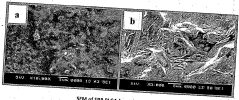
Examining Sulphanilic Acid-Incorporated Nanostructures for Drug Delivery Applications
JUL 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulphanilic Acid Nanostructures: Background and Objectives
Sulphanilic acid-incorporated nanostructures have emerged as a promising field in drug delivery applications, representing a convergence of nanotechnology and pharmaceutical sciences. This innovative approach leverages the unique properties of sulphanilic acid, a compound known for its versatile chemical reactivity and biocompatibility, to enhance drug delivery systems at the nanoscale.
The development of these nanostructures is rooted in the broader context of nanomedicine, which has seen exponential growth over the past two decades. As researchers sought to overcome limitations in traditional drug delivery methods, such as poor solubility, limited bioavailability, and undesired side effects, nanostructured carriers emerged as a potential solution.
Sulphanilic acid, with its aromatic amine and sulfonic acid functional groups, offers unique opportunities for molecular interactions and modifications. Its incorporation into nanostructures aims to exploit these properties to create more effective and targeted drug delivery vehicles.
The primary objectives of research in this field are multifaceted. Firstly, there is a focus on enhancing drug loading capacity and release kinetics through the strategic integration of sulphanilic acid into various nanostructures, such as nanoparticles, nanogels, and nanofibers. This aims to improve the therapeutic index of drugs by increasing their concentration at target sites while minimizing systemic exposure.
Secondly, researchers are exploring the potential of sulphanilic acid-incorporated nanostructures to overcome biological barriers. The unique surface properties imparted by sulphanilic acid may enhance the nanostructures' ability to penetrate cell membranes or cross the blood-brain barrier, opening new avenues for treating challenging conditions like brain tumors or neurodegenerative diseases.
Another key objective is to develop stimuli-responsive drug delivery systems. The pH-sensitive nature of sulphanilic acid could be exploited to create nanostructures that selectively release their payload in specific physiological environments, such as the acidic milieu of tumor tissues or the varying pH along the gastrointestinal tract.
Furthermore, there is growing interest in utilizing sulphanilic acid-incorporated nanostructures for theranostic applications, combining therapeutic delivery with diagnostic capabilities. This could potentially lead to more personalized and effective treatment strategies, allowing real-time monitoring of drug distribution and efficacy.
As research in this field progresses, it is anticipated that these nanostructures will play a crucial role in addressing current challenges in drug delivery and pave the way for more sophisticated, efficient, and patient-friendly therapeutic interventions. The ongoing technological advancements and interdisciplinary collaborations in this area promise to yield innovative solutions that could significantly impact the future of healthcare and personalized medicine.
The development of these nanostructures is rooted in the broader context of nanomedicine, which has seen exponential growth over the past two decades. As researchers sought to overcome limitations in traditional drug delivery methods, such as poor solubility, limited bioavailability, and undesired side effects, nanostructured carriers emerged as a potential solution.
Sulphanilic acid, with its aromatic amine and sulfonic acid functional groups, offers unique opportunities for molecular interactions and modifications. Its incorporation into nanostructures aims to exploit these properties to create more effective and targeted drug delivery vehicles.
The primary objectives of research in this field are multifaceted. Firstly, there is a focus on enhancing drug loading capacity and release kinetics through the strategic integration of sulphanilic acid into various nanostructures, such as nanoparticles, nanogels, and nanofibers. This aims to improve the therapeutic index of drugs by increasing their concentration at target sites while minimizing systemic exposure.
Secondly, researchers are exploring the potential of sulphanilic acid-incorporated nanostructures to overcome biological barriers. The unique surface properties imparted by sulphanilic acid may enhance the nanostructures' ability to penetrate cell membranes or cross the blood-brain barrier, opening new avenues for treating challenging conditions like brain tumors or neurodegenerative diseases.
Another key objective is to develop stimuli-responsive drug delivery systems. The pH-sensitive nature of sulphanilic acid could be exploited to create nanostructures that selectively release their payload in specific physiological environments, such as the acidic milieu of tumor tissues or the varying pH along the gastrointestinal tract.
