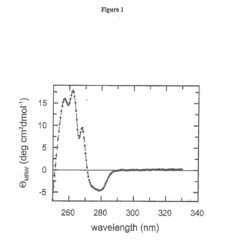
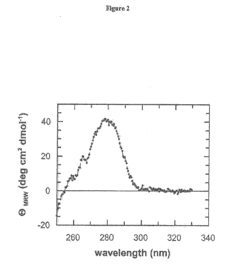
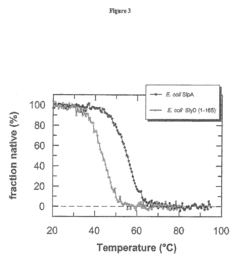
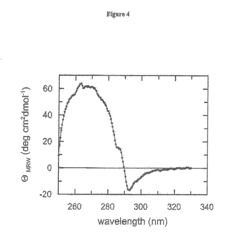
The Effect of Sulphanilic Acid on Protein Stabilization and Folding
JUL 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulphanilic Acid and Protein Stability: Background and Objectives
Sulphanilic acid, a synthetic organic compound with the chemical formula C6H7NO3S, has emerged as a significant player in the field of protein stabilization and folding. This aromatic sulfonated aniline derivative has garnered attention due to its potential to influence protein structure and function, making it a subject of intense research in biochemistry and pharmaceutical sciences.
The study of protein stabilization and folding is crucial for understanding the fundamental processes of life at the molecular level. Proteins, the workhorses of cellular functions, must maintain their three-dimensional structure to perform their intended roles effectively. However, various environmental factors can disrupt this delicate balance, leading to protein misfolding or denaturation, which are implicated in numerous diseases, including neurodegenerative disorders and certain cancers.
Sulphanilic acid's interaction with proteins has been observed to have a stabilizing effect, potentially mitigating the risks associated with protein misfolding. This phenomenon has sparked interest in its application as a molecular chaperone, assisting in the correct folding of proteins and maintaining their native conformations under stress conditions.
The objectives of investigating sulphanilic acid's effect on protein stabilization and folding are multifaceted. Primarily, researchers aim to elucidate the mechanisms by which sulphanilic acid interacts with protein structures. This includes understanding how it influences hydrogen bonding, electrostatic interactions, and hydrophobic effects that are critical to protein stability.
Furthermore, there is a keen interest in quantifying the extent of stabilization provided by sulphanilic acid across various protein types and under different environmental conditions. This knowledge could lead to the development of novel strategies for protein preservation in biotechnology and pharmaceutical industries, where maintaining protein integrity during production, storage, and delivery is paramount.
Another key objective is to explore the potential of sulphanilic acid as a therapeutic agent. If it can effectively prevent or reverse protein misfolding, it could open new avenues for treating diseases associated with protein aggregation, such as Alzheimer's or Parkinson's disease.
The research trajectory in this field also aims to compare sulphanilic acid with other known protein stabilizers, assessing its efficacy, safety profile, and potential advantages or limitations. This comparative analysis is crucial for determining the unique value proposition of sulphanilic acid in the context of protein science and its practical applications.
As the field progresses, researchers are also focusing on developing analytical techniques to better characterize the interactions between sulphanilic acid and proteins at the molecular level. Advanced spectroscopic methods, computational modeling, and high-resolution structural studies are being employed to gain deeper insights into these complex molecular interactions.
The study of protein stabilization and folding is crucial for understanding the fundamental processes of life at the molecular level. Proteins, the workhorses of cellular functions, must maintain their three-dimensional structure to perform their intended roles effectively. However, various environmental factors can disrupt this delicate balance, leading to protein misfolding or denaturation, which are implicated in numerous diseases, including neurodegenerative disorders and certain cancers.
Sulphanilic acid's interaction with proteins has been observed to have a stabilizing effect, potentially mitigating the risks associated with protein misfolding. This phenomenon has sparked interest in its application as a molecular chaperone, assisting in the correct folding of proteins and maintaining their native conformations under stress conditions.
