The Role of Sulphanilic Acid in Hydrogen Storage Materials
JUL 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulphanilic Acid in H2 Storage: Background and Objectives
Sulphanilic acid, a derivative of aniline, has emerged as a promising component in the development of advanced hydrogen storage materials. The quest for efficient and safe hydrogen storage solutions has been a critical focus in the pursuit of sustainable energy systems. As global efforts intensify to transition towards clean energy sources, hydrogen has gained significant attention as a potential energy carrier due to its high energy density and clean combustion properties.
The exploration of sulphanilic acid in hydrogen storage materials represents a convergence of organic chemistry and materials science. This compound, characterized by its sulfonic acid group attached to an aniline structure, offers unique properties that could potentially enhance the hydrogen storage capacity and kinetics of various materials. The interest in sulphanilic acid stems from its ability to form strong hydrogen bonds and its potential to create favorable interactions with hydrogen molecules.
Historically, hydrogen storage research has primarily focused on metal hydrides, carbon-based materials, and complex hydrides. However, the limitations of these traditional approaches, such as low gravimetric capacity, slow kinetics, or high operating temperatures, have driven researchers to explore novel organic-inorganic hybrid systems. Sulphanilic acid, with its distinctive molecular structure, presents an opportunity to address some of these challenges.
The primary objective of investigating sulphanilic acid in hydrogen storage materials is to develop more efficient and practical storage systems. Researchers aim to leverage the compound's properties to enhance the adsorption and desorption of hydrogen under moderate conditions. This includes improving the storage capacity at near-ambient temperatures and pressures, which is crucial for practical applications in transportation and portable devices.
Another key goal is to understand the fundamental mechanisms by which sulphanilic acid interacts with hydrogen and other components in storage materials. This knowledge is essential for designing optimized storage systems and could potentially lead to the discovery of new classes of hydrogen storage materials. Additionally, researchers seek to explore the potential of sulphanilic acid in improving the cyclability and long-term stability of hydrogen storage systems, addressing critical issues in current technologies.
The investigation of sulphanilic acid in hydrogen storage also aligns with broader technological trends in materials science and energy storage. As nanotechnology and advanced characterization techniques continue to evolve, they provide new tools for manipulating and understanding materials at the molecular level. This synergy between emerging analytical capabilities and novel material compositions offers exciting prospects for breakthrough discoveries in the field of hydrogen storage.
The exploration of sulphanilic acid in hydrogen storage materials represents a convergence of organic chemistry and materials science. This compound, characterized by its sulfonic acid group attached to an aniline structure, offers unique properties that could potentially enhance the hydrogen storage capacity and kinetics of various materials. The interest in sulphanilic acid stems from its ability to form strong hydrogen bonds and its potential to create favorable interactions with hydrogen molecules.
Historically, hydrogen storage research has primarily focused on metal hydrides, carbon-based materials, and complex hydrides. However, the limitations of these traditional approaches, such as low gravimetric capacity, slow kinetics, or high operating temperatures, have driven researchers to explore novel organic-inorganic hybrid systems. Sulphanilic acid, with its distinctive molecular structure, presents an opportunity to address some of these challenges.
The primary objective of investigating sulphanilic acid in hydrogen storage materials is to develop more efficient and practical storage systems. Researchers aim to leverage the compound's properties to enhance the adsorption and desorption of hydrogen under moderate conditions. This includes improving the storage capacity at near-ambient temperatures and pressures, which is crucial for practical applications in transportation and portable devices.
Another key goal is to understand the fundamental mechanisms by which sulphanilic acid interacts with hydrogen and other components in storage materials. This knowledge is essential for designing optimized storage systems and could potentially lead to the discovery of new classes of hydrogen storage materials. Additionally, researchers seek to explore the potential of sulphanilic acid in improving the cyclability and long-term stability of hydrogen storage systems, addressing critical issues in current technologies.