Furthermore, there is growing interest in utilizing sulphanilic acid-incorporated nanostructures for theranostic applications, combining therapeutic delivery with diagnostic capabilities. This could potentially lead to more personalized and effective treatment strategies, allowing real-time monitoring of drug distribution and efficacy.
As research in this field progresses, it is anticipated that these nanostructures will play a crucial role in addressing current challenges in drug delivery and pave the way for more sophisticated, efficient, and patient-friendly therapeutic interventions. The ongoing technological advancements and interdisciplinary collaborations in this area promise to yield innovative solutions that could significantly impact the future of healthcare and personalized medicine.
Market Analysis for Nanostructure-Based Drug Delivery
The market for nanostructure-based drug delivery systems has been experiencing significant growth in recent years, driven by the increasing demand for targeted and efficient drug delivery methods. This market segment is expected to continue its upward trajectory, with a compound annual growth rate projected to remain in the double digits through 2028.
The adoption of nanostructure-based drug delivery systems is particularly prominent in oncology, where the need for precise targeting of cancer cells while minimizing damage to healthy tissues is crucial. Other therapeutic areas showing substantial market potential include cardiovascular diseases, neurological disorders, and infectious diseases.
Geographically, North America currently leads the market, owing to its advanced healthcare infrastructure and substantial investments in research and development. However, the Asia-Pacific region is anticipated to witness the fastest growth rate in the coming years, fueled by increasing healthcare expenditure, growing awareness of advanced drug delivery technologies, and a rising prevalence of chronic diseases.
Key factors driving market growth include the ability of nanostructure-based systems to enhance drug solubility, improve bioavailability, and enable controlled release profiles. These advantages address many of the limitations associated with conventional drug delivery methods, making nanostructure-based systems increasingly attractive to pharmaceutical companies and healthcare providers.
The incorporation of sulphanilic acid into nanostructures for drug delivery applications represents a niche but promising segment within this market. Sulphanilic acid's unique properties, including its potential for improving drug stability and enhancing targeted delivery, align well with the broader trends in the nanostructure-based drug delivery market.
However, challenges such as high development costs, complex regulatory pathways, and potential safety concerns related to long-term use of nanoparticles in the body may impact market growth. These factors necessitate ongoing research and clinical trials to establish the safety and efficacy of sulphanilic acid-incorporated nanostructures for drug delivery.
Despite these challenges, the market outlook remains positive. The increasing focus on personalized medicine and the growing pipeline of biologic drugs that require advanced delivery systems are expected to create new opportunities for nanostructure-based drug delivery technologies, including those incorporating sulphanilic acid.
The adoption of nanostructure-based drug delivery systems is particularly prominent in oncology, where the need for precise targeting of cancer cells while minimizing damage to healthy tissues is crucial. Other therapeutic areas showing substantial market potential include cardiovascular diseases, neurological disorders, and infectious diseases.
Geographically, North America currently leads the market, owing to its advanced healthcare infrastructure and substantial investments in research and development. However, the Asia-Pacific region is anticipated to witness the fastest growth rate in the coming years, fueled by increasing healthcare expenditure, growing awareness of advanced drug delivery technologies, and a rising prevalence of chronic diseases.
Key factors driving market growth include the ability of nanostructure-based systems to enhance drug solubility, improve bioavailability, and enable controlled release profiles. These advantages address many of the limitations associated with conventional drug delivery methods, making nanostructure-based systems increasingly attractive to pharmaceutical companies and healthcare providers.
The incorporation of sulphanilic acid into nanostructures for drug delivery applications represents a niche but promising segment within this market. Sulphanilic acid's unique properties, including its potential for improving drug stability and enhancing targeted delivery, align well with the broader trends in the nanostructure-based drug delivery market.
However, challenges such as high development costs, complex regulatory pathways, and potential safety concerns related to long-term use of nanoparticles in the body may impact market growth. These factors necessitate ongoing research and clinical trials to establish the safety and efficacy of sulphanilic acid-incorporated nanostructures for drug delivery.
