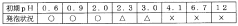
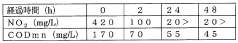
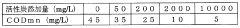
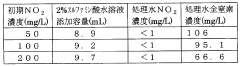
Application of Sulphanilic Acid in Water Treatment: Nitrite Removal Mechanisms
JUL 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulphanilic Acid in Water Treatment: Background and Objectives
Sulphanilic acid, a synthetic organic compound with the chemical formula C6H7NO3S, has emerged as a promising agent in water treatment processes, particularly for nitrite removal. The evolution of water treatment technologies has been driven by the increasing global demand for clean water and the need to address various contaminants, including nitrites, which pose significant health risks when present in drinking water.
The application of sulphanilic acid in water treatment represents a convergence of chemical engineering and environmental science. This technology has its roots in the broader field of advanced oxidation processes (AOPs), which have been developed over the past few decades to tackle persistent water pollutants. The use of sulphanilic acid for nitrite removal is a relatively recent development, building upon the understanding of azo dye degradation mechanisms and the reactivity of aromatic amines.
The primary objective of employing sulphanilic acid in water treatment is to effectively and efficiently remove nitrite ions from water sources. Nitrites are intermediate compounds in the nitrogen cycle and can accumulate in water due to incomplete nitrification or denitrification processes. Their presence in drinking water has been linked to methemoglobinemia, especially in infants, and the formation of carcinogenic nitrosamines.
Research into sulphanilic acid's application for nitrite removal aims to develop a cost-effective, environmentally friendly, and scalable solution that can be integrated into existing water treatment infrastructure. The technology seeks to overcome limitations of conventional nitrite removal methods, such as biological denitrification or ion exchange, which may be slow, expensive, or produce secondary waste streams.
The exploration of sulphanilic acid in this context aligns with the broader trends in water treatment technology, including the development of sustainable treatment processes, the minimization of chemical inputs, and the enhancement of treatment efficiency. As water scarcity and quality issues become more pressing globally, innovative approaches like the use of sulphanilic acid for targeted contaminant removal are gaining importance.
Understanding the mechanisms by which sulphanilic acid interacts with nitrite ions is crucial for optimizing its application in water treatment systems. This involves investigating the chemical kinetics, reaction pathways, and potential by-products of the nitrite removal process. Additionally, research efforts are focused on determining the optimal conditions for sulphanilic acid's effectiveness, including pH, temperature, and concentration ratios.
The development of this technology also necessitates consideration of its integration with other water treatment processes, its long-term stability and performance, and any potential environmental impacts. As such, the research objectives extend beyond mere nitrite removal to encompass a holistic approach to water purification and safety.
The application of sulphanilic acid in water treatment represents a convergence of chemical engineering and environmental science. This technology has its roots in the broader field of advanced oxidation processes (AOPs), which have been developed over the past few decades to tackle persistent water pollutants. The use of sulphanilic acid for nitrite removal is a relatively recent development, building upon the understanding of azo dye degradation mechanisms and the reactivity of aromatic amines.
The primary objective of employing sulphanilic acid in water treatment is to effectively and efficiently remove nitrite ions from water sources. Nitrites are intermediate compounds in the nitrogen cycle and can accumulate in water due to incomplete nitrification or denitrification processes. Their presence in drinking water has been linked to methemoglobinemia, especially in infants, and the formation of carcinogenic nitrosamines.
Research into sulphanilic acid's application for nitrite removal aims to develop a cost-effective, environmentally friendly, and scalable solution that can be integrated into existing water treatment infrastructure. The technology seeks to overcome limitations of conventional nitrite removal methods, such as biological denitrification or ion exchange, which may be slow, expensive, or produce secondary waste streams.
The exploration of sulphanilic acid in this context aligns with the broader trends in water treatment technology, including the development of sustainable treatment processes, the minimization of chemical inputs, and the enhancement of treatment efficiency. As water scarcity and quality issues become more pressing globally, innovative approaches like the use of sulphanilic acid for targeted contaminant removal are gaining importance.