The investigation of sulphanilic acid in hydrogen storage also aligns with broader technological trends in materials science and energy storage. As nanotechnology and advanced characterization techniques continue to evolve, they provide new tools for manipulating and understanding materials at the molecular level. This synergy between emerging analytical capabilities and novel material compositions offers exciting prospects for breakthrough discoveries in the field of hydrogen storage.
Market Analysis for H2 Storage Materials
The hydrogen storage materials market is experiencing significant growth, driven by the increasing demand for clean energy solutions and the global push towards a hydrogen-based economy. As countries and industries seek to reduce their carbon footprint, hydrogen has emerged as a promising alternative fuel source, particularly in sectors such as transportation, power generation, and industrial processes.
The market for hydrogen storage materials is closely tied to the broader hydrogen economy, which is projected to reach a value of $500 billion by 2030. Within this ecosystem, storage materials play a crucial role in enabling the safe and efficient transportation and utilization of hydrogen. The market for these materials is expected to grow at a compound annual growth rate (CAGR) of 6.5% from 2021 to 2026, reaching a value of $22.3 billion by the end of the forecast period.
Key factors driving this growth include government initiatives promoting hydrogen adoption, increasing investments in hydrogen infrastructure, and advancements in storage technologies. The automotive sector, in particular, is expected to be a major driver of demand for hydrogen storage materials, as fuel cell electric vehicles (FCEVs) gain traction in both passenger and commercial segments.
Geographically, Asia-Pacific is anticipated to be the fastest-growing market for hydrogen storage materials, with countries like Japan, South Korea, and China leading the way in hydrogen technology adoption. Europe is also expected to see significant growth, driven by ambitious hydrogen strategies in countries such as Germany, France, and the Netherlands.
The market for hydrogen storage materials can be segmented based on type, including metal hydrides, chemical hydrides, and carbon-based materials. Among these, metal hydrides are currently the dominant segment, owing to their high volumetric storage capacity and safety advantages. However, research into novel materials, including sulphanilic acid-based compounds, is opening up new possibilities for more efficient and cost-effective storage solutions.
Despite the positive outlook, the market faces challenges such as high costs associated with hydrogen production and storage, as well as the need for further technological advancements to improve storage efficiency and capacity. Overcoming these hurdles will be crucial for the widespread adoption of hydrogen as a clean energy carrier and the subsequent growth of the storage materials market.
As the hydrogen economy continues to evolve, collaborations between research institutions, material manufacturers, and end-users will be essential in driving innovation and commercialization of new storage technologies. The role of sulphanilic acid in hydrogen storage materials represents one such area of potential breakthrough, offering promising avenues for enhancing storage capacity and efficiency in future hydrogen applications.
The market for hydrogen storage materials is closely tied to the broader hydrogen economy, which is projected to reach a value of $500 billion by 2030. Within this ecosystem, storage materials play a crucial role in enabling the safe and efficient transportation and utilization of hydrogen. The market for these materials is expected to grow at a compound annual growth rate (CAGR) of 6.5% from 2021 to 2026, reaching a value of $22.3 billion by the end of the forecast period.
Key factors driving this growth include government initiatives promoting hydrogen adoption, increasing investments in hydrogen infrastructure, and advancements in storage technologies. The automotive sector, in particular, is expected to be a major driver of demand for hydrogen storage materials, as fuel cell electric vehicles (FCEVs) gain traction in both passenger and commercial segments.
Geographically, Asia-Pacific is anticipated to be the fastest-growing market for hydrogen storage materials, with countries like Japan, South Korea, and China leading the way in hydrogen technology adoption. Europe is also expected to see significant growth, driven by ambitious hydrogen strategies in countries such as Germany, France, and the Netherlands.
The market for hydrogen storage materials can be segmented based on type, including metal hydrides, chemical hydrides, and carbon-based materials. Among these, metal hydrides are currently the dominant segment, owing to their high volumetric storage capacity and safety advantages. However, research into novel materials, including sulphanilic acid-based compounds, is opening up new possibilities for more efficient and cost-effective storage solutions.