Despite these challenges, the market outlook remains positive. The increasing focus on personalized medicine and the growing pipeline of biologic drugs that require advanced delivery systems are expected to create new opportunities for nanostructure-based drug delivery technologies, including those incorporating sulphanilic acid.
Current Challenges in Sulphanilic Acid Nanostructure Development
The development of sulphanilic acid-incorporated nanostructures for drug delivery applications faces several significant challenges that hinder their widespread adoption and clinical translation. One of the primary obstacles is the complexity of designing nanostructures that can effectively encapsulate and deliver sulphanilic acid while maintaining its therapeutic efficacy. The chemical properties of sulphanilic acid, including its solubility and stability, pose difficulties in achieving optimal drug loading and controlled release profiles.
Another major challenge lies in the biocompatibility and biodegradability of the nanostructures. Ensuring that these drug delivery systems are non-toxic and can be safely metabolized by the body without causing adverse effects is crucial. This requires extensive in vitro and in vivo testing, which can be time-consuming and resource-intensive. Additionally, the potential long-term effects of these nanostructures on human health and the environment remain a concern that needs to be thoroughly addressed.
The scalability of production processes for sulphanilic acid-incorporated nanostructures presents another significant hurdle. Developing manufacturing methods that can consistently produce high-quality nanostructures with uniform size, shape, and drug loading capacity at an industrial scale is challenging. This issue is compounded by the need to maintain batch-to-batch consistency and meet regulatory standards for pharmaceutical production.
Targeting and delivery efficiency are also areas of ongoing research and development. Creating nanostructures that can selectively target specific tissues or cells while minimizing off-target effects remains a complex task. This challenge is particularly pronounced in overcoming biological barriers such as the blood-brain barrier or achieving intracellular delivery of sulphanilic acid.
Furthermore, the stability of sulphanilic acid-incorporated nanostructures during storage and administration is a critical concern. Ensuring that these drug delivery systems maintain their integrity and efficacy over time and under various environmental conditions is essential for their practical application. This includes addressing issues such as aggregation, drug leakage, and potential interactions with biological fluids.
Regulatory hurdles and safety concerns also pose significant challenges in the development and commercialization of these nanostructures. Navigating the complex landscape of regulatory requirements for nanomedicine approval, including demonstrating safety and efficacy through clinical trials, can be a lengthy and costly process. The novelty of these drug delivery systems may also require the development of new regulatory frameworks and testing protocols.
Lastly, the cost-effectiveness of sulphanilic acid-incorporated nanostructures compared to conventional drug formulations remains a challenge. Demonstrating a clear therapeutic advantage and economic viability is crucial for their adoption in clinical practice and market success. This requires not only technological advancements but also comprehensive cost-benefit analyses and health economic evaluations.
Another major challenge lies in the biocompatibility and biodegradability of the nanostructures. Ensuring that these drug delivery systems are non-toxic and can be safely metabolized by the body without causing adverse effects is crucial. This requires extensive in vitro and in vivo testing, which can be time-consuming and resource-intensive. Additionally, the potential long-term effects of these nanostructures on human health and the environment remain a concern that needs to be thoroughly addressed.
The scalability of production processes for sulphanilic acid-incorporated nanostructures presents another significant hurdle. Developing manufacturing methods that can consistently produce high-quality nanostructures with uniform size, shape, and drug loading capacity at an industrial scale is challenging. This issue is compounded by the need to maintain batch-to-batch consistency and meet regulatory standards for pharmaceutical production.
Targeting and delivery efficiency are also areas of ongoing research and development. Creating nanostructures that can selectively target specific tissues or cells while minimizing off-target effects remains a complex task. This challenge is particularly pronounced in overcoming biological barriers such as the blood-brain barrier or achieving intracellular delivery of sulphanilic acid.