Understanding the mechanisms by which sulphanilic acid interacts with nitrite ions is crucial for optimizing its application in water treatment systems. This involves investigating the chemical kinetics, reaction pathways, and potential by-products of the nitrite removal process. Additionally, research efforts are focused on determining the optimal conditions for sulphanilic acid's effectiveness, including pH, temperature, and concentration ratios.
The development of this technology also necessitates consideration of its integration with other water treatment processes, its long-term stability and performance, and any potential environmental impacts. As such, the research objectives extend beyond mere nitrite removal to encompass a holistic approach to water purification and safety.
Market Analysis for Nitrite Removal Technologies
The market for nitrite removal technologies in water treatment has been experiencing significant growth due to increasing awareness of the health risks associated with nitrite contamination and stricter environmental regulations. The global water treatment chemicals market, which includes nitrite removal solutions, is projected to reach a substantial value in the coming years, driven by urbanization, industrialization, and the growing demand for clean water.
Sulphanilic acid, as a potential agent for nitrite removal, enters a competitive landscape dominated by established technologies such as ion exchange, reverse osmosis, and biological denitrification. However, the unique properties of sulphanilic acid in nitrite removal mechanisms present an opportunity for market differentiation and potential disruption of existing solutions.
The demand for nitrite removal technologies spans various sectors, including municipal water treatment, industrial wastewater management, and aquaculture. Municipal water treatment represents the largest market segment, as governments worldwide invest in upgrading water infrastructure to meet increasingly stringent water quality standards. The industrial sector, particularly food and beverage processing, chemical manufacturing, and mining, also contributes significantly to the market demand for nitrite removal solutions.
Geographically, North America and Europe currently lead the market for nitrite removal technologies, owing to advanced water treatment infrastructure and stringent regulatory frameworks. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by rapid industrialization, population growth, and increasing government initiatives to address water pollution.
The market is characterized by a mix of large multinational corporations and specialized water treatment companies. Key players in the nitrite removal market include Dow Chemical Company, BASF SE, Evoqua Water Technologies, and Veolia Water Technologies. These companies offer a range of solutions, from chemical treatments to advanced filtration systems.
The introduction of sulphanilic acid-based nitrite removal technologies could potentially disrupt the existing market dynamics. Its effectiveness, cost-efficiency, and environmental compatibility could appeal to both municipal and industrial customers seeking alternatives to traditional nitrite removal methods. However, market penetration would depend on factors such as scalability, regulatory approval, and the ability to demonstrate long-term performance and cost benefits over existing technologies.
As water scarcity and quality issues continue to be global concerns, the market for nitrite removal technologies is expected to expand. The potential application of sulphanilic acid in this field aligns with the growing trend towards more sustainable and efficient water treatment solutions, positioning it as a promising contender in the evolving landscape of water purification technologies.
Sulphanilic acid, as a potential agent for nitrite removal, enters a competitive landscape dominated by established technologies such as ion exchange, reverse osmosis, and biological denitrification. However, the unique properties of sulphanilic acid in nitrite removal mechanisms present an opportunity for market differentiation and potential disruption of existing solutions.
The demand for nitrite removal technologies spans various sectors, including municipal water treatment, industrial wastewater management, and aquaculture. Municipal water treatment represents the largest market segment, as governments worldwide invest in upgrading water infrastructure to meet increasingly stringent water quality standards. The industrial sector, particularly food and beverage processing, chemical manufacturing, and mining, also contributes significantly to the market demand for nitrite removal solutions.
Geographically, North America and Europe currently lead the market for nitrite removal technologies, owing to advanced water treatment infrastructure and stringent regulatory frameworks. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by rapid industrialization, population growth, and increasing government initiatives to address water pollution.
