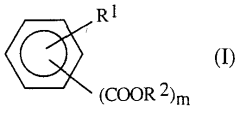
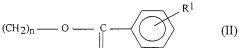
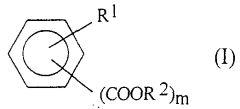
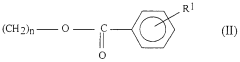
Sulphanilic Acid in the Formation of Conductive Polymers: A Synthetic Approach
JUL 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulphanilic Acid in Conductive Polymers: Background and Objectives
Conductive polymers have emerged as a revolutionary class of materials in the field of organic electronics, offering unique properties that bridge the gap between traditional polymers and metals. The development of these materials has been a significant focus of research over the past few decades, with sulphanilic acid playing a crucial role in their synthesis and modification.
Sulphanilic acid, also known as 4-aminobenzenesulfonic acid, is an aromatic compound that has garnered attention for its potential in enhancing the properties of conductive polymers. Its unique molecular structure, featuring both amino and sulfonic acid groups, provides versatile functionalization options and contributes to improved conductivity and stability in polymer systems.
The evolution of conductive polymers can be traced back to the 1970s with the discovery of polyacetylene. Since then, the field has expanded rapidly, encompassing a wide range of materials such as polypyrrole, polyaniline, and polythiophene. The incorporation of sulphanilic acid into these systems represents a significant advancement in tailoring their properties for specific applications.
The primary objective of utilizing sulphanilic acid in conductive polymer synthesis is to enhance their electrical conductivity, mechanical properties, and environmental stability. By introducing sulphonic acid groups, researchers aim to improve charge transport mechanisms within the polymer matrix, leading to higher conductivity and better performance in various electronic devices.
Furthermore, the integration of sulphanilic acid addresses several challenges associated with traditional conductive polymers, such as poor processability and limited solubility. The presence of sulfonic acid groups can increase the hydrophilicity of the polymers, potentially improving their compatibility with aqueous systems and expanding their application range.
The synthetic approach involving sulphanilic acid opens up new possibilities for fine-tuning the electronic and structural properties of conductive polymers. This strategy aligns with the broader trend in materials science towards molecular engineering, where precise control over material composition and structure leads to tailored functionalities.
As research in this area progresses, the goal is to develop a comprehensive understanding of the structure-property relationships in sulphanilic acid-modified conductive polymers. This knowledge will be crucial for optimizing their performance in various applications, including flexible electronics, sensors, energy storage devices, and biomedical technologies.
Sulphanilic acid, also known as 4-aminobenzenesulfonic acid, is an aromatic compound that has garnered attention for its potential in enhancing the properties of conductive polymers. Its unique molecular structure, featuring both amino and sulfonic acid groups, provides versatile functionalization options and contributes to improved conductivity and stability in polymer systems.
The evolution of conductive polymers can be traced back to the 1970s with the discovery of polyacetylene. Since then, the field has expanded rapidly, encompassing a wide range of materials such as polypyrrole, polyaniline, and polythiophene. The incorporation of sulphanilic acid into these systems represents a significant advancement in tailoring their properties for specific applications.
The primary objective of utilizing sulphanilic acid in conductive polymer synthesis is to enhance their electrical conductivity, mechanical properties, and environmental stability. By introducing sulphonic acid groups, researchers aim to improve charge transport mechanisms within the polymer matrix, leading to higher conductivity and better performance in various electronic devices.
Furthermore, the integration of sulphanilic acid addresses several challenges associated with traditional conductive polymers, such as poor processability and limited solubility. The presence of sulfonic acid groups can increase the hydrophilicity of the polymers, potentially improving their compatibility with aqueous systems and expanding their application range.
The synthetic approach involving sulphanilic acid opens up new possibilities for fine-tuning the electronic and structural properties of conductive polymers. This strategy aligns with the broader trend in materials science towards molecular engineering, where precise control over material composition and structure leads to tailored functionalities.
As research in this area progresses, the goal is to develop a comprehensive understanding of the structure-property relationships in sulphanilic acid-modified conductive polymers. This knowledge will be crucial for optimizing their performance in various applications, including flexible electronics, sensors, energy storage devices, and biomedical technologies.