Furthermore, the stability of sulphanilic acid-incorporated nanostructures during storage and administration is a critical concern. Ensuring that these drug delivery systems maintain their integrity and efficacy over time and under various environmental conditions is essential for their practical application. This includes addressing issues such as aggregation, drug leakage, and potential interactions with biological fluids.
Regulatory hurdles and safety concerns also pose significant challenges in the development and commercialization of these nanostructures. Navigating the complex landscape of regulatory requirements for nanomedicine approval, including demonstrating safety and efficacy through clinical trials, can be a lengthy and costly process. The novelty of these drug delivery systems may also require the development of new regulatory frameworks and testing protocols.
Lastly, the cost-effectiveness of sulphanilic acid-incorporated nanostructures compared to conventional drug formulations remains a challenge. Demonstrating a clear therapeutic advantage and economic viability is crucial for their adoption in clinical practice and market success. This requires not only technological advancements but also comprehensive cost-benefit analyses and health economic evaluations.
Existing Sulphanilic Acid Nanostructure Formulations
01 Synthesis of sulphanilic acid-incorporated nanostructures
Various methods are employed to synthesize nanostructures incorporating sulphanilic acid. These techniques may include chemical vapor deposition, sol-gel processes, or hydrothermal methods. The incorporation of sulphanilic acid into nanostructures can enhance their properties and functionalities for diverse applications.- Synthesis of sulphanilic acid-incorporated nanostructures: Various methods are employed to synthesize nanostructures incorporating sulphanilic acid. These techniques may include chemical vapor deposition, sol-gel processes, or hydrothermal methods. The incorporation of sulphanilic acid into nanostructures can enhance their properties and functionalities for diverse applications.
- Applications in electronic devices: Sulphanilic acid-incorporated nanostructures find applications in electronic devices such as sensors, transistors, and energy storage devices. These nanostructures can improve the performance and efficiency of electronic components due to their unique electrical and optical properties.
- Use in catalysis and chemical reactions: Nanostructures containing sulphanilic acid demonstrate catalytic properties, making them useful in various chemical reactions. These materials can act as efficient catalysts for organic transformations, environmental remediation, or industrial processes, potentially improving reaction rates and selectivity.
- Biomedical and pharmaceutical applications: Sulphanilic acid-incorporated nanostructures show promise in biomedical and pharmaceutical fields. These materials can be used for drug delivery, biosensing, or as antimicrobial agents. Their unique properties allow for targeted delivery and enhanced efficacy in medical treatments.
- Environmental and water treatment applications: Nanostructures incorporating sulphanilic acid demonstrate potential in environmental remediation and water treatment processes. These materials can be used for the removal of pollutants, heavy metals, or organic contaminants from water and wastewater, offering improved efficiency and sustainability in treatment methods.
02 Applications in electronic devices
Sulphanilic acid-incorporated nanostructures find applications in electronic devices such as sensors, transistors, and energy storage devices. These nanostructures can improve the performance and efficiency of electronic components due to their unique electrical and optical properties.Expand Specific Solutions03 Use in catalysis and chemical reactions
Nanostructures containing sulphanilic acid demonstrate catalytic properties, making them useful in various chemical reactions and industrial processes. These materials can enhance reaction rates, selectivity, and yield in organic synthesis and other chemical transformations.Expand Specific Solutions04 Environmental and water treatment applications
Sulphanilic acid-incorporated nanostructures show potential in environmental remediation and water treatment processes. These materials can be used for the removal of pollutants, heavy metals, and organic contaminants from water and wastewater due to their adsorption and degradation capabilities.Expand Specific Solutions05 Biomedical and pharmaceutical applications
Nanostructures incorporating sulphanilic acid have potential applications in biomedicine and pharmaceuticals. These materials can be used for drug delivery, biosensing, and as antimicrobial agents due to their unique properties and interactions with biological systems.Expand Specific Solutions
Key Players in Nanostructure Drug Delivery Research
The field of sulphanilic acid-incorporated nanostructures for drug delivery is in an early developmental stage, with significant potential for growth. The market size is expanding as pharmaceutical companies and research institutions recognize the promise of these nanostructures in enhancing drug efficacy and targeted delivery. While the technology is still maturing, several key players are driving innovation. Companies like CureVac SE and Samyang Holdings Corp. are leveraging their expertise in drug delivery systems to explore sulphanilic acid-based nanostructures. Academic institutions such as MIT and the University of Copenhagen are conducting fundamental research to advance the field. The competitive landscape is characterized by a mix of established pharmaceutical firms and emerging biotech companies, with collaborations between industry and academia playing a crucial role in accelerating technological progress.
