ions dates back to the mid-20th century, with early research focusing on its potential as a chelating agent. As environmental concerns grew, particularly regarding heavy metal pollution in water bodies, the interest in sulphanilic acid's metal-binding properties intensified.
Sulphanilic acid's structure plays a crucial role in its interaction with metal ions. The sulfonic acid group (-SO3H) and the amino group (-NH2) on the benzene ring provide potential binding sites for metal ions. This structural characteristic allows sulphanilic acid to form complexes with various heavy metals, including but not limited to copper, zinc, lead, and cadmium.
The interaction between sulphanilic acid and metal ions is primarily governed by coordination chemistry principles. The electron-rich nitrogen of the amino group and the oxygen atoms of the sulfonic acid group can donate electrons to the metal ions, forming coordinate covalent bonds. This process often results in the formation of stable metal-ligand complexes.
The pH of the aqueous solution significantly influences the nature and strength of these interactions. At lower pH values, the protonation of the amino group can reduce its metal-binding capacity, while at higher pH, the deprotonation of the sulfonic acid group enhances the overall metal-binding ability of sulphanilic acid.
Temperature, ionic strength, and the presence of competing ligands in the solution also play crucial roles in determining the efficiency and selectivity of sulphanilic acid-metal ion interactions. These factors can affect the stability constants of the formed complexes and the overall thermodynamics of the interaction process.
Understanding these interactions has led to the development of various analytical methods for heavy metal detection and quantification. Spectrophotometric techniques, in particular, have been widely employed, utilizing the changes in absorption spectra that occur when sulphanilic acid complexes with different metal ions.
Moreover, the metal-binding properties of sulphanilic acid have been explored for potential applications in wastewater treatment. Its ability to form stable complexes with heavy metals suggests its use as a chelating agent for metal removal from contaminated water sources.
The study of sulphanilic acid's interactions with heavy metal ions dates back to the mid-20th century, with early research focusing on its potential as a chelating agent. As environmental concerns grew, particularly regarding heavy metal pollution in water bodies, the interest in sulphanilic acid's metal-binding properties intensified.
Sulphanilic acid's structure plays a crucial role in its interaction with metal ions. The sulfonic acid group (-SO3H) and the amino group (-NH2) on the benzene ring provide potential binding sites for metal ions. This structural characteristic allows sulphanilic acid to form complexes with various heavy metals, including but not limited to copper, zinc, lead, and cadmium.
The interaction between sulphanilic acid and metal ions is primarily governed by coordination chemistry principles. The electron-rich nitrogen of the amino group and the oxygen atoms of the sulfonic acid group can donate electrons to the metal ions, forming coordinate covalent bonds. This process often results in the formation of stable metal-ligand complexes.
The pH of the aqueous solution significantly influences the nature and strength of these interactions. At lower pH values, the protonation of the amino group can reduce its metal-binding capacity, while at higher pH, the deprotonation of the sulfonic acid group enhances the overall metal-binding ability of sulphanilic acid.
Temperature, ionic strength, and the presence of competing ligands in the solution also play crucial roles in determining the efficiency and selectivity of sulphanilic acid-metal ion interactions. These factors can affect the stability constants of the formed complexes and the overall thermodynamics of the interaction process.
Understanding these interactions has led to the development of various analytical methods for heavy metal detection and quantification. Spectrophotometric techniques, in particular, have been widely employed, utilizing the changes in absorption spectra that occur when sulphanilic acid complexes with different metal ions.
Moreover, the metal-binding properties of sulphanilic acid have been explored for potential applications in wastewater treatment. Its ability to form stable complexes with heavy metals suggests its use as a chelating agent for metal removal from contaminated water sources.
Environmental Remediation Market Analysis
The environmental remediation market has been experiencing significant growth in recent years, driven by increasing awareness of environmental pollution and stricter regulations worldwide. The global market for environmental remediation was valued at approximately $85 billion in 2020 and is projected to reach $142 billion by 2027, growing at a CAGR of 7.5% during the forecast period.
The analysis of sulphanilic acid interactions with heavy metal ions in aqueous solutions plays a crucial role in this market, particularly in water treatment and soil remediation sectors. Heavy metal contamination remains a persistent environmental issue, with industrial activities, mining operations, and agricultural practices being major contributors. The demand for effective and cost-efficient remediation technologies is on the rise, creating opportunities for innovative solutions.