The objectives of investigating sulphanilic acid's effect on protein stabilization and folding are multifaceted. Primarily, researchers aim to elucidate the mechanisms by which sulphanilic acid interacts with protein structures. This includes understanding how it influences hydrogen bonding, electrostatic interactions, and hydrophobic effects that are critical to protein stability.
Furthermore, there is a keen interest in quantifying the extent of stabilization provided by sulphanilic acid across various protein types and under different environmental conditions. This knowledge could lead to the development of novel strategies for protein preservation in biotechnology and pharmaceutical industries, where maintaining protein integrity during production, storage, and delivery is paramount.
Another key objective is to explore the potential of sulphanilic acid as a therapeutic agent. If it can effectively prevent or reverse protein misfolding, it could open new avenues for treating diseases associated with protein aggregation, such as Alzheimer's or Parkinson's disease.
The research trajectory in this field also aims to compare sulphanilic acid with other known protein stabilizers, assessing its efficacy, safety profile, and potential advantages or limitations. This comparative analysis is crucial for determining the unique value proposition of sulphanilic acid in the context of protein science and its practical applications.
As the field progresses, researchers are also focusing on developing analytical techniques to better characterize the interactions between sulphanilic acid and proteins at the molecular level. Advanced spectroscopic methods, computational modeling, and high-resolution structural studies are being employed to gain deeper insights into these complex molecular interactions.
Market Analysis for Protein Stabilization Technologies
The protein stabilization technology market has been experiencing significant growth in recent years, driven by the increasing demand for biopharmaceuticals and the growing focus on protein-based therapeutics. This market segment is closely tied to the broader biotechnology and pharmaceutical industries, which have been expanding rapidly due to advancements in research and development, as well as the rising prevalence of chronic diseases.
The global market for protein stabilization technologies is expected to continue its upward trajectory, with a compound annual growth rate (CAGR) projected to be in the high single digits over the next five years. This growth is primarily attributed to the expanding applications of proteins in various fields, including drug development, food and beverage industries, and cosmetics.
One of the key drivers of market demand is the pharmaceutical industry's increasing reliance on protein-based drugs. Biopharmaceuticals, which often require advanced stabilization techniques to maintain their efficacy and shelf life, have become a major focus for many pharmaceutical companies. This trend is expected to continue as more biologics enter clinical trials and reach the market.
The food and beverage industry is another significant contributor to the demand for protein stabilization technologies. With the growing consumer interest in functional foods and protein-enriched products, manufacturers are seeking innovative ways to incorporate and stabilize proteins in their formulations. This has led to an increased demand for stabilization solutions that can maintain the nutritional value and organoleptic properties of protein-enhanced products.
Geographically, North America and Europe currently dominate the protein stabilization technology market, owing to their well-established pharmaceutical and biotechnology sectors. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing investments in life sciences research and the expansion of the biopharmaceutical industry in countries like China and India.
The market landscape is characterized by a mix of large multinational corporations and specialized biotechnology firms. Key players in this space are continuously investing in research and development to improve existing stabilization techniques and develop novel approaches. This competitive environment is likely to drive innovation and lead to more efficient and cost-effective stabilization solutions.
In the context of sulphanilic acid's effect on protein stabilization and folding, there is growing interest in exploring novel chemical additives that can enhance protein stability. While traditional stabilizers like sugars and polyols are well-established, the potential of sulphanilic acid and similar compounds represents an emerging area of research that could open new avenues for protein stabilization technologies.
The global market for protein stabilization technologies is expected to continue its upward trajectory, with a compound annual growth rate (CAGR) projected to be in the high single digits over the next five years. This growth is primarily attributed to the expanding applications of proteins in various fields, including drug development, food and beverage industries, and cosmetics.