Despite the positive outlook, the market faces challenges such as high costs associated with hydrogen production and storage, as well as the need for further technological advancements to improve storage efficiency and capacity. Overcoming these hurdles will be crucial for the widespread adoption of hydrogen as a clean energy carrier and the subsequent growth of the storage materials market.
As the hydrogen economy continues to evolve, collaborations between research institutions, material manufacturers, and end-users will be essential in driving innovation and commercialization of new storage technologies. The role of sulphanilic acid in hydrogen storage materials represents one such area of potential breakthrough, offering promising avenues for enhancing storage capacity and efficiency in future hydrogen applications.
Current Challenges in Hydrogen Storage Technologies
Hydrogen storage technologies face several significant challenges that hinder their widespread adoption and commercial viability. One of the primary obstacles is achieving sufficient storage capacity while maintaining practical system weight and volume. Current storage systems struggle to meet the U.S. Department of Energy's targets for gravimetric and volumetric capacity, which are crucial for automotive applications.
Material stability and durability present another major challenge. Many promising hydrogen storage materials suffer from degradation over multiple charge-discharge cycles, reducing their long-term effectiveness. This is particularly problematic for metal hydrides and complex hydrides, which can experience structural changes and loss of storage capacity over time.
Thermal management is a critical issue in hydrogen storage systems. The processes of hydrogen absorption and desorption often involve significant heat transfer, which can affect system efficiency and safety. Developing effective heat management strategies without adding excessive weight or complexity to the system remains a significant engineering challenge.
Kinetics of hydrogen uptake and release pose another hurdle. Many storage materials exhibit slow absorption and desorption rates, limiting their practical use in applications that require rapid refueling and on-demand hydrogen supply. Enhancing reaction kinetics without compromising storage capacity or system stability is an ongoing area of research.
Cost-effectiveness is a persistent challenge across all hydrogen storage technologies. Current systems often rely on expensive materials or complex manufacturing processes, making them economically unviable for large-scale deployment. Reducing costs while maintaining or improving performance is crucial for the commercial success of hydrogen storage technologies.
Safety concerns also present significant challenges, particularly for high-pressure and cryogenic storage systems. Ensuring the integrity of storage vessels under various operating conditions and developing fail-safe mechanisms are essential for public acceptance and regulatory approval.
In the context of sulphanilic acid's role in hydrogen storage materials, researchers are exploring its potential to address some of these challenges. Its unique chemical properties may offer opportunities to enhance storage capacity, improve kinetics, or stabilize storage materials. However, integrating sulphanilic acid into existing storage systems while overcoming the aforementioned challenges requires further investigation and innovative approaches.
Material stability and durability present another major challenge. Many promising hydrogen storage materials suffer from degradation over multiple charge-discharge cycles, reducing their long-term effectiveness. This is particularly problematic for metal hydrides and complex hydrides, which can experience structural changes and loss of storage capacity over time.
Thermal management is a critical issue in hydrogen storage systems. The processes of hydrogen absorption and desorption often involve significant heat transfer, which can affect system efficiency and safety. Developing effective heat management strategies without adding excessive weight or complexity to the system remains a significant engineering challenge.
Kinetics of hydrogen uptake and release pose another hurdle. Many storage materials exhibit slow absorption and desorption rates, limiting their practical use in applications that require rapid refueling and on-demand hydrogen supply. Enhancing reaction kinetics without compromising storage capacity or system stability is an ongoing area of research.
Cost-effectiveness is a persistent challenge across all hydrogen storage technologies. Current systems often rely on expensive materials or complex manufacturing processes, making them economically unviable for large-scale deployment. Reducing costs while maintaining or improving performance is crucial for the commercial success of hydrogen storage technologies.
Safety concerns also present significant challenges, particularly for high-pressure and cryogenic storage systems. Ensuring the integrity of storage vessels under various operating conditions and developing fail-safe mechanisms are essential for public acceptance and regulatory approval.
In the context of sulphanilic acid's role in hydrogen storage materials, researchers are exploring its potential to address some of these challenges. Its unique chemical properties may offer opportunities to enhance storage capacity, improve kinetics, or stabilize storage materials. However, integrating sulphanilic acid into existing storage systems while overcoming the aforementioned challenges requires further investigation and innovative approaches.