In the water treatment segment, which accounts for the largest share of the environmental remediation market, sulphanilic acid-based technologies are gaining traction due to their ability to form stable complexes with heavy metal ions. This property makes them particularly useful in removing toxic metals such as lead, cadmium, and mercury from wastewater and contaminated water bodies. The market for water treatment is expected to grow at a CAGR of 8.2% from 2021 to 2028, driven by increasing water scarcity and the need for clean water resources.
Soil remediation is another significant segment where sulphanilic acid interactions with heavy metal ions find application. The global soil remediation market is projected to reach $38 billion by 2025, growing at a CAGR of 9.7%. The increasing focus on brownfield redevelopment and the remediation of contaminated industrial sites are key factors driving this growth.
Geographically, North America dominates the environmental remediation market, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years due to rapid industrialization, urbanization, and increasing environmental concerns in countries like China and India.
The market landscape is characterized by a mix of large multinational corporations and specialized environmental service providers. Key players in the market are investing heavily in research and development to develop more efficient and sustainable remediation technologies, including those based on sulphanilic acid interactions with heavy metal ions.
Emerging trends in the market include the adoption of in-situ remediation techniques, which are more cost-effective and less disruptive than traditional ex-situ methods. Additionally, there is a growing interest in green remediation technologies that minimize the environmental footprint of the remediation process itself. The integration of sulphanilic acid-based solutions with other advanced technologies, such as nanotechnology and bioremediation, is expected to create new opportunities in the market.
The analysis of sulphanilic acid interactions with heavy metal ions in aqueous solutions plays a crucial role in this market, particularly in water treatment and soil remediation sectors. Heavy metal contamination remains a persistent environmental issue, with industrial activities, mining operations, and agricultural practices being major contributors. The demand for effective and cost-efficient remediation technologies is on the rise, creating opportunities for innovative solutions.
In the water treatment segment, which accounts for the largest share of the environmental remediation market, sulphanilic acid-based technologies are gaining traction due to their ability to form stable complexes with heavy metal ions. This property makes them particularly useful in removing toxic metals such as lead, cadmium, and mercury from wastewater and contaminated water bodies. The market for water treatment is expected to grow at a CAGR of 8.2% from 2021 to 2028, driven by increasing water scarcity and the need for clean water resources.
Soil remediation is another significant segment where sulphanilic acid interactions with heavy metal ions find application. The global soil remediation market is projected to reach $38 billion by 2025, growing at a CAGR of 9.7%. The increasing focus on brownfield redevelopment and the remediation of contaminated industrial sites are key factors driving this growth.
Geographically, North America dominates the environmental remediation market, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years due to rapid industrialization, urbanization, and increasing environmental concerns in countries like China and India.
The market landscape is characterized by a mix of large multinational corporations and specialized environmental service providers. Key players in the market are investing heavily in research and development to develop more efficient and sustainable remediation technologies, including those based on sulphanilic acid interactions with heavy metal ions.
Emerging trends in the market include the adoption of in-situ remediation techniques, which are more cost-effective and less disruptive than traditional ex-situ methods. Additionally, there is a growing interest in green remediation technologies that minimize the environmental footprint of the remediation process itself. The integration of sulphanilic acid-based solutions with other advanced technologies, such as nanotechnology and bioremediation, is expected to create new opportunities in the market.
Current Challenges in Heavy Metal Detection
The detection of heavy metal ions in aqueous solutions remains a significant challenge in environmental monitoring and public health protection. Despite advancements in analytical techniques, several obstacles persist in achieving accurate, sensitive, and cost-effective detection methods.
One of the primary challenges is the low concentration of heavy metal ions in many environmental samples. Trace amounts of these contaminants can have severe health impacts, necessitating detection limits in the parts per billion (ppb) or even parts per trillion (ppt) range. Achieving such high sensitivity while maintaining accuracy and precision is technically demanding and often requires sophisticated instrumentation.
Interference from other ions and organic compounds present in complex environmental matrices poses another significant hurdle. These interfering species can mask the signal of target heavy metal ions or produce false positives, compromising the reliability of detection methods. Developing selective detection techniques that can discriminate between different metal ions and overcome matrix effects is crucial for accurate analysis.
The need for on-site and real-time monitoring presents additional challenges. Many current detection methods rely on laboratory-based techniques that require sample collection, transportation, and preparation. This approach is time-consuming and may not capture rapid fluctuations in metal ion concentrations. Developing portable, robust, and user-friendly detection systems for field use remains an active area of research.