The market is characterized by a mix of large multinational corporations and specialized water treatment companies. Key players in the nitrite removal market include Dow Chemical Company, BASF SE, Evoqua Water Technologies, and Veolia Water Technologies. These companies offer a range of solutions, from chemical treatments to advanced filtration systems.
The introduction of sulphanilic acid-based nitrite removal technologies could potentially disrupt the existing market dynamics. Its effectiveness, cost-efficiency, and environmental compatibility could appeal to both municipal and industrial customers seeking alternatives to traditional nitrite removal methods. However, market penetration would depend on factors such as scalability, regulatory approval, and the ability to demonstrate long-term performance and cost benefits over existing technologies.
As water scarcity and quality issues continue to be global concerns, the market for nitrite removal technologies is expected to expand. The potential application of sulphanilic acid in this field aligns with the growing trend towards more sustainable and efficient water treatment solutions, positioning it as a promising contender in the evolving landscape of water purification technologies.
Current Challenges in Nitrite Removal Techniques
Despite significant advancements in water treatment technologies, nitrite removal remains a persistent challenge in the field. One of the primary obstacles is the complex nature of nitrite chemistry in aqueous environments. Nitrite ions exhibit varying stability and reactivity under different pH and temperature conditions, making it difficult to develop a universally effective removal method.
The presence of competing ions in water sources further complicates nitrite removal processes. Sulfate, chloride, and other anions can interfere with the selectivity of removal techniques, reducing their efficiency and necessitating additional treatment steps. This interference not only decreases the overall effectiveness of nitrite removal but also increases operational costs and complexity.
Another significant challenge is the limited capacity and longevity of current nitrite removal materials. Many adsorbents and ion exchange resins used for nitrite removal suffer from rapid saturation and require frequent regeneration or replacement. This leads to increased operational downtime and higher maintenance costs, making large-scale implementation less economically viable.
The environmental impact of nitrite removal techniques is also a growing concern. Some methods, particularly those involving chemical reduction, can produce harmful by-products or require the use of potentially hazardous reagents. Balancing effective nitrite removal with environmental sustainability remains a significant challenge for researchers and water treatment professionals.
Moreover, the variability in nitrite concentrations across different water sources poses a challenge for developing standardized treatment protocols. Water sources with fluctuating nitrite levels require adaptive treatment systems, which are often more complex and expensive to implement and maintain.
The energy intensity of some nitrite removal techniques, especially those involving advanced oxidation processes or electrochemical methods, presents another hurdle. High energy consumption not only increases operational costs but also contributes to the carbon footprint of water treatment facilities, conflicting with sustainability goals.
Lastly, the integration of nitrite removal processes into existing water treatment infrastructure without disrupting other treatment steps remains a significant engineering challenge. Retrofitting existing plants or designing new facilities that can effectively incorporate nitrite removal while maintaining overall water quality and treatment efficiency is an ongoing area of research and development in the water treatment industry.
The presence of competing ions in water sources further complicates nitrite removal processes. Sulfate, chloride, and other anions can interfere with the selectivity of removal techniques, reducing their efficiency and necessitating additional treatment steps. This interference not only decreases the overall effectiveness of nitrite removal but also increases operational costs and complexity.
Another significant challenge is the limited capacity and longevity of current nitrite removal materials. Many adsorbents and ion exchange resins used for nitrite removal suffer from rapid saturation and require frequent regeneration or replacement. This leads to increased operational downtime and higher maintenance costs, making large-scale implementation less economically viable.
The environmental impact of nitrite removal techniques is also a growing concern. Some methods, particularly those involving chemical reduction, can produce harmful by-products or require the use of potentially hazardous reagents. Balancing effective nitrite removal with environmental sustainability remains a significant challenge for researchers and water treatment professionals.
Moreover, the variability in nitrite concentrations across different water sources poses a challenge for developing standardized treatment protocols. Water sources with fluctuating nitrite levels require adaptive treatment systems, which are often more complex and expensive to implement and maintain.