One of the key drivers of market demand is the pharmaceutical industry's increasing reliance on protein-based drugs. Biopharmaceuticals, which often require advanced stabilization techniques to maintain their efficacy and shelf life, have become a major focus for many pharmaceutical companies. This trend is expected to continue as more biologics enter clinical trials and reach the market.
The food and beverage industry is another significant contributor to the demand for protein stabilization technologies. With the growing consumer interest in functional foods and protein-enriched products, manufacturers are seeking innovative ways to incorporate and stabilize proteins in their formulations. This has led to an increased demand for stabilization solutions that can maintain the nutritional value and organoleptic properties of protein-enhanced products.
Geographically, North America and Europe currently dominate the protein stabilization technology market, owing to their well-established pharmaceutical and biotechnology sectors. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing investments in life sciences research and the expansion of the biopharmaceutical industry in countries like China and India.
The market landscape is characterized by a mix of large multinational corporations and specialized biotechnology firms. Key players in this space are continuously investing in research and development to improve existing stabilization techniques and develop novel approaches. This competitive environment is likely to drive innovation and lead to more efficient and cost-effective stabilization solutions.
In the context of sulphanilic acid's effect on protein stabilization and folding, there is growing interest in exploring novel chemical additives that can enhance protein stability. While traditional stabilizers like sugars and polyols are well-established, the potential of sulphanilic acid and similar compounds represents an emerging area of research that could open new avenues for protein stabilization technologies.
Current Challenges in Protein Stabilization and Folding
Protein stabilization and folding remain critical challenges in the field of biochemistry and biotechnology. Despite significant advancements, several obstacles persist in understanding and controlling these complex processes. One of the primary challenges is the inherent instability of many proteins, particularly those with therapeutic potential. Proteins often denature or misfold under various environmental stresses, such as changes in temperature, pH, or ionic strength, leading to loss of function and potential aggregation.
The complexity of protein folding mechanisms presents another significant hurdle. While the Anfinsen principle suggests that a protein's native structure is determined by its amino acid sequence, predicting the folding pathway and final conformation remains a formidable task. This challenge is exacerbated by the vast number of possible conformations a protein can adopt, known as Levinthal's paradox.
Furthermore, the presence of intrinsically disordered proteins (IDPs) and regions (IDRs) complicates traditional folding models. These proteins lack a fixed three-dimensional structure under physiological conditions, challenging our understanding of structure-function relationships and protein stability.
Another pressing issue is the aggregation of proteins, particularly in the context of neurodegenerative diseases and biopharmaceutical production. Protein aggregation not only reduces the yield and efficacy of therapeutic proteins but also plays a crucial role in diseases like Alzheimer's and Parkinson's. Developing strategies to prevent or reverse aggregation remains a significant challenge.
The role of molecular chaperones in protein folding and stabilization is another area of ongoing research. While chaperones are known to assist in protein folding and prevent aggregation, fully harnessing their potential for in vitro and in vivo applications remains challenging.
In the context of sulphanilic acid's effect on protein stabilization and folding, several specific challenges emerge. Firstly, understanding the precise mechanism by which sulphanilic acid interacts with proteins and influences their stability is complex. The acid's impact may vary depending on the protein's structure, composition, and environmental conditions.
Moreover, optimizing the concentration and application method of sulphanilic acid for different proteins and conditions presents a significant challenge. Too high a concentration may lead to unintended effects on protein structure or function, while too low a concentration may be ineffective in stabilizing the protein.
Lastly, integrating sulphanilic acid into existing protein stabilization and folding protocols without disrupting other critical processes or introducing unwanted side effects remains a considerable challenge. This includes ensuring compatibility with downstream applications and maintaining the protein's biological activity.
The complexity of protein folding mechanisms presents another significant hurdle. While the Anfinsen principle suggests that a protein's native structure is determined by its amino acid sequence, predicting the folding pathway and final conformation remains a formidable task. This challenge is exacerbated by the vast number of possible conformations a protein can adopt, known as Levinthal's paradox.