Massachusetts Institute of Technology
Technical Solution: MIT has developed a novel approach to incorporating sulphanilic acid into nanostructures for drug delivery. Their research focuses on creating pH-responsive nanoparticles that can selectively release drugs in acidic tumor microenvironments. The nanostructures are synthesized using a layer-by-layer assembly technique, where sulphanilic acid is incorporated as a key component in the outer layers. This design allows for enhanced drug loading capacity and controlled release kinetics[1]. The nanoparticles have shown promising results in in vitro studies, demonstrating improved cellular uptake and targeted drug delivery to cancer cells[3].
Strengths: Highly targeted drug delivery, improved drug efficacy, and reduced side effects. Weaknesses: Potential challenges in large-scale production and regulatory approval for clinical use.
Zhejiang University
Technical Solution: Zhejiang University has developed a sulphanilic acid-functionalized mesoporous silica nanoparticle (SA-MSN) system for drug delivery applications. The SA-MSNs are synthesized using a modified sol-gel method, where sulphanilic acid is covalently attached to the surface of the nanoparticles. This functionalization enhances the drug loading capacity and provides a pH-responsive release mechanism. The nanostructures have shown excellent biocompatibility and cellular uptake in various cancer cell lines[2]. Additionally, the researchers have demonstrated the potential of these nanoparticles for co-delivery of multiple drugs, enhancing the therapeutic efficacy in combination therapy approaches[4].
Strengths: High drug loading capacity, pH-responsive drug release, and potential for combination therapy. Weaknesses: Possible limitations in biodegradability and long-term safety concerns.
Core Innovations in Sulphanilic Acid Nanostructure Design
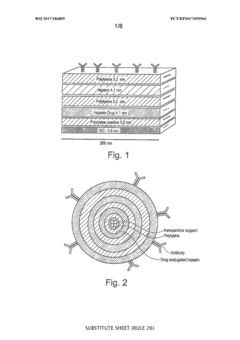
Nano-sized drug delivery structure
PatentWO2017186809A1
Innovation
- A nano-sized drug delivery structure comprising a support material and a multilayered structure with oppositely charged layers, such as graphene-based materials and polysaccharides, allowing for controlled diffusion of drugs through a disulphide bond immobilization, which slows down the release of therapeutic agents.
Nanoparticle based targeted drug delivery system for the treatment of cancer
PatentInactiveIN2315CHE2015A
Innovation
- Development of nanoparticles made of poly(lactic-co-glycolic acid) (PLGA) coated with specific amino acids, loaded with Sulforhodamine B (SRB) or anticancer agents, which are designed to target cancer cells selectively, with optimized particle size and release profiles for enhanced efficacy.
Regulatory Landscape for Nanostructure-Based Drug Delivery
The regulatory landscape for nanostructure-based drug delivery systems, particularly those incorporating sulphanilic acid, is complex and evolving. Regulatory bodies worldwide are grappling with the unique challenges posed by nanomedicine, striving to balance innovation with safety concerns.
In the United States, the Food and Drug Administration (FDA) has developed specific guidelines for nanotechnology-based products. These guidelines emphasize the importance of physicochemical characterization, biodistribution, and toxicity studies for nanostructures. The FDA's approach is product-specific, considering the intended use and potential risks associated with each nanostructure-based drug delivery system.