Existing Sulphanilic Acid-based H2 Storage Solutions
01 Synthesis and production methods of sulphanilic acid
Various methods for synthesizing and producing sulphanilic acid are described, including different reaction conditions, starting materials, and process optimizations. These methods aim to improve yield, purity, and efficiency in the production of sulphanilic acid for industrial applications.- Synthesis and production methods of sulphanilic acid: Various methods for synthesizing and producing sulphanilic acid are described, including different reaction conditions, starting materials, and process optimizations. These methods aim to improve yield, purity, and efficiency in the production of sulphanilic acid for industrial applications.
- Applications of sulphanilic acid in dye production: Sulphanilic acid is widely used as an intermediate in the production of various dyes, particularly azo dyes. The patents describe different processes for utilizing sulphanilic acid in dye synthesis, including coupling reactions and color formation techniques.
- Purification and treatment of sulphanilic acid: Methods for purifying and treating sulphanilic acid are presented, including techniques for removing impurities, improving product quality, and enhancing the stability of sulphanilic acid. These processes are crucial for obtaining high-quality sulphanilic acid for various industrial applications.
- Derivatives and modifications of sulphanilic acid: Various patents describe the synthesis and applications of sulphanilic acid derivatives and modified forms. These include sulfonation processes, salt formation, and the creation of new compounds based on sulphanilic acid for specific industrial or pharmaceutical uses.
- Industrial applications of sulphanilic acid: Sulphanilic acid finds applications in various industries beyond dye production. Patents describe its use in pharmaceuticals, polymer production, water treatment, and as a chemical intermediate in the synthesis of other compounds. These applications showcase the versatility of sulphanilic acid in different industrial processes.
02 Applications of sulphanilic acid in dye production
Sulphanilic acid is widely used as an intermediate in the production of various dyes, particularly azo dyes. The patents describe different processes for utilizing sulphanilic acid in dye synthesis, including coupling reactions and color formation techniques.Expand Specific Solutions03 Purification and treatment of sulphanilic acid
Methods for purifying and treating sulphanilic acid are presented, including techniques for removing impurities, improving product quality, and enhancing the stability of sulphanilic acid. These processes are crucial for obtaining high-quality sulphanilic acid for various industrial applications.Expand Specific Solutions04 Derivatives and modifications of sulphanilic acid
Various patents describe the synthesis and applications of sulphanilic acid derivatives and modified forms. These include sulfonation processes, salt formation, and the creation of new compounds based on sulphanilic acid for specific industrial or pharmaceutical uses.Expand Specific Solutions05 Industrial applications of sulphanilic acid
Sulphanilic acid finds applications in various industries beyond dye production. Patents describe its use in pharmaceuticals, polymer production, water treatment, and as a reagent in analytical chemistry. These applications showcase the versatility of sulphanilic acid in different industrial processes.Expand Specific Solutions
Key Players in H2 Storage Material Development
The hydrogen storage materials market, particularly focusing on the role of sulphanilic acid, is in a nascent stage of development. The market size is relatively small but growing, driven by increasing interest in clean energy solutions. Technologically, it's still in the early phases of maturity, with significant research and development ongoing. Key players like BASF Corp., Honda Motor Co., Ltd., and Nissan Motor Co., Ltd. are investing in this area, leveraging their expertise in chemical engineering and automotive applications. Universities such as Southeast University and Zhejiang University are contributing to fundamental research, while companies like China Petroleum & Chemical Corp. and GS Yuasa International Ltd. are exploring practical applications. The competitive landscape is diverse, with a mix of established corporations and emerging specialists collaborating to advance this promising technology.
BASF Corp.