Cost considerations also play a significant role in the widespread implementation of heavy metal detection technologies. While highly sensitive and accurate methods exist, they often involve expensive equipment and require skilled operators. Balancing performance with affordability is essential for broader adoption, especially in resource-limited settings.
The stability and longevity of detection systems, particularly for continuous monitoring applications, present another challenge. Sensors and detection platforms must maintain their performance over extended periods and under varying environmental conditions. Issues such as sensor fouling, drift, and degradation need to be addressed to ensure reliable long-term operation.
Furthermore, the simultaneous detection of multiple heavy metal ions in a single analysis remains challenging. Many current methods are optimized for specific metal ions, requiring separate tests for each analyte. Developing multiplexed detection platforms that can accurately quantify several metal ions concurrently would greatly enhance the efficiency and practicality of environmental monitoring efforts.
One of the primary challenges is the low concentration of heavy metal ions in many environmental samples. Trace amounts of these contaminants can have severe health impacts, necessitating detection limits in the parts per billion (ppb) or even parts per trillion (ppt) range. Achieving such high sensitivity while maintaining accuracy and precision is technically demanding and often requires sophisticated instrumentation.
Interference from other ions and organic compounds present in complex environmental matrices poses another significant hurdle. These interfering species can mask the signal of target heavy metal ions or produce false positives, compromising the reliability of detection methods. Developing selective detection techniques that can discriminate between different metal ions and overcome matrix effects is crucial for accurate analysis.
The need for on-site and real-time monitoring presents additional challenges. Many current detection methods rely on laboratory-based techniques that require sample collection, transportation, and preparation. This approach is time-consuming and may not capture rapid fluctuations in metal ion concentrations. Developing portable, robust, and user-friendly detection systems for field use remains an active area of research.
Cost considerations also play a significant role in the widespread implementation of heavy metal detection technologies. While highly sensitive and accurate methods exist, they often involve expensive equipment and require skilled operators. Balancing performance with affordability is essential for broader adoption, especially in resource-limited settings.
The stability and longevity of detection systems, particularly for continuous monitoring applications, present another challenge. Sensors and detection platforms must maintain their performance over extended periods and under varying environmental conditions. Issues such as sensor fouling, drift, and degradation need to be addressed to ensure reliable long-term operation.
Furthermore, the simultaneous detection of multiple heavy metal ions in a single analysis remains challenging. Many current methods are optimized for specific metal ions, requiring separate tests for each analyte. Developing multiplexed detection platforms that can accurately quantify several metal ions concurrently would greatly enhance the efficiency and practicality of environmental monitoring efforts.
Existing Sulphanilic Acid-Metal Complexation Techniques
01 Sulphanilic acid in dye synthesis
Sulphanilic acid is widely used in the synthesis of various dyes, particularly azo dyes. It serves as an important intermediate in the production of colorants for textiles, paper, and other industries. The acid's ability to form stable diazonium salts makes it valuable in creating a wide range of color compounds.- Synthesis and reactions of sulphanilic acid derivatives: Various methods for synthesizing sulphanilic acid derivatives and their reactions with other compounds are explored. These processes involve different reaction conditions and catalysts to produce desired products with specific properties.
- Applications in dye and pigment industry: Sulphanilic acid and its derivatives are widely used in the production of dyes and pigments. The interactions of these compounds with other chemicals lead to the development of new colorants with improved properties such as stability and color intensity.
- Use in pharmaceutical and medicinal chemistry: Sulphanilic acid interactions are studied for their potential applications in pharmaceutical and medicinal chemistry. These compounds serve as intermediates or active ingredients in the synthesis of various drugs and therapeutic agents.
- Analytical methods and detection techniques: Various analytical methods and detection techniques are developed to study sulphanilic acid interactions. These include spectroscopic methods, chromatography, and electrochemical techniques for quantitative and qualitative analysis of sulphanilic acid and its derivatives.
- Industrial applications and material science: Sulphanilic acid interactions are explored for their potential in industrial applications and material science. This includes their use in polymer synthesis, surface treatments, and the development of functional materials with specific properties.