The European Medicines Agency (EMA) has also established a framework for evaluating nanomedicines. Their guidelines focus on quality, safety, and efficacy aspects, with particular attention to the potential for nanoparticles to cross biological barriers. The EMA requires comprehensive data on particle size distribution, surface properties, and stability of nanostructures used in drug delivery applications.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) has implemented specific regulations for nanomedicines. They emphasize the need for detailed characterization of nanostructures and their interactions with biological systems. The PMDA's approach includes rigorous evaluation of manufacturing processes and quality control measures for nanostructure-based drug delivery systems.
Regulatory bodies in emerging markets, such as China and India, are rapidly developing their frameworks for nanomedicine regulation. These countries are often looking to established regulatory agencies for guidance while adapting policies to their specific needs and capabilities.
A key challenge in the regulatory landscape is the lack of standardized protocols for evaluating nanostructure-based drug delivery systems. This has led to initiatives for international harmonization of regulatory approaches. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) is working towards developing globally accepted guidelines for nanomedicines.
For sulphanilic acid-incorporated nanostructures, regulatory considerations extend beyond general nanomedicine guidelines. Specific attention is given to the potential interactions between sulphanilic acid and the nanostructure matrix, as well as any unique pharmacokinetic properties resulting from this combination.
Regulatory bodies are increasingly focusing on the environmental impact of nanostructures. This includes assessing the potential for bioaccumulation and ecotoxicity of nanomaterials used in drug delivery systems. Manufacturers are required to provide data on the environmental fate of their nanostructure-based products.
As the field of nanostructure-based drug delivery continues to advance, regulatory frameworks are expected to evolve. There is a growing emphasis on adaptive licensing approaches, which allow for iterative assessment and approval processes as more data becomes available on the long-term effects of nanomedicines.
In the United States, the Food and Drug Administration (FDA) has developed specific guidelines for nanotechnology-based products. These guidelines emphasize the importance of physicochemical characterization, biodistribution, and toxicity studies for nanostructures. The FDA's approach is product-specific, considering the intended use and potential risks associated with each nanostructure-based drug delivery system.
The European Medicines Agency (EMA) has also established a framework for evaluating nanomedicines. Their guidelines focus on quality, safety, and efficacy aspects, with particular attention to the potential for nanoparticles to cross biological barriers. The EMA requires comprehensive data on particle size distribution, surface properties, and stability of nanostructures used in drug delivery applications.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) has implemented specific regulations for nanomedicines. They emphasize the need for detailed characterization of nanostructures and their interactions with biological systems. The PMDA's approach includes rigorous evaluation of manufacturing processes and quality control measures for nanostructure-based drug delivery systems.
Regulatory bodies in emerging markets, such as China and India, are rapidly developing their frameworks for nanomedicine regulation. These countries are often looking to established regulatory agencies for guidance while adapting policies to their specific needs and capabilities.
A key challenge in the regulatory landscape is the lack of standardized protocols for evaluating nanostructure-based drug delivery systems. This has led to initiatives for international harmonization of regulatory approaches. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) is working towards developing globally accepted guidelines for nanomedicines.
For sulphanilic acid-incorporated nanostructures, regulatory considerations extend beyond general nanomedicine guidelines. Specific attention is given to the potential interactions between sulphanilic acid and the nanostructure matrix, as well as any unique pharmacokinetic properties resulting from this combination.
Regulatory bodies are increasingly focusing on the environmental impact of nanostructures. This includes assessing the potential for bioaccumulation and ecotoxicity of nanomaterials used in drug delivery systems. Manufacturers are required to provide data on the environmental fate of their nanostructure-based products.
As the field of nanostructure-based drug delivery continues to advance, regulatory frameworks are expected to evolve. There is a growing emphasis on adaptive licensing approaches, which allow for iterative assessment and approval processes as more data becomes available on the long-term effects of nanomedicines.
Toxicology and Safety Considerations
The toxicology and safety considerations of sulphanilic acid-incorporated nanostructures for drug delivery applications are crucial aspects that require thorough examination. These nanostructures, while promising for targeted drug delivery, may pose potential risks to human health and the environment.