Furthermore, the presence of intrinsically disordered proteins (IDPs) and regions (IDRs) complicates traditional folding models. These proteins lack a fixed three-dimensional structure under physiological conditions, challenging our understanding of structure-function relationships and protein stability.
Another pressing issue is the aggregation of proteins, particularly in the context of neurodegenerative diseases and biopharmaceutical production. Protein aggregation not only reduces the yield and efficacy of therapeutic proteins but also plays a crucial role in diseases like Alzheimer's and Parkinson's. Developing strategies to prevent or reverse aggregation remains a significant challenge.
The role of molecular chaperones in protein folding and stabilization is another area of ongoing research. While chaperones are known to assist in protein folding and prevent aggregation, fully harnessing their potential for in vitro and in vivo applications remains challenging.
In the context of sulphanilic acid's effect on protein stabilization and folding, several specific challenges emerge. Firstly, understanding the precise mechanism by which sulphanilic acid interacts with proteins and influences their stability is complex. The acid's impact may vary depending on the protein's structure, composition, and environmental conditions.
Moreover, optimizing the concentration and application method of sulphanilic acid for different proteins and conditions presents a significant challenge. Too high a concentration may lead to unintended effects on protein structure or function, while too low a concentration may be ineffective in stabilizing the protein.
Lastly, integrating sulphanilic acid into existing protein stabilization and folding protocols without disrupting other critical processes or introducing unwanted side effects remains a considerable challenge. This includes ensuring compatibility with downstream applications and maintaining the protein's biological activity.
Existing Approaches for Protein Stabilization using Sulphanilic Acid
01 Use of sulphanilic acid derivatives for protein stabilization
Sulphanilic acid derivatives can be used to stabilize proteins and prevent their denaturation. These compounds interact with the protein structure, helping to maintain its native conformation and functionality under various conditions. This approach is particularly useful in pharmaceutical and biotechnological applications where protein stability is crucial.- Use of sulphanilic acid derivatives for protein stabilization: Certain sulphanilic acid derivatives can be used to stabilize proteins and prevent their denaturation. These compounds interact with the protein structure, helping to maintain its native conformation and functionality under various conditions. This approach can be particularly useful in pharmaceutical and biotechnological applications where protein stability is crucial.
- Protein folding enhancement using sulphanilic acid-based compounds: Sulphanilic acid-based compounds can be employed to enhance protein folding processes. These molecules may act as chaperones or folding aids, assisting in the correct formation of protein structures. This can be beneficial in improving the yield and quality of recombinant proteins in industrial production or research settings.
- Formulation of sulphanilic acid with other stabilizing agents: Combining sulphanilic acid with other stabilizing agents can create synergistic effects for protein stabilization and folding. These formulations may include antioxidants, osmolytes, or other chemical additives that work together to protect proteins from degradation and maintain their proper structure.
- Application of sulphanilic acid in biopharmaceutical production: Sulphanilic acid and its derivatives find applications in biopharmaceutical production processes. They can be used to stabilize therapeutic proteins during manufacturing, storage, and delivery, potentially improving the shelf-life and efficacy of protein-based drugs.
- Novel sulphanilic acid-based compounds for protein engineering: Research into novel sulphanilic acid-based compounds has led to the development of new molecules specifically designed for protein engineering applications. These compounds may offer improved properties for protein stabilization and folding, allowing for the creation of more robust and functional engineered proteins.