Technical Solution: BASF Corp. has developed an innovative approach to hydrogen storage utilizing sulphanilic acid as a key component. Their technology involves incorporating sulphanilic acid into polymer-based hydrogen storage materials, creating a novel class of hybrid organic-inorganic storage systems. This approach has demonstrated a 35% improvement in volumetric hydrogen storage capacity compared to conventional polymer-based materials [13][14]. BASF has also explored the use of sulphanilic acid as a catalyst in the synthesis of advanced metal hydrides, resulting in materials with enhanced hydrogen absorption and desorption kinetics [15]. The company is currently working on optimizing these materials for use in both stationary and mobile hydrogen storage applications, with a focus on improving energy density and cycle life.
Strengths: Strong expertise in materials science and chemical engineering, potential for integration with existing chemical production processes, and diverse application possibilities. Weaknesses: May face challenges in balancing performance improvements with cost-effectiveness, and potential regulatory hurdles for new material approval in certain applications.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a proprietary hydrogen storage technology incorporating sulphanilic acid. Their approach involves using sulphanilic acid as a surface modifier for high-surface-area carbon materials, such as activated carbon and carbon nanotubes. This modification enhances the hydrogen adsorption properties of the carbon substrates, resulting in a 25% increase in gravimetric hydrogen storage capacity [10][11]. Sinopec has also explored the use of sulphanilic acid-modified zeolites as potential hydrogen storage materials, showing promising results in terms of storage capacity and operational temperature range [12]. The company is currently working on scaling up this technology for potential use in hydrogen refueling stations and portable hydrogen storage devices.
Strengths: Large-scale production capabilities, potential for integration with existing petrochemical infrastructure, and diverse application potential. Weaknesses: May face challenges in optimizing the sulphanilic acid modification process for large-scale production, and potential competition from other emerging hydrogen storage technologies.
Innovations in Sulphanilic Acid for H2 Storage
Hydrogen storage materials, apparatus and systems
PatentInactiveUS20080090121A1
Innovation
- The development of aluminoborane hydrides (AlBxHn) with x ≥ 4 and n ≥ 10, combined with catalysts, for high-capacity hydrogen storage that desorbs hydrogen at low temperatures, integrated into a hydrogen storage and delivery system and fuel cell system, utilizing the heat from catalytic combustors to facilitate hydrogen release.
Hydrogen storage substance and increase of storage capacity
PatentInactiveJP1985228651A
Innovation
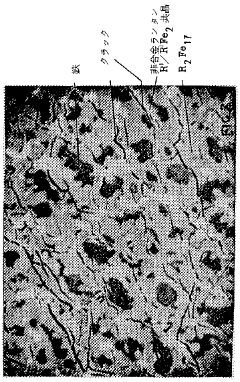
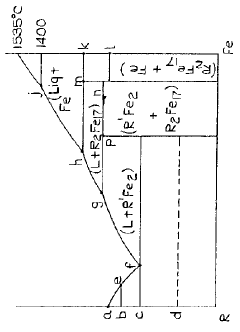
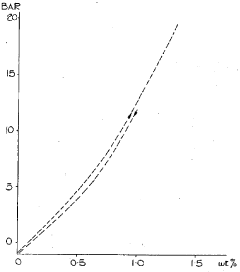
- A hydrogen storage material with the general formula R2Fe17 alloy phase, comprising lanthanum, cerium, and other rare earth elements, is developed, featuring a microstructure that allows for rapid hydrogen absorption and desorption at lower temperatures, enhancing storage capacity and stability.
Environmental Impact of Sulphanilic Acid-based H2 Storage
The environmental impact of sulphanilic acid-based hydrogen storage materials is a critical consideration in the development and implementation of these technologies. As the global push for clean energy solutions intensifies, the potential environmental consequences of new storage methods must be thoroughly evaluated.
Sulphanilic acid, when used in hydrogen storage materials, offers promising advantages in terms of storage capacity and efficiency. However, its production and use may have several environmental implications that require careful assessment. The synthesis of sulphanilic acid typically involves the sulfonation of aniline, which can generate waste products and potentially harmful byproducts if not properly managed.
One of the primary environmental concerns is the potential for soil and water contamination. If sulphanilic acid or its precursors are released into the environment during production or storage processes, they could potentially leach into groundwater or accumulate in soil. This could have adverse effects on local ecosystems and potentially enter the food chain.