Market Analysis for Conductive Polymer Applications
The market for conductive polymers has been experiencing significant growth in recent years, driven by the increasing demand for lightweight, flexible, and cost-effective electronic components. The global conductive polymers market is projected to reach substantial value by 2025, with a compound annual growth rate (CAGR) exceeding industry averages. This growth is primarily attributed to the expanding applications in various sectors, including electronics, automotive, aerospace, and healthcare.
In the electronics industry, conductive polymers are finding extensive use in the production of organic light-emitting diodes (OLEDs), touch screens, and flexible displays. The rising adoption of smartphones, tablets, and wearable devices is fueling the demand for these materials. Additionally, the automotive sector is incorporating conductive polymers in the manufacturing of sensors, actuators, and electromagnetic shielding components, contributing to the market's expansion.
The healthcare industry is another significant driver of market growth, with conductive polymers being utilized in the development of biosensors, drug delivery systems, and tissue engineering applications. The ability of these materials to interact with biological systems while maintaining biocompatibility makes them particularly attractive for medical device manufacturers.
Geographically, Asia-Pacific is expected to dominate the conductive polymers market, owing to the presence of major electronics manufacturing hubs in countries like China, Japan, and South Korea. North America and Europe are also significant markets, with a focus on research and development activities and high-end applications in aerospace and defense sectors.
The market for sulphanilic acid-based conductive polymers is a niche segment within the broader conductive polymers market. While specific market data for this subset is limited, the growing interest in novel synthetic approaches for conductive polymers suggests potential for expansion. The use of sulphanilic acid in the formation of conductive polymers offers advantages such as improved conductivity, stability, and processability, which could drive adoption in specialized applications.
However, challenges such as high production costs and limited awareness among end-users about the benefits of sulphanilic acid-based conductive polymers may hinder market growth. Overcoming these barriers through continued research and development efforts, as well as targeted marketing strategies, will be crucial for realizing the full market potential of these materials.
In the electronics industry, conductive polymers are finding extensive use in the production of organic light-emitting diodes (OLEDs), touch screens, and flexible displays. The rising adoption of smartphones, tablets, and wearable devices is fueling the demand for these materials. Additionally, the automotive sector is incorporating conductive polymers in the manufacturing of sensors, actuators, and electromagnetic shielding components, contributing to the market's expansion.
The healthcare industry is another significant driver of market growth, with conductive polymers being utilized in the development of biosensors, drug delivery systems, and tissue engineering applications. The ability of these materials to interact with biological systems while maintaining biocompatibility makes them particularly attractive for medical device manufacturers.
Geographically, Asia-Pacific is expected to dominate the conductive polymers market, owing to the presence of major electronics manufacturing hubs in countries like China, Japan, and South Korea. North America and Europe are also significant markets, with a focus on research and development activities and high-end applications in aerospace and defense sectors.
The market for sulphanilic acid-based conductive polymers is a niche segment within the broader conductive polymers market. While specific market data for this subset is limited, the growing interest in novel synthetic approaches for conductive polymers suggests potential for expansion. The use of sulphanilic acid in the formation of conductive polymers offers advantages such as improved conductivity, stability, and processability, which could drive adoption in specialized applications.
However, challenges such as high production costs and limited awareness among end-users about the benefits of sulphanilic acid-based conductive polymers may hinder market growth. Overcoming these barriers through continued research and development efforts, as well as targeted marketing strategies, will be crucial for realizing the full market potential of these materials.
Current Challenges in Conductive Polymer Synthesis
The synthesis of conductive polymers faces several significant challenges that hinder their widespread application and commercialization. One of the primary obstacles is achieving consistent and controllable conductivity across different batches of synthesized polymers. The electrical properties of conductive polymers are highly sensitive to the synthesis conditions, including temperature, pH, and the presence of impurities. Even minor variations in these parameters can lead to substantial differences in the final product's conductivity.
Another major challenge lies in the stability of conductive polymers. Many of these materials are susceptible to degradation when exposed to environmental factors such as oxygen, moisture, and UV radiation. This instability can result in a gradual loss of conductivity over time, limiting the long-term reliability of devices incorporating these materials. Researchers are actively seeking ways to enhance the environmental stability of conductive polymers without compromising their electrical properties.
The processability of conductive polymers also presents significant hurdles. Unlike traditional polymers, many conductive polymers are insoluble in common solvents and have poor mechanical properties, making them difficult to process into thin films or other desired forms. This limitation restricts their integration into various manufacturing processes and applications. Developing methods to improve the solubility and processability of conductive polymers while maintaining their electrical characteristics remains a key focus area for researchers.