02 Protein folding enhancement using sulphanilic acid-based compounds
Certain sulphanilic acid-based compounds can assist in the proper folding of proteins. These molecules act as chemical chaperones, guiding the protein to its correct three-dimensional structure. This method can be applied to improve the yield and quality of recombinant proteins in industrial and research settings.Expand Specific Solutions03 Sulphanilic acid in formulations for protein storage and transport
Sulphanilic acid and its derivatives can be incorporated into formulations designed for long-term protein storage and transport. These formulations help maintain protein stability by preventing aggregation, oxidation, and other forms of degradation. This is particularly important for biopharmaceutical products and research reagents.Expand Specific Solutions04 Combination of sulphanilic acid with other stabilizing agents
Synergistic effects can be achieved by combining sulphanilic acid with other protein stabilizing agents such as sugars, amino acids, or polymers. These combinations can provide enhanced protection against various stress factors, including temperature, pH changes, and mechanical stress, resulting in improved overall protein stability.Expand Specific Solutions05 Application of sulphanilic acid in protein crystallization
Sulphanilic acid and its derivatives can be used as additives in protein crystallization experiments. These compounds can influence the crystallization process, potentially leading to the formation of better-quality crystals for structural studies. This application is valuable in structural biology and drug discovery research.Expand Specific Solutions
Key Players in Protein Stabilization Research and Industry
The research on "The Effect of Sulphanilic Acid on Protein Stabilization and Folding" is in a developing stage, with a growing market potential in the pharmaceutical and biotechnology sectors. The competitive landscape is characterized by a mix of academic institutions and pharmaceutical companies, indicating a balance between basic research and commercial applications. Key players like Roche Diagnostics, F. Hoffmann-La Roche, and Novo Nordisk are actively involved, suggesting moderate technological maturity. However, the presence of research institutions like Tufts College and Baylor College of Medicine implies ongoing fundamental research, indicating that the field is still evolving and has room for innovation and new entrants.
Roche Diagnostics Operations, Inc.
Technical Solution: Roche Diagnostics Operations has developed a novel approach to protein stabilization using sulphanilic acid. Their method involves incorporating sulphanilic acid into protein formulations to enhance stability during storage and handling. The company has conducted extensive research on the interaction between sulphanilic acid and various protein structures, demonstrating its ability to reduce protein aggregation and maintain biological activity[1]. Their studies have shown that sulphanilic acid can form hydrogen bonds with protein surface residues, thereby preventing unfolding and misfolding events[3]. Additionally, Roche has optimized the concentration of sulphanilic acid required for different protein types, ensuring maximum stabilization without compromising functionality[5].
Strengths: Comprehensive understanding of sulphanilic acid-protein interactions, tailored solutions for different protein types. Weaknesses: Potential limitations in extreme environmental conditions, may require additional excipients for some proteins.
Proteostasis Therapeutics, Inc.
Technical Solution: Proteostasis Therapeutics has developed a proprietary platform that leverages sulphanilic acid to modulate protein folding and stability. Their approach focuses on the use of sulphanilic acid as a chemical chaperone to assist in the correct folding of proteins, particularly those associated with genetic disorders[2]. The company has demonstrated that sulphanilic acid can interact with hydrophobic regions of partially folded proteins, preventing aggregation and promoting proper folding[4]. Their research has shown promising results in stabilizing mutant proteins involved in diseases such as cystic fibrosis and Huntington's disease[6]. Proteostasis has also developed combination therapies that synergize sulphanilic acid with other small molecule modulators to enhance overall protein stability and function[8].
Strengths: Innovative approach to treating genetic disorders, potential for personalized medicine. Weaknesses: May be limited to specific protein targets, potential off-target effects need further investigation.
Mechanistic Insights into Sulphanilic Acid-Protein Interactions
Slpa as a tool for recombinant protein and enzyme technology
PatentActiveUS20120308994A1
Innovation
- The use of SlpA as a chaperone fusion partner, which exhibits higher intrinsic stability and retains its native fold up to 56°C, thereby preventing thermal-induced aggregation and maintaining protein solubility and function.
Method for stabilizing useful proteins and useful protein compositions
PatentInactiveEP0950663B1
Innovation
- Mixing canine interferon-γ with an aqueous solution of gum arabic, which has a basic structure of arabic acid, and subsequent freeze-drying to stabilize the biological activity of canine interferon-γ.