Air quality is another important factor to consider. The production of sulphanilic acid may release volatile organic compounds (VOCs) and other air pollutants. While modern manufacturing processes have significantly reduced these emissions, the large-scale production required for widespread hydrogen storage applications could still have a cumulative impact on air quality if not properly controlled.
Energy consumption in the production of sulphanilic acid-based storage materials is also a key environmental consideration. The energy-intensive processes involved in synthesizing these materials could potentially offset some of the environmental benefits of hydrogen as a clean energy carrier if not sourced from renewable energy.
On the positive side, sulphanilic acid-based hydrogen storage materials could contribute to a significant reduction in greenhouse gas emissions by enabling more widespread adoption of hydrogen as a clean fuel. This could lead to a net positive environmental impact when considering the lifecycle analysis of these materials compared to conventional fossil fuel technologies.
Recycling and disposal of sulphanilic acid-based storage materials at the end of their life cycle present both challenges and opportunities. Developing efficient recycling processes for these materials could minimize waste and reduce the need for new raw materials, thereby lessening the overall environmental footprint.
In conclusion, while sulphanilic acid-based hydrogen storage materials show promise for advancing clean energy solutions, their environmental impact must be carefully managed throughout the entire lifecycle. This includes optimizing production processes, implementing stringent safety measures to prevent environmental releases, and developing effective recycling strategies. Ongoing research and development efforts should focus on minimizing the potential negative environmental effects while maximizing the benefits of this technology in the transition to a more sustainable energy future.
Sulphanilic acid, when used in hydrogen storage materials, offers promising advantages in terms of storage capacity and efficiency. However, its production and use may have several environmental implications that require careful assessment. The synthesis of sulphanilic acid typically involves the sulfonation of aniline, which can generate waste products and potentially harmful byproducts if not properly managed.
One of the primary environmental concerns is the potential for soil and water contamination. If sulphanilic acid or its precursors are released into the environment during production or storage processes, they could potentially leach into groundwater or accumulate in soil. This could have adverse effects on local ecosystems and potentially enter the food chain.
Air quality is another important factor to consider. The production of sulphanilic acid may release volatile organic compounds (VOCs) and other air pollutants. While modern manufacturing processes have significantly reduced these emissions, the large-scale production required for widespread hydrogen storage applications could still have a cumulative impact on air quality if not properly controlled.
Energy consumption in the production of sulphanilic acid-based storage materials is also a key environmental consideration. The energy-intensive processes involved in synthesizing these materials could potentially offset some of the environmental benefits of hydrogen as a clean energy carrier if not sourced from renewable energy.
On the positive side, sulphanilic acid-based hydrogen storage materials could contribute to a significant reduction in greenhouse gas emissions by enabling more widespread adoption of hydrogen as a clean fuel. This could lead to a net positive environmental impact when considering the lifecycle analysis of these materials compared to conventional fossil fuel technologies.
Recycling and disposal of sulphanilic acid-based storage materials at the end of their life cycle present both challenges and opportunities. Developing efficient recycling processes for these materials could minimize waste and reduce the need for new raw materials, thereby lessening the overall environmental footprint.
In conclusion, while sulphanilic acid-based hydrogen storage materials show promise for advancing clean energy solutions, their environmental impact must be carefully managed throughout the entire lifecycle. This includes optimizing production processes, implementing stringent safety measures to prevent environmental releases, and developing effective recycling strategies. Ongoing research and development efforts should focus on minimizing the potential negative environmental effects while maximizing the benefits of this technology in the transition to a more sustainable energy future.
Safety Regulations for H2 Storage Materials
Safety regulations for hydrogen storage materials, including those incorporating sulphanilic acid, are critical for ensuring the safe handling, storage, and use of these materials. The primary focus of these regulations is to mitigate the risks associated with hydrogen's high flammability and potential for explosive reactions.