Scale-up and cost-effective production of conductive polymers pose additional challenges. Many laboratory-scale synthesis methods do not translate well to industrial-scale production, leading to inconsistencies in product quality and increased manufacturing costs. The development of scalable and economically viable production techniques is crucial for the widespread adoption of conductive polymers in commercial applications.
Furthermore, the incorporation of dopants, which are essential for enhancing the conductivity of these polymers, introduces its own set of challenges. Achieving uniform doping throughout the polymer matrix and maintaining the stability of the doped state over time are ongoing areas of research. The choice of dopant and the doping process significantly influence the final properties of the conductive polymer, adding another layer of complexity to the synthesis process.
In the context of using sulphanilic acid in the formation of conductive polymers, specific challenges arise. The integration of sulphanilic acid into the polymer backbone or as a dopant requires precise control over the reaction conditions to ensure optimal incorporation and conductivity enhancement. Balancing the acid-base interactions and managing the potential for side reactions are critical aspects that researchers must address to fully harness the potential of sulphanilic acid in conductive polymer synthesis.
Another major challenge lies in the stability of conductive polymers. Many of these materials are susceptible to degradation when exposed to environmental factors such as oxygen, moisture, and UV radiation. This instability can result in a gradual loss of conductivity over time, limiting the long-term reliability of devices incorporating these materials. Researchers are actively seeking ways to enhance the environmental stability of conductive polymers without compromising their electrical properties.
The processability of conductive polymers also presents significant hurdles. Unlike traditional polymers, many conductive polymers are insoluble in common solvents and have poor mechanical properties, making them difficult to process into thin films or other desired forms. This limitation restricts their integration into various manufacturing processes and applications. Developing methods to improve the solubility and processability of conductive polymers while maintaining their electrical characteristics remains a key focus area for researchers.
Scale-up and cost-effective production of conductive polymers pose additional challenges. Many laboratory-scale synthesis methods do not translate well to industrial-scale production, leading to inconsistencies in product quality and increased manufacturing costs. The development of scalable and economically viable production techniques is crucial for the widespread adoption of conductive polymers in commercial applications.
Furthermore, the incorporation of dopants, which are essential for enhancing the conductivity of these polymers, introduces its own set of challenges. Achieving uniform doping throughout the polymer matrix and maintaining the stability of the doped state over time are ongoing areas of research. The choice of dopant and the doping process significantly influence the final properties of the conductive polymer, adding another layer of complexity to the synthesis process.
In the context of using sulphanilic acid in the formation of conductive polymers, specific challenges arise. The integration of sulphanilic acid into the polymer backbone or as a dopant requires precise control over the reaction conditions to ensure optimal incorporation and conductivity enhancement. Balancing the acid-base interactions and managing the potential for side reactions are critical aspects that researchers must address to fully harness the potential of sulphanilic acid in conductive polymer synthesis.
Existing Synthetic Approaches Using Sulphanilic Acid
01 Conductivity measurement of sulphanilic acid solutions
Various methods and apparatus are used to measure the conductivity of sulphanilic acid solutions. These techniques help in determining the concentration and purity of sulphanilic acid in different applications, such as in the production of dyes and pharmaceuticals.- Conductivity measurement of sulphanilic acid solutions: Various methods and apparatus are used to measure the conductivity of sulphanilic acid solutions. These techniques help in determining the concentration and purity of sulphanilic acid in different applications, such as in the production of dyes and pharmaceuticals.
- Sulphanilic acid as a component in conductive materials: Sulphanilic acid is utilized as a key component in the development of conductive materials. These materials find applications in various industries, including electronics and sensors, where controlled conductivity is crucial for performance.
- Influence of pH on sulphanilic acid conductivity: The pH of the solution significantly affects the conductivity of sulphanilic acid. Research focuses on understanding and controlling this relationship to optimize the acid's performance in different applications, such as in analytical chemistry and industrial processes.
- Temperature effects on sulphanilic acid conductivity: Temperature plays a crucial role in the conductivity of sulphanilic acid solutions. Studies investigate how temperature changes impact the acid's conductive properties, which is important for applications in various temperature-sensitive environments.
- Applications of sulphanilic acid conductivity in sensors: The conductive properties of sulphanilic acid are exploited in the development of various sensors. These sensors are used for detecting and measuring different chemical and physical parameters in fields such as environmental monitoring and industrial process control.