The energy intensity of some nitrite removal techniques, especially those involving advanced oxidation processes or electrochemical methods, presents another hurdle. High energy consumption not only increases operational costs but also contributes to the carbon footprint of water treatment facilities, conflicting with sustainability goals.
Lastly, the integration of nitrite removal processes into existing water treatment infrastructure without disrupting other treatment steps remains a significant engineering challenge. Retrofitting existing plants or designing new facilities that can effectively incorporate nitrite removal while maintaining overall water quality and treatment efficiency is an ongoing area of research and development in the water treatment industry.
Existing Sulphanilic Acid-based Nitrite Removal Solutions
01 Chemical treatment for sulphanilic acid nitrite removal
Various chemical treatments can be employed to remove sulphanilic acid nitrite from solutions. These methods may involve oxidation, reduction, or other chemical reactions to convert the nitrite into less harmful compounds or facilitate its separation from the solution.- Chemical treatment methods for sulphanilic acid nitrite removal: Various chemical treatment methods can be employed for the removal of sulphanilic acid nitrite. These methods may involve oxidation, reduction, or neutralization processes to convert the nitrite into less harmful compounds or to facilitate its separation from the solution.
- Adsorption techniques for nitrite removal: Adsorption techniques can be effective for removing nitrite from solutions containing sulphanilic acid. This may involve the use of activated carbon, ion exchange resins, or other adsorbent materials that can selectively capture nitrite ions from the solution.
- Biological treatment for nitrite removal: Biological treatment methods can be employed to remove nitrite from sulphanilic acid-containing wastewater. This may involve the use of specific microorganisms or enzymes that can convert nitrite into nitrogen gas or other harmless compounds.
- Membrane-based separation techniques: Membrane-based separation techniques, such as reverse osmosis or nanofiltration, can be used to remove nitrite from sulphanilic acid solutions. These methods rely on selective permeation of molecules through specialized membranes to achieve separation.
- Combined treatment approaches for enhanced nitrite removal: Combining multiple treatment methods can lead to more effective removal of nitrite from sulphanilic acid solutions. This may involve sequential application of different techniques or the development of hybrid processes that integrate multiple removal mechanisms.
02 Adsorption techniques for nitrite removal
Adsorption methods can be effective for removing sulphanilic acid nitrite from water or other solutions. This may involve the use of activated carbon, ion exchange resins, or other adsorbent materials that can selectively capture the nitrite compounds.Expand Specific Solutions03 Biological treatment for nitrite removal
Biological processes can be used to remove sulphanilic acid nitrite from wastewater or contaminated solutions. This may involve the use of specific microorganisms or enzymes that can metabolize or transform the nitrite compounds into less harmful substances.Expand Specific Solutions04 Membrane-based separation for nitrite removal
Membrane technologies, such as reverse osmosis or nanofiltration, can be employed to separate sulphanilic acid nitrite from solutions. These methods rely on selective permeability of membranes to remove the nitrite compounds while allowing other components to pass through.Expand Specific Solutions05 Electrochemical methods for nitrite removal
Electrochemical techniques can be used to remove sulphanilic acid nitrite from water or other solutions. These methods may involve electrolysis, electrocoagulation, or other electrochemical processes to convert or separate the nitrite compounds.Expand Specific Solutions
Key Players in Water Treatment Industry
The application of sulphanilic acid in water treatment for nitrite removal is an emerging field with growing market potential. The industry is in its early growth stage, driven by increasing environmental regulations and water quality concerns. The global market for advanced water treatment technologies is expanding, with a projected CAGR of 6-8% over the next five years. Technologically, the field is still developing, with ongoing research to optimize removal mechanisms and efficiency. Key players like Evoqua Water Technologies, BASF Corp., and Schlumberger Technologies are investing in R&D to enhance their offerings. Academic institutions such as Harbin Institute of Technology and Tsinghua University are contributing to fundamental research, while companies like BioSO4 Oy are focusing on innovative applications. The competitive landscape is diverse, with both established water treatment companies and specialized startups vying for market share.