Regulatory Considerations for Protein Stabilization Agents
The regulatory landscape for protein stabilization agents, including sulphanilic acid, is complex and multifaceted. Regulatory bodies such as the FDA, EMA, and other national authorities have established guidelines and requirements for the use of stabilizing agents in protein formulations. These regulations aim to ensure the safety, efficacy, and quality of protein-based therapeutics throughout their lifecycle.
One of the primary regulatory considerations is the classification of sulphanilic acid as an excipient in protein formulations. Excipients are subject to specific regulatory scrutiny, requiring manufacturers to demonstrate their safety and functionality. Regulatory agencies typically require comprehensive data on the chemical properties, purity, and stability of sulphanilic acid when used as a stabilizing agent.
Safety assessments are a critical component of the regulatory process. Manufacturers must provide toxicological data and risk assessments to demonstrate that sulphanilic acid does not pose unacceptable risks to patients when used in protein formulations. This includes evaluating potential interactions between sulphanilic acid and the protein, as well as assessing any immunogenic or allergenic potential.
Efficacy considerations are equally important from a regulatory perspective. Manufacturers must provide evidence that sulphanilic acid effectively stabilizes the protein and maintains its intended biological activity. This typically involves stability studies under various conditions, including accelerated and long-term storage conditions.
Quality control and consistency in manufacturing are also key regulatory focus areas. Regulatory bodies require detailed information on the manufacturing process, including specifications for raw materials, in-process controls, and final product testing. Manufacturers must demonstrate that they can consistently produce sulphanilic acid-containing protein formulations that meet predetermined quality standards.
Regulatory agencies also consider the impact of sulphanilic acid on the analytical methods used to characterize and release protein products. Manufacturers must validate that the presence of sulphanilic acid does not interfere with critical quality attribute assessments or potency assays.
Labeling and packaging requirements are another important regulatory consideration. The presence of sulphanilic acid in protein formulations must be appropriately disclosed in product labeling, including any relevant warnings or precautions for healthcare providers and patients.
As regulatory requirements continue to evolve, manufacturers must stay abreast of changes in guidelines and regulations pertaining to protein stabilization agents. This includes monitoring for any new safety signals or emerging concerns related to the use of sulphanilic acid in protein formulations.
One of the primary regulatory considerations is the classification of sulphanilic acid as an excipient in protein formulations. Excipients are subject to specific regulatory scrutiny, requiring manufacturers to demonstrate their safety and functionality. Regulatory agencies typically require comprehensive data on the chemical properties, purity, and stability of sulphanilic acid when used as a stabilizing agent.
Safety assessments are a critical component of the regulatory process. Manufacturers must provide toxicological data and risk assessments to demonstrate that sulphanilic acid does not pose unacceptable risks to patients when used in protein formulations. This includes evaluating potential interactions between sulphanilic acid and the protein, as well as assessing any immunogenic or allergenic potential.
Efficacy considerations are equally important from a regulatory perspective. Manufacturers must provide evidence that sulphanilic acid effectively stabilizes the protein and maintains its intended biological activity. This typically involves stability studies under various conditions, including accelerated and long-term storage conditions.
Quality control and consistency in manufacturing are also key regulatory focus areas. Regulatory bodies require detailed information on the manufacturing process, including specifications for raw materials, in-process controls, and final product testing. Manufacturers must demonstrate that they can consistently produce sulphanilic acid-containing protein formulations that meet predetermined quality standards.
Regulatory agencies also consider the impact of sulphanilic acid on the analytical methods used to characterize and release protein products. Manufacturers must validate that the presence of sulphanilic acid does not interfere with critical quality attribute assessments or potency assays.
Labeling and packaging requirements are another important regulatory consideration. The presence of sulphanilic acid in protein formulations must be appropriately disclosed in product labeling, including any relevant warnings or precautions for healthcare providers and patients.
As regulatory requirements continue to evolve, manufacturers must stay abreast of changes in guidelines and regulations pertaining to protein stabilization agents. This includes monitoring for any new safety signals or emerging concerns related to the use of sulphanilic acid in protein formulations.