Regulatory bodies such as the International Organization for Standardization (ISO) and the National Fire Protection Association (NFPA) have established comprehensive guidelines for hydrogen storage. These guidelines cover various aspects, including material selection, design specifications, and operational procedures. For instance, ISO 16111 provides specific requirements for transportable gas storage devices containing hydrogen absorbed in reversible metal hydrides.
The storage of hydrogen in materials containing sulphanilic acid requires additional safety considerations due to the chemical properties of sulphanilic acid. Regulations typically mandate the use of appropriate containment systems that are resistant to corrosion and capable of withstanding high pressures. These systems must be designed to prevent leakage and minimize the risk of accidental release.
Safety regulations also address the importance of proper ventilation in storage areas. Given hydrogen's propensity to accumulate in enclosed spaces, storage facilities must be equipped with adequate ventilation systems to prevent the buildup of potentially explosive concentrations. Additionally, regulations often require the installation of hydrogen detection systems to provide early warning of any leaks.
Fire safety is another crucial aspect covered by these regulations. Storage areas must be equipped with appropriate fire suppression systems, and materials used in the construction of storage facilities must be fire-resistant. The regulations also specify minimum safe distances between hydrogen storage units and other structures or potential ignition sources.
Personal protective equipment (PPE) requirements are typically outlined in safety regulations for handling hydrogen storage materials. This may include specialized clothing, gloves, and respiratory protection when working with materials containing sulphanilic acid. Training requirements for personnel involved in the handling and storage of these materials are also typically specified to ensure proper understanding of safety protocols.
Emergency response procedures form a significant part of safety regulations. These procedures outline the steps to be taken in case of leaks, fires, or other incidents involving hydrogen storage materials. They often include evacuation plans, communication protocols, and coordination with local emergency services.
Regular inspection and maintenance requirements are typically mandated to ensure the ongoing integrity and safety of hydrogen storage systems. This includes periodic testing of storage vessels, monitoring of pressure relief devices, and checks for any signs of material degradation or corrosion, particularly in systems incorporating sulphanilic acid.
Regulatory bodies such as the International Organization for Standardization (ISO) and the National Fire Protection Association (NFPA) have established comprehensive guidelines for hydrogen storage. These guidelines cover various aspects, including material selection, design specifications, and operational procedures. For instance, ISO 16111 provides specific requirements for transportable gas storage devices containing hydrogen absorbed in reversible metal hydrides.
The storage of hydrogen in materials containing sulphanilic acid requires additional safety considerations due to the chemical properties of sulphanilic acid. Regulations typically mandate the use of appropriate containment systems that are resistant to corrosion and capable of withstanding high pressures. These systems must be designed to prevent leakage and minimize the risk of accidental release.
Safety regulations also address the importance of proper ventilation in storage areas. Given hydrogen's propensity to accumulate in enclosed spaces, storage facilities must be equipped with adequate ventilation systems to prevent the buildup of potentially explosive concentrations. Additionally, regulations often require the installation of hydrogen detection systems to provide early warning of any leaks.
Fire safety is another crucial aspect covered by these regulations. Storage areas must be equipped with appropriate fire suppression systems, and materials used in the construction of storage facilities must be fire-resistant. The regulations also specify minimum safe distances between hydrogen storage units and other structures or potential ignition sources.
Personal protective equipment (PPE) requirements are typically outlined in safety regulations for handling hydrogen storage materials. This may include specialized clothing, gloves, and respiratory protection when working with materials containing sulphanilic acid. Training requirements for personnel involved in the handling and storage of these materials are also typically specified to ensure proper understanding of safety protocols.
Emergency response procedures form a significant part of safety regulations. These procedures outline the steps to be taken in case of leaks, fires, or other incidents involving hydrogen storage materials. They often include evacuation plans, communication protocols, and coordination with local emergency services.
Regular inspection and maintenance requirements are typically mandated to ensure the ongoing integrity and safety of hydrogen storage systems. This includes periodic testing of storage vessels, monitoring of pressure relief devices, and checks for any signs of material degradation or corrosion, particularly in systems incorporating sulphanilic acid.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!