Evoqua Water Technologies LLC
Technical Solution: Evoqua Water Technologies LLC has pioneered a sulphanilic acid-based nitrite removal system that combines chemical reduction with advanced oxidation processes. Their technology utilizes a two-stage treatment approach: first, sulphanilic acid is used to reduce nitrite to nitrogen gas, followed by an UV-assisted oxidation step to break down any remaining organic compounds, including excess sulphanilic acid [2]. This dual-action process ensures comprehensive water purification. Evoqua's system also incorporates smart dosing technology that adjusts sulphanilic acid application based on real-time nitrite concentration measurements, optimizing treatment efficiency and reducing operational costs [4].
Strengths: Comprehensive water treatment beyond nitrite removal, automated dosing for improved efficiency. Weaknesses: Higher energy consumption due to UV treatment, may require more complex maintenance.
Harbin Institute of Technology
Technical Solution: Researchers at Harbin Institute of Technology have developed a novel approach to nitrite removal using sulphanilic acid-modified biochar. This innovative method combines the reducing properties of sulphanilic acid with the adsorptive capabilities of biochar. The biochar is functionalized with sulphanilic acid through a simple and cost-effective process, creating a hybrid material that effectively removes nitrite from water [5]. Laboratory studies have shown that this material can achieve up to 95% nitrite removal efficiency in various water matrices, including groundwater and surface water [6]. The mechanism involves both adsorption of nitrite onto the biochar surface and subsequent reduction by the sulphanilic acid moieties.
Strengths: High removal efficiency, utilizes sustainable biochar, applicable to various water sources. Weaknesses: May require frequent regeneration or replacement of the adsorbent material, potential for secondary pollution if not properly managed.
Core Mechanisms of Sulphanilic Acid in Nitrite Removal
Method for treating nitrite based anticorrosive-containing waste water
PatentInactiveJP2006305462A
Innovation
- The method involves adding sulfamic acid in an amount of 0.8 to 1.2 times the nitrite ion concentration, adjusting the pH to 2 or less with a nitrogen-free acid, and using activated carbon to adsorb residual COD components, ensuring complete decomposition of nitrite ions and removal of nitrogen and COD.
System and method for removing nitrate from water
PatentActiveUS20210276891A1
Innovation
- A system comprising a porous oxide-derived silver electrode (OD-Ag) for electrocatalytic reduction of nitrate to nitrite, followed by a Pd-based catalyst for catalytic reduction of nitrite, achieving high selectivity and efficiency in converting nitrate to nitrite with minimal ammonia production.
Environmental Impact Assessment
The application of sulphanilic acid in water treatment for nitrite removal has significant environmental implications that warrant careful consideration. The use of this chemical compound in water treatment processes can lead to both positive and negative impacts on the environment.
On the positive side, the effective removal of nitrites from water bodies contributes to the overall improvement of water quality. Nitrites are known to be harmful to aquatic ecosystems and human health, particularly when present in high concentrations. By reducing nitrite levels, sulphanilic acid treatment helps maintain the ecological balance of water systems and protects various aquatic species from potential toxic effects. This, in turn, supports biodiversity and the overall health of aquatic habitats.
However, the environmental impact of sulphanilic acid usage is not without concerns. The chemical itself, if not properly managed, can become a pollutant. Residual sulphanilic acid or its byproducts in treated water may have unintended consequences on the receiving environment. There is a need for comprehensive studies to assess the long-term effects of these residues on aquatic flora and fauna, as well as potential bioaccumulation in the food chain.
The production and transportation of sulphanilic acid also contribute to the overall environmental footprint of this water treatment method. Manufacturing processes may involve energy-intensive steps and the use of other chemicals, potentially leading to greenhouse gas emissions and other forms of industrial pollution. Additionally, the transportation of the chemical to water treatment facilities adds to carbon emissions and the risk of accidental spills during transit.