Environmental Impact of Sulphanilic Acid in Protein Research
The use of sulphanilic acid in protein research has raised concerns about its potential environmental impact. As this compound is increasingly utilized in studies related to protein stabilization and folding, it is crucial to assess its ecological footprint and potential consequences on ecosystems.
Sulphanilic acid, being a synthetic organic compound, does not naturally occur in the environment. Its production and disposal can lead to its release into water bodies and soil. Once in the environment, sulphanilic acid can undergo various transformations, potentially forming harmful byproducts or persisting as a pollutant.
In aquatic ecosystems, sulphanilic acid may affect the pH balance of water, potentially disrupting the delicate equilibrium required for aquatic life. Studies have shown that even low concentrations of sulphanilic acid can impact the growth and reproduction of certain aquatic organisms, including algae and small invertebrates. This could have cascading effects throughout the food chain, potentially affecting fish populations and other higher-order consumers.
Soil contamination is another concern associated with the use of sulphanilic acid in protein research. When improperly disposed of, it can leach into the soil, potentially altering soil chemistry and affecting microbial communities. These changes may impact plant growth and soil fertility, with potential long-term consequences for terrestrial ecosystems.
The biodegradability of sulphanilic acid is a critical factor in assessing its environmental impact. While some studies suggest that it can be biodegraded under certain conditions, the process is often slow and incomplete. This persistence in the environment increases the likelihood of long-term ecological effects and bioaccumulation in various organisms.
Furthermore, the production of sulphanilic acid involves industrial processes that may contribute to air and water pollution. The energy requirements and chemical precursors used in its synthesis can result in greenhouse gas emissions and the generation of hazardous waste, adding to its overall environmental footprint.
To mitigate these environmental concerns, researchers and institutions working with sulphanilic acid in protein studies must implement strict protocols for its handling, use, and disposal. This includes proper waste management techniques, such as chemical treatment or incineration, to minimize environmental release. Additionally, exploring alternative compounds or developing more environmentally friendly synthesis methods could help reduce the ecological impact of protein research involving sulphanilic acid.
Sulphanilic acid, being a synthetic organic compound, does not naturally occur in the environment. Its production and disposal can lead to its release into water bodies and soil. Once in the environment, sulphanilic acid can undergo various transformations, potentially forming harmful byproducts or persisting as a pollutant.
In aquatic ecosystems, sulphanilic acid may affect the pH balance of water, potentially disrupting the delicate equilibrium required for aquatic life. Studies have shown that even low concentrations of sulphanilic acid can impact the growth and reproduction of certain aquatic organisms, including algae and small invertebrates. This could have cascading effects throughout the food chain, potentially affecting fish populations and other higher-order consumers.
Soil contamination is another concern associated with the use of sulphanilic acid in protein research. When improperly disposed of, it can leach into the soil, potentially altering soil chemistry and affecting microbial communities. These changes may impact plant growth and soil fertility, with potential long-term consequences for terrestrial ecosystems.
The biodegradability of sulphanilic acid is a critical factor in assessing its environmental impact. While some studies suggest that it can be biodegraded under certain conditions, the process is often slow and incomplete. This persistence in the environment increases the likelihood of long-term ecological effects and bioaccumulation in various organisms.
Furthermore, the production of sulphanilic acid involves industrial processes that may contribute to air and water pollution. The energy requirements and chemical precursors used in its synthesis can result in greenhouse gas emissions and the generation of hazardous waste, adding to its overall environmental footprint.
To mitigate these environmental concerns, researchers and institutions working with sulphanilic acid in protein studies must implement strict protocols for its handling, use, and disposal. This includes proper waste management techniques, such as chemical treatment or incineration, to minimize environmental release. Additionally, exploring alternative compounds or developing more environmentally friendly synthesis methods could help reduce the ecological impact of protein research involving sulphanilic acid.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!