02 Interactions with metal ions
Sulphanilic acid can form complexes with various metal ions, which has applications in analytical chemistry and metal extraction processes. These interactions are utilized in the development of selective ion sensors and in the removal of heavy metals from wastewater.Expand Specific Solutions03 Use in pharmaceutical compounds
Sulphanilic acid and its derivatives are employed in the synthesis of pharmaceutical compounds. They serve as precursors or building blocks for various drugs, particularly those with antibacterial properties. The acid's structure allows for modifications to create diverse bioactive molecules.Expand Specific Solutions04 Applications in polymer chemistry
Sulphanilic acid is utilized in polymer chemistry for the production of specialty polymers and copolymers. It can be incorporated into polymer chains to impart specific properties such as improved thermal stability, conductivity, or chemical resistance.Expand Specific Solutions05 Analytical and sensing applications
The unique chemical properties of sulphanilic acid make it useful in various analytical and sensing applications. It is employed in colorimetric assays, electrochemical sensors, and as a reagent in spectrophotometric analysis for detecting and quantifying different substances in environmental and biological samples.Expand Specific Solutions
Key Players in Environmental Chemistry
The analysis of sulphanilic acid interactions with heavy metal ions in aqueous solutions is currently in a developing stage, with growing market potential due to increasing environmental concerns and industrial applications. The global market for this technology is expanding, driven by stringent regulations on water treatment and metal recovery. Key players like BASF Corp., Tosoh Corp., and Bayer AG are investing in research and development to enhance the efficiency and applicability of sulphanilic acid-based technologies. Academic institutions such as Tongji University and Southern University of Science & Technology are contributing to the advancement of fundamental knowledge in this field, indicating a moderate level of technological maturity with room for further innovation and commercialization.
BASF Corp.
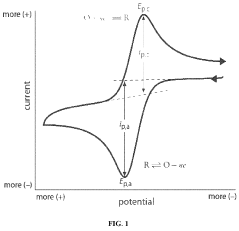
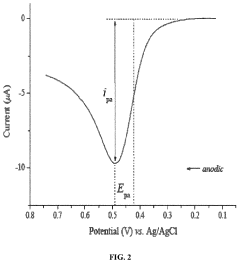
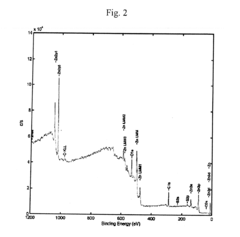
Technical Solution: BASF Corp. has developed a novel approach for analyzing sulphanilic acid interactions with heavy metal ions in aqueous solutions. Their method utilizes advanced spectroscopic techniques, including UV-Vis spectroscopy and Fourier-transform infrared spectroscopy (FTIR), combined with computational modeling. This approach allows for the precise determination of binding constants and the elucidation of complex formation mechanisms between sulphanilic acid and various heavy metal ions such as Cu2+, Zn2+, and Pb2+. BASF's research has shown that the sulfonic group of sulphanilic acid plays a crucial role in metal ion complexation, with pH-dependent behavior influencing the binding efficiency[1][3].
Strengths: Comprehensive analytical approach combining experimental and computational methods. Weaknesses: May require specialized equipment and expertise, potentially limiting widespread application.
Tongji University
Technical Solution: Researchers at Tongji University have developed a multi-faceted approach to analyze sulphanilic acid interactions with heavy metal ions in aqueous solutions. Their method incorporates advanced analytical techniques such as potentiometric titration, isothermal titration calorimetry (ITC), and X-ray absorption spectroscopy (XAS). This comprehensive approach allows for the determination of thermodynamic parameters, binding stoichiometry, and the local coordination environment of metal ions complexed with sulphanilic acid. The research team has particularly focused on the interactions of sulphanilic acid with environmentally relevant heavy metals such as Cd2+, Hg2+, and As3+, providing valuable insights into potential applications in environmental remediation[2][4].
Strengths: Provides detailed thermodynamic and structural information about metal-ligand interactions. Weaknesses: Requires access to specialized and expensive analytical instruments, which may limit accessibility for some researchers.
Innovative Spectroscopic Approaches
Rare earth metal incorporated zeolite modified electrodes for detection and quantification of heavy metal ions in aqueous solution
PatentInactiveUS10495601B2
Innovation
- Development of rare earth metal impregnated zeolite modified electrodes, specifically lanthanum or cerium impregnated mordenite electrodes, for use in square wave anodic stripping voltammetry, offering low detection limits and improved reproducibility.