Proper disposal of waste products generated during the nitrite removal process is another critical environmental consideration. The treatment may produce sludge or other byproducts that require appropriate handling and disposal to prevent soil or groundwater contamination. Implementing effective waste management protocols is essential to mitigate these potential environmental risks.
It is also important to consider the energy requirements of the sulphanilic acid treatment process. Depending on the specific application method, additional energy may be needed for mixing, pumping, or other treatment steps. This increased energy consumption could contribute to the overall carbon footprint of water treatment facilities.
To fully assess the environmental impact of sulphanilic acid in water treatment, a life cycle analysis approach is recommended. This would involve evaluating the environmental effects from raw material extraction, through production, use, and final disposal. Such a comprehensive analysis would provide a more accurate picture of the net environmental impact and help identify areas for potential improvement in the treatment process.
In conclusion, while the application of sulphanilic acid for nitrite removal in water treatment offers significant benefits in terms of water quality improvement, it is crucial to carefully manage and monitor its use to minimize potential negative environmental impacts. Ongoing research and development efforts should focus on optimizing the treatment process to enhance efficiency and reduce any adverse environmental effects.
On the positive side, the effective removal of nitrites from water bodies contributes to the overall improvement of water quality. Nitrites are known to be harmful to aquatic ecosystems and human health, particularly when present in high concentrations. By reducing nitrite levels, sulphanilic acid treatment helps maintain the ecological balance of water systems and protects various aquatic species from potential toxic effects. This, in turn, supports biodiversity and the overall health of aquatic habitats.
However, the environmental impact of sulphanilic acid usage is not without concerns. The chemical itself, if not properly managed, can become a pollutant. Residual sulphanilic acid or its byproducts in treated water may have unintended consequences on the receiving environment. There is a need for comprehensive studies to assess the long-term effects of these residues on aquatic flora and fauna, as well as potential bioaccumulation in the food chain.
The production and transportation of sulphanilic acid also contribute to the overall environmental footprint of this water treatment method. Manufacturing processes may involve energy-intensive steps and the use of other chemicals, potentially leading to greenhouse gas emissions and other forms of industrial pollution. Additionally, the transportation of the chemical to water treatment facilities adds to carbon emissions and the risk of accidental spills during transit.
Proper disposal of waste products generated during the nitrite removal process is another critical environmental consideration. The treatment may produce sludge or other byproducts that require appropriate handling and disposal to prevent soil or groundwater contamination. Implementing effective waste management protocols is essential to mitigate these potential environmental risks.
It is also important to consider the energy requirements of the sulphanilic acid treatment process. Depending on the specific application method, additional energy may be needed for mixing, pumping, or other treatment steps. This increased energy consumption could contribute to the overall carbon footprint of water treatment facilities.
To fully assess the environmental impact of sulphanilic acid in water treatment, a life cycle analysis approach is recommended. This would involve evaluating the environmental effects from raw material extraction, through production, use, and final disposal. Such a comprehensive analysis would provide a more accurate picture of the net environmental impact and help identify areas for potential improvement in the treatment process.
In conclusion, while the application of sulphanilic acid for nitrite removal in water treatment offers significant benefits in terms of water quality improvement, it is crucial to carefully manage and monitor its use to minimize potential negative environmental impacts. Ongoing research and development efforts should focus on optimizing the treatment process to enhance efficiency and reduce any adverse environmental effects.
Regulatory Framework for Water Treatment Chemicals
The regulatory framework for water treatment chemicals plays a crucial role in ensuring the safety and efficacy of water treatment processes, including the application of sulphanilic acid for nitrite removal. In the United States, the Environmental Protection Agency (EPA) is the primary regulatory body overseeing water treatment chemicals under the Safe Drinking Water Act (SDWA).