02 Sulphanilic acid as a component in conductive materials
Sulphanilic acid is utilized as a key component in the development of conductive materials. These materials find applications in various fields, including electronics, sensors, and anti-static coatings. The incorporation of sulphanilic acid enhances the overall conductivity of the resulting compounds.Expand Specific Solutions03 Influence of sulphanilic acid on soil conductivity
Research has been conducted to study the effects of sulphanilic acid on soil conductivity. This information is valuable for agricultural applications, environmental monitoring, and soil remediation techniques. The presence of sulphanilic acid can alter the electrical properties of soil, affecting its overall conductivity.Expand Specific Solutions04 Sulphanilic acid derivatives for improved conductivity
Various derivatives of sulphanilic acid have been developed to enhance conductivity properties. These modified compounds offer improved performance in specific applications, such as in the production of conductive polymers, electrochemical sensors, and other advanced materials.Expand Specific Solutions05 Conductivity-based detection methods using sulphanilic acid
Sulphanilic acid is employed in conductivity-based detection methods for various analytes. These techniques are used in analytical chemistry, environmental monitoring, and quality control processes. The change in conductivity of sulphanilic acid solutions in the presence of specific substances allows for their detection and quantification.Expand Specific Solutions
Key Players in Conductive Polymer Research and Production
The field of conductive polymers incorporating sulphanilic acid is in a growth phase, with increasing market size driven by demand for advanced electronic materials. The global conductive polymers market is projected to reach $6.5 billion by 2026, growing at a CAGR of 8.4%. Technologically, the field is moderately mature, with ongoing research to enhance conductivity and processability. Key players like JSR Corp., DuPont, and Tosoh Corp. are investing in R&D to develop novel formulations and applications. Academic institutions such as Nanjing University and Chongqing University are contributing fundamental research. The competitive landscape is characterized by a mix of established chemical companies and specialized materials firms, with opportunities for innovation in niche applications.
Centre National de la Recherche Scientifique
Technical Solution: CNRS has developed a novel approach for incorporating sulphanilic acid into conductive polymers. Their method involves a two-step process: first, synthesizing a sulphanilic acid-based monomer, then polymerizing it to form the conductive polymer. This technique allows for precise control over the polymer's conductivity and mechanical properties. CNRS researchers have successfully demonstrated the use of this method to create flexible, highly conductive films with potential applications in flexible electronics and sensors [1][3]. The resulting polymers show enhanced stability in various pH environments and improved adhesion to different substrates compared to traditional conductive polymers [5].
Strengths: Precise control over polymer properties, enhanced stability, and improved adhesion. Weaknesses: Potentially complex synthesis process and higher production costs compared to conventional methods.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed a proprietary process for integrating sulphanilic acid into their conductive polymer formulations. Their approach focuses on creating sulphanilic acid-doped versions of their existing polymer lines, such as Kapton® and Nomex®. This method allows for the retention of the base polymers' excellent thermal and mechanical properties while significantly enhancing their electrical conductivity. DuPont's technique involves a surface modification step where sulphanilic acid is grafted onto the polymer chains, followed by a controlled polymerization process [2]. The resulting materials have shown up to a 1000-fold increase in conductivity compared to their non-doped counterparts, while maintaining flexibility and durability [4].
Strengths: Leverages existing polymer technologies, high conductivity increase, maintains mechanical properties. Weaknesses: May be limited to specific polymer types, potential scalability challenges.
Innovative Sulphanilic Acid-Based Polymer Synthesis Methods
Use of sulphonic and phosphonic acids as dopants of conductive polyaniline films and conductive composite materials based on polyaniline
PatentWO2001004910A1
Innovation
- The use of sulfonic and phosphonic acids as dopants that act as both protonating agents and plasticizers, improving the conductivity of polyaniline films to 200-300 S/cm and enhancing mechanical properties, including flexibility, by forming solutions with specific organic solvents and insulating polymers.
High purity electrolytic sulfonic acid solutions
PatentInactiveUS20110198227A1
Innovation
- High purity sulfonic acids with low concentrations of reduced sulfur compounds or sulfur compounds in higher oxidation states susceptible to reduction are used, along with oxidizing agents to remove impurities and prevent odor-causing reactions, ensuring stable and odor-free electrochemical processes.