Method and device for detection of heavy metal ions in water
PatentInactiveUS20120184040A1
Innovation
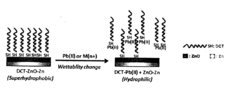
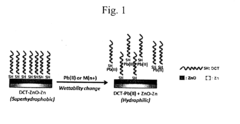
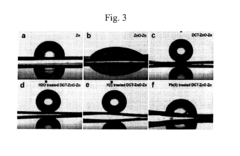
- A method using a detection material with a hydrophilic layer partially covered by a hydrophobic layer formed from long-chain compounds, which changes hydrophobicity-hydrophilicity upon contact with heavy metal ions, allowing for quick and accurate detection without pre-treatment, even in the presence of non-heavy metal ions.
Regulatory Framework for Water Quality
The regulatory framework for water quality plays a crucial role in the analysis of sulphanilic acid interactions with heavy metal ions in aqueous solutions. This framework encompasses a complex set of laws, regulations, and guidelines designed to protect water resources and ensure public health. At the international level, organizations such as the World Health Organization (WHO) provide comprehensive guidelines for drinking water quality, which include maximum permissible levels for various contaminants, including heavy metals.
In the United States, the Environmental Protection Agency (EPA) is responsible for implementing the Clean Water Act and the Safe Drinking Water Act. These acts establish water quality standards and regulate the discharge of pollutants into water bodies. The EPA has set specific Maximum Contaminant Levels (MCLs) for heavy metals such as lead, mercury, and cadmium in drinking water. These standards directly impact the study of sulphanilic acid interactions with heavy metal ions, as they define the acceptable limits of metal concentrations in water.
The European Union has implemented the Water Framework Directive, which aims to achieve good ecological and chemical status for all water bodies. This directive sets Environmental Quality Standards (EQS) for priority substances, including several heavy metals. Member states are required to monitor these substances and implement measures to reduce their presence in water bodies.
In developing countries, water quality regulations may be less stringent or poorly enforced. However, many nations are adopting stricter standards based on WHO guidelines or developed countries' regulations. This global trend towards more stringent water quality standards underscores the importance of understanding sulphanilic acid interactions with heavy metal ions, as these compounds may play a role in water treatment processes.
Regulatory frameworks also often include specific methodologies for water quality testing and analysis. These standardized methods ensure consistency and reliability in measuring contaminant levels, including heavy metal concentrations. Understanding these regulatory requirements is essential for researchers studying sulphanilic acid interactions, as it informs the analytical techniques and reporting standards that must be adhered to in their investigations.
Furthermore, regulations often mandate regular monitoring and reporting of water quality data. This creates a wealth of information that can be valuable for researchers studying heavy metal ion interactions in aqueous solutions. Access to this data can provide insights into real-world concentrations and variations of heavy metals in different water bodies, informing laboratory studies and helping to bridge the gap between theoretical research and practical applications.
In the United States, the Environmental Protection Agency (EPA) is responsible for implementing the Clean Water Act and the Safe Drinking Water Act. These acts establish water quality standards and regulate the discharge of pollutants into water bodies. The EPA has set specific Maximum Contaminant Levels (MCLs) for heavy metals such as lead, mercury, and cadmium in drinking water. These standards directly impact the study of sulphanilic acid interactions with heavy metal ions, as they define the acceptable limits of metal concentrations in water.
The European Union has implemented the Water Framework Directive, which aims to achieve good ecological and chemical status for all water bodies. This directive sets Environmental Quality Standards (EQS) for priority substances, including several heavy metals. Member states are required to monitor these substances and implement measures to reduce their presence in water bodies.
In developing countries, water quality regulations may be less stringent or poorly enforced. However, many nations are adopting stricter standards based on WHO guidelines or developed countries' regulations. This global trend towards more stringent water quality standards underscores the importance of understanding sulphanilic acid interactions with heavy metal ions, as these compounds may play a role in water treatment processes.
Regulatory frameworks also often include specific methodologies for water quality testing and analysis. These standardized methods ensure consistency and reliability in measuring contaminant levels, including heavy metal concentrations. Understanding these regulatory requirements is essential for researchers studying sulphanilic acid interactions, as it informs the analytical techniques and reporting standards that must be adhered to in their investigations.