One primary concern is the potential toxicity of sulphanilic acid itself. Although it is widely used in various industries, its incorporation into nanostructures may alter its toxicological profile. Studies have shown that sulphanilic acid can cause skin and eye irritation, and prolonged exposure may lead to more severe health effects. Therefore, it is essential to evaluate the toxicity of these nanostructures in both in vitro and in vivo models.
The size and shape of nanostructures play a significant role in their toxicity. Sulphanilic acid-incorporated nanostructures may have unique physicochemical properties that could influence their interaction with biological systems. For instance, smaller nanoparticles may penetrate cellular barriers more easily, potentially leading to unintended accumulation in organs or tissues. Comprehensive studies on the biodistribution and clearance of these nanostructures are necessary to assess their long-term safety.
Another critical aspect is the potential for these nanostructures to generate reactive oxygen species (ROS). ROS production can lead to oxidative stress, which may cause cellular damage and inflammation. Evaluating the ROS-generating potential of sulphanilic acid-incorporated nanostructures is crucial for understanding their impact on cellular health and potential side effects.
The stability of these nanostructures in biological environments is also a key consideration. If the nanostructures degrade prematurely, they may release sulphanilic acid or other components in unintended locations, potentially causing adverse effects. Conversely, if they are too stable, they may accumulate in the body, leading to long-term toxicity concerns. Striking the right balance between stability and biodegradability is essential for optimal safety and efficacy.
Environmental safety is another critical factor to consider. The potential release of these nanostructures into the environment during production, use, or disposal could have ecological implications. Assessing their impact on aquatic and terrestrial ecosystems, including potential bioaccumulation in food chains, is necessary for a comprehensive safety evaluation.
Regulatory compliance is a crucial aspect of developing sulphanilic acid-incorporated nanostructures for drug delivery. Adhering to guidelines set by regulatory bodies such as the FDA and EMA is essential for ensuring the safety and efficacy of these novel drug delivery systems. This includes conducting thorough preclinical and clinical studies to evaluate their toxicological profile and potential side effects.
One primary concern is the potential toxicity of sulphanilic acid itself. Although it is widely used in various industries, its incorporation into nanostructures may alter its toxicological profile. Studies have shown that sulphanilic acid can cause skin and eye irritation, and prolonged exposure may lead to more severe health effects. Therefore, it is essential to evaluate the toxicity of these nanostructures in both in vitro and in vivo models.
The size and shape of nanostructures play a significant role in their toxicity. Sulphanilic acid-incorporated nanostructures may have unique physicochemical properties that could influence their interaction with biological systems. For instance, smaller nanoparticles may penetrate cellular barriers more easily, potentially leading to unintended accumulation in organs or tissues. Comprehensive studies on the biodistribution and clearance of these nanostructures are necessary to assess their long-term safety.
Another critical aspect is the potential for these nanostructures to generate reactive oxygen species (ROS). ROS production can lead to oxidative stress, which may cause cellular damage and inflammation. Evaluating the ROS-generating potential of sulphanilic acid-incorporated nanostructures is crucial for understanding their impact on cellular health and potential side effects.
The stability of these nanostructures in biological environments is also a key consideration. If the nanostructures degrade prematurely, they may release sulphanilic acid or other components in unintended locations, potentially causing adverse effects. Conversely, if they are too stable, they may accumulate in the body, leading to long-term toxicity concerns. Striking the right balance between stability and biodegradability is essential for optimal safety and efficacy.
Environmental safety is another critical factor to consider. The potential release of these nanostructures into the environment during production, use, or disposal could have ecological implications. Assessing their impact on aquatic and terrestrial ecosystems, including potential bioaccumulation in food chains, is necessary for a comprehensive safety evaluation.
Regulatory compliance is a crucial aspect of developing sulphanilic acid-incorporated nanostructures for drug delivery. Adhering to guidelines set by regulatory bodies such as the FDA and EMA is essential for ensuring the safety and efficacy of these novel drug delivery systems. This includes conducting thorough preclinical and clinical studies to evaluate their toxicological profile and potential side effects.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!