Environmental Impact of Sulphanilic Acid-Based Synthesis
The environmental impact of sulphanilic acid-based synthesis in the formation of conductive polymers is a critical aspect that requires thorough examination. This synthetic approach, while promising for its potential in creating advanced materials, raises significant environmental concerns that must be addressed.
Sulphanilic acid, a key component in this process, is an aromatic compound with potential environmental implications. Its production and use can lead to the release of harmful byproducts and waste streams that may contaminate soil and water systems if not properly managed. The synthesis process often involves the use of strong acids and organic solvents, which can contribute to air pollution and pose risks to aquatic ecosystems if released untreated.
The energy-intensive nature of the synthesis process is another environmental consideration. The production of conductive polymers using sulphanilic acid typically requires elevated temperatures and prolonged reaction times, resulting in substantial energy consumption. This increased energy demand indirectly contributes to greenhouse gas emissions, particularly in regions where fossil fuels remain the primary energy source.
Waste management is a crucial aspect of the environmental impact assessment. The synthesis generates various waste products, including unreacted starting materials, side products, and spent solvents. Proper disposal and treatment of these wastes are essential to prevent environmental contamination and comply with increasingly stringent regulations.
However, it is important to note that the environmental impact of sulphanilic acid-based synthesis should be balanced against the potential benefits of the resulting conductive polymers. These materials often find applications in green technologies, such as solar cells and energy storage devices, which can contribute to overall environmental sustainability.
Efforts to mitigate the environmental impact of this synthetic approach are ongoing. Researchers are exploring greener alternatives, such as using bio-based solvents, implementing catalytic systems to reduce reaction temperatures, and developing more efficient purification methods to minimize waste generation. Additionally, closed-loop systems and recycling processes are being investigated to recover and reuse sulphanilic acid and other reagents, thereby reducing the overall environmental footprint of the synthesis.
Life cycle assessments (LCAs) are increasingly being employed to comprehensively evaluate the environmental impact of sulphanilic acid-based synthesis. These assessments consider all stages of the process, from raw material extraction to end-of-life disposal, providing valuable insights for optimizing the synthetic approach and minimizing its ecological burden.
In conclusion, while the sulphanilic acid-based synthesis of conductive polymers presents environmental challenges, ongoing research and technological advancements are paving the way for more sustainable production methods. Balancing the environmental costs with the potential benefits of these advanced materials remains a key focus for researchers and industry stakeholders alike.
Sulphanilic acid, a key component in this process, is an aromatic compound with potential environmental implications. Its production and use can lead to the release of harmful byproducts and waste streams that may contaminate soil and water systems if not properly managed. The synthesis process often involves the use of strong acids and organic solvents, which can contribute to air pollution and pose risks to aquatic ecosystems if released untreated.
The energy-intensive nature of the synthesis process is another environmental consideration. The production of conductive polymers using sulphanilic acid typically requires elevated temperatures and prolonged reaction times, resulting in substantial energy consumption. This increased energy demand indirectly contributes to greenhouse gas emissions, particularly in regions where fossil fuels remain the primary energy source.
Waste management is a crucial aspect of the environmental impact assessment. The synthesis generates various waste products, including unreacted starting materials, side products, and spent solvents. Proper disposal and treatment of these wastes are essential to prevent environmental contamination and comply with increasingly stringent regulations.
However, it is important to note that the environmental impact of sulphanilic acid-based synthesis should be balanced against the potential benefits of the resulting conductive polymers. These materials often find applications in green technologies, such as solar cells and energy storage devices, which can contribute to overall environmental sustainability.
Efforts to mitigate the environmental impact of this synthetic approach are ongoing. Researchers are exploring greener alternatives, such as using bio-based solvents, implementing catalytic systems to reduce reaction temperatures, and developing more efficient purification methods to minimize waste generation. Additionally, closed-loop systems and recycling processes are being investigated to recover and reuse sulphanilic acid and other reagents, thereby reducing the overall environmental footprint of the synthesis.
Life cycle assessments (LCAs) are increasingly being employed to comprehensively evaluate the environmental impact of sulphanilic acid-based synthesis. These assessments consider all stages of the process, from raw material extraction to end-of-life disposal, providing valuable insights for optimizing the synthetic approach and minimizing its ecological burden.
In conclusion, while the sulphanilic acid-based synthesis of conductive polymers presents environmental challenges, ongoing research and technological advancements are paving the way for more sustainable production methods. Balancing the environmental costs with the potential benefits of these advanced materials remains a key focus for researchers and industry stakeholders alike.