Furthermore, regulations often mandate regular monitoring and reporting of water quality data. This creates a wealth of information that can be valuable for researchers studying heavy metal ion interactions in aqueous solutions. Access to this data can provide insights into real-world concentrations and variations of heavy metals in different water bodies, informing laboratory studies and helping to bridge the gap between theoretical research and practical applications.
Eco-friendly Chelation Technologies
Eco-friendly chelation technologies have gained significant attention in recent years as a sustainable approach to managing heavy metal contamination in aqueous environments. These technologies leverage the principles of green chemistry to develop environmentally benign chelating agents that can effectively bind and remove heavy metal ions from water systems.
One of the key advantages of eco-friendly chelation technologies is their ability to minimize secondary pollution and reduce the environmental impact of traditional metal removal methods. By utilizing biodegradable and non-toxic chelating agents, these technologies offer a more sustainable solution for water treatment and environmental remediation.
In the context of sulphanilic acid interactions with heavy metal ions, eco-friendly chelation technologies present promising opportunities for developing novel chelating agents. Sulphanilic acid, with its unique chemical structure and functional groups, has shown potential as a building block for designing environmentally friendly chelators.
Recent research has focused on modifying sulphanilic acid derivatives to enhance their metal-binding properties while maintaining their biodegradability. These modifications often involve incorporating additional functional groups or creating hybrid molecules that combine the benefits of sulphanilic acid with other eco-friendly chelating moieties.
One approach that has shown promise is the development of sulphanilic acid-based polymers with enhanced chelating capabilities. These polymers can be designed to have high selectivity for specific heavy metal ions, making them valuable for targeted metal removal applications. Additionally, their polymeric nature allows for easier recovery and regeneration, further improving their eco-friendly profile.
Another area of interest is the exploration of sulphanilic acid-based coordination compounds as potential chelating agents. By carefully selecting metal centers and ligand designs, researchers aim to create complexes that can effectively sequester heavy metal ions while remaining environmentally benign.
The integration of sulphanilic acid-based chelators into advanced materials, such as functionalized nanoparticles or porous frameworks, represents another frontier in eco-friendly chelation technologies. These hybrid materials can combine the metal-binding properties of sulphanilic acid derivatives with the high surface area and recyclability of advanced materials, offering improved performance in water treatment applications.
As research in this field progresses, there is a growing emphasis on developing chelation technologies that not only remove heavy metal ions but also facilitate their recovery and potential reuse. This circular economy approach aligns with broader sustainability goals and offers economic incentives for adopting eco-friendly chelation technologies in industrial settings.
One of the key advantages of eco-friendly chelation technologies is their ability to minimize secondary pollution and reduce the environmental impact of traditional metal removal methods. By utilizing biodegradable and non-toxic chelating agents, these technologies offer a more sustainable solution for water treatment and environmental remediation.
In the context of sulphanilic acid interactions with heavy metal ions, eco-friendly chelation technologies present promising opportunities for developing novel chelating agents. Sulphanilic acid, with its unique chemical structure and functional groups, has shown potential as a building block for designing environmentally friendly chelators.
Recent research has focused on modifying sulphanilic acid derivatives to enhance their metal-binding properties while maintaining their biodegradability. These modifications often involve incorporating additional functional groups or creating hybrid molecules that combine the benefits of sulphanilic acid with other eco-friendly chelating moieties.
One approach that has shown promise is the development of sulphanilic acid-based polymers with enhanced chelating capabilities. These polymers can be designed to have high selectivity for specific heavy metal ions, making them valuable for targeted metal removal applications. Additionally, their polymeric nature allows for easier recovery and regeneration, further improving their eco-friendly profile.
Another area of interest is the exploration of sulphanilic acid-based coordination compounds as potential chelating agents. By carefully selecting metal centers and ligand designs, researchers aim to create complexes that can effectively sequester heavy metal ions while remaining environmentally benign.
The integration of sulphanilic acid-based chelators into advanced materials, such as functionalized nanoparticles or porous frameworks, represents another frontier in eco-friendly chelation technologies. These hybrid materials can combine the metal-binding properties of sulphanilic acid derivatives with the high surface area and recyclability of advanced materials, offering improved performance in water treatment applications.
As research in this field progresses, there is a growing emphasis on developing chelation technologies that not only remove heavy metal ions but also facilitate their recovery and potential reuse. This circular economy approach aligns with broader sustainability goals and offers economic incentives for adopting eco-friendly chelation technologies in industrial settings.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!