The EPA has established the National Primary Drinking Water Regulations (NPDWRs), which set legally enforceable standards for various contaminants in drinking water. These regulations include Maximum Contaminant Levels (MCLs) for nitrite, which is relevant to the application of sulphanilic acid in water treatment. The current MCL for nitrite is set at 1 mg/L as nitrogen.
In addition to the EPA, state-level environmental agencies often have their own regulations and guidelines for water treatment chemicals. These state-specific regulations may impose additional requirements or stricter standards than those set by the EPA. Water treatment facilities must comply with both federal and state regulations to ensure the safety of treated water.
The use of sulphanilic acid in water treatment must also adhere to the National Sanitation Foundation (NSF) standards, particularly NSF/ANSI Standard 60: Drinking Water Treatment Chemicals - Health Effects. This standard evaluates the safety of chemicals used in water treatment and establishes maximum use levels to protect public health.
Internationally, the World Health Organization (WHO) provides guidelines for drinking water quality, which many countries use as a basis for their own regulations. The WHO guideline value for nitrite in drinking water is 3 mg/L (short-term exposure) and 0.2 mg/L (long-term exposure).
The European Union's Drinking Water Directive (98/83/EC) sets standards for drinking water quality across EU member states. The directive establishes a parametric value of 0.5 mg/L for nitrite, which is more stringent than the EPA's standard.
Regulatory bodies also require extensive testing and documentation for water treatment chemicals. Manufacturers of sulphanilic acid and related products must provide safety data sheets, toxicological studies, and efficacy data to demonstrate compliance with regulatory standards. Regular monitoring and reporting of water quality parameters, including nitrite levels, are mandatory for water treatment facilities.
As research on water treatment technologies advances, regulatory frameworks continue to evolve. Emerging contaminants and new treatment methods are regularly evaluated for potential inclusion in updated regulations. This dynamic regulatory environment ensures that water treatment processes, including those utilizing sulphanilic acid for nitrite removal, remain effective and safe for public consumption.
The EPA has established the National Primary Drinking Water Regulations (NPDWRs), which set legally enforceable standards for various contaminants in drinking water. These regulations include Maximum Contaminant Levels (MCLs) for nitrite, which is relevant to the application of sulphanilic acid in water treatment. The current MCL for nitrite is set at 1 mg/L as nitrogen.
In addition to the EPA, state-level environmental agencies often have their own regulations and guidelines for water treatment chemicals. These state-specific regulations may impose additional requirements or stricter standards than those set by the EPA. Water treatment facilities must comply with both federal and state regulations to ensure the safety of treated water.
The use of sulphanilic acid in water treatment must also adhere to the National Sanitation Foundation (NSF) standards, particularly NSF/ANSI Standard 60: Drinking Water Treatment Chemicals - Health Effects. This standard evaluates the safety of chemicals used in water treatment and establishes maximum use levels to protect public health.
Internationally, the World Health Organization (WHO) provides guidelines for drinking water quality, which many countries use as a basis for their own regulations. The WHO guideline value for nitrite in drinking water is 3 mg/L (short-term exposure) and 0.2 mg/L (long-term exposure).
The European Union's Drinking Water Directive (98/83/EC) sets standards for drinking water quality across EU member states. The directive establishes a parametric value of 0.5 mg/L for nitrite, which is more stringent than the EPA's standard.
Regulatory bodies also require extensive testing and documentation for water treatment chemicals. Manufacturers of sulphanilic acid and related products must provide safety data sheets, toxicological studies, and efficacy data to demonstrate compliance with regulatory standards. Regular monitoring and reporting of water quality parameters, including nitrite levels, are mandatory for water treatment facilities.
As research on water treatment technologies advances, regulatory frameworks continue to evolve. Emerging contaminants and new treatment methods are regularly evaluated for potential inclusion in updated regulations. This dynamic regulatory environment ensures that water treatment processes, including those utilizing sulphanilic acid for nitrite removal, remain effective and safe for public consumption.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!