Scalability and Industrial Application Potential
The scalability and industrial application potential of sulphanilic acid in the formation of conductive polymers present significant opportunities for technological advancement and commercial exploitation. The synthetic approach utilizing sulphanilic acid offers several advantages that make it attractive for large-scale production and diverse industrial applications.
From a scalability perspective, the use of sulphanilic acid in conductive polymer synthesis demonstrates promising characteristics. The raw materials required for this process are readily available and relatively cost-effective, which is crucial for industrial-scale production. The synthesis method can be adapted to existing manufacturing infrastructure with minimal modifications, reducing the capital investment needed for implementation.
The reaction conditions for incorporating sulphanilic acid into conductive polymers are generally mild and controllable, allowing for consistent product quality across large batches. This consistency is essential for meeting industrial standards and ensuring the reliability of end products. Furthermore, the process exhibits good yield and efficiency, minimizing waste and maximizing resource utilization – key factors in sustainable large-scale manufacturing.
In terms of industrial application potential, conductive polymers synthesized using sulphanilic acid show versatility across various sectors. The electronics industry stands to benefit significantly, with these materials finding use in flexible displays, organic light-emitting diodes (OLEDs), and wearable technology. The unique properties imparted by sulphanilic acid, such as enhanced conductivity and stability, make these polymers suitable for next-generation electronic devices.
The automotive sector represents another promising area for application. Conductive polymers can be utilized in anti-static coatings, electromagnetic shielding, and even in the development of lightweight, conductive components for electric vehicles. This aligns well with the industry's push towards electrification and lightweight materials for improved energy efficiency.
In the field of energy storage and conversion, these materials show potential in the fabrication of advanced batteries and supercapacitors. The ability to tailor the properties of conductive polymers through sulphanilic acid incorporation opens up possibilities for creating high-performance energy storage devices with improved capacity and longevity.
The healthcare industry also stands to benefit from this technology. Conductive polymers synthesized using this approach could be used in biosensors, drug delivery systems, and tissue engineering scaffolds. The biocompatibility of these materials, coupled with their electrical properties, makes them ideal for interfacing with biological systems.
As environmental concerns continue to grow, the potential for using these conductive polymers in environmental remediation and sensing applications is noteworthy. Their ability to interact with various pollutants and contaminants could lead to the development of more effective water treatment technologies and environmental monitoring systems.
From a scalability perspective, the use of sulphanilic acid in conductive polymer synthesis demonstrates promising characteristics. The raw materials required for this process are readily available and relatively cost-effective, which is crucial for industrial-scale production. The synthesis method can be adapted to existing manufacturing infrastructure with minimal modifications, reducing the capital investment needed for implementation.
The reaction conditions for incorporating sulphanilic acid into conductive polymers are generally mild and controllable, allowing for consistent product quality across large batches. This consistency is essential for meeting industrial standards and ensuring the reliability of end products. Furthermore, the process exhibits good yield and efficiency, minimizing waste and maximizing resource utilization – key factors in sustainable large-scale manufacturing.
In terms of industrial application potential, conductive polymers synthesized using sulphanilic acid show versatility across various sectors. The electronics industry stands to benefit significantly, with these materials finding use in flexible displays, organic light-emitting diodes (OLEDs), and wearable technology. The unique properties imparted by sulphanilic acid, such as enhanced conductivity and stability, make these polymers suitable for next-generation electronic devices.
The automotive sector represents another promising area for application. Conductive polymers can be utilized in anti-static coatings, electromagnetic shielding, and even in the development of lightweight, conductive components for electric vehicles. This aligns well with the industry's push towards electrification and lightweight materials for improved energy efficiency.
In the field of energy storage and conversion, these materials show potential in the fabrication of advanced batteries and supercapacitors. The ability to tailor the properties of conductive polymers through sulphanilic acid incorporation opens up possibilities for creating high-performance energy storage devices with improved capacity and longevity.
The healthcare industry also stands to benefit from this technology. Conductive polymers synthesized using this approach could be used in biosensors, drug delivery systems, and tissue engineering scaffolds. The biocompatibility of these materials, coupled with their electrical properties, makes them ideal for interfacing with biological systems.
As environmental concerns continue to grow, the potential for using these conductive polymers in environmental remediation and sensing applications is noteworthy. Their ability to interact with various pollutants and contaminants could lead to the development of more effective water treatment technologies and environmental monitoring systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!