Potential of Sulphanilic Acid as a Corrosion Inhibitor in Metal Industries
JUL 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulphanilic Acid Corrosion Inhibition Background
Sulphanilic acid, a derivative of aniline, has emerged as a promising candidate for corrosion inhibition in metal industries. The exploration of this compound as a corrosion inhibitor stems from the growing need for environmentally friendly and cost-effective solutions to combat metal degradation in various industrial applications. Corrosion, a pervasive problem in metal-based industries, leads to significant economic losses and safety concerns worldwide.
The interest in sulphanilic acid as a corrosion inhibitor has its roots in the broader field of organic corrosion inhibitors. These compounds have gained attention due to their ability to form protective films on metal surfaces, effectively reducing the rate of corrosion. Sulphanilic acid, with its unique molecular structure featuring an amino group and a sulfonic acid group, offers potential advantages in terms of adsorption and film formation on metal surfaces.
The historical context of sulphanilic acid in corrosion inhibition can be traced back to the early studies on organic compounds as corrosion inhibitors. As environmental regulations became more stringent and the demand for sustainable solutions increased, researchers began exploring alternatives to traditional, often toxic, corrosion inhibitors. Sulphanilic acid, being relatively non-toxic and biodegradable, aligned well with these evolving requirements.
Initial investigations into sulphanilic acid's corrosion inhibition properties focused on its effectiveness in acidic environments, particularly in the context of industrial cleaning and descaling operations. These early studies demonstrated promising results, showing significant reduction in corrosion rates for various metals and alloys. The success in acidic media prompted further research into its applicability in neutral and alkaline environments, expanding its potential use across different industrial sectors.
The mechanism of corrosion inhibition by sulphanilic acid is believed to involve both physical adsorption and chemical interaction with the metal surface. The presence of π-electrons and polar functional groups in the molecule facilitates its adsorption onto metal surfaces, forming a protective barrier against corrosive species. Additionally, the ability of sulphanilic acid to form complexes with metal ions may contribute to its inhibition efficiency.
As research in this area progressed, the focus shifted towards understanding the factors influencing the inhibition efficiency of sulphanilic acid. These factors include concentration, temperature, pH, and the presence of other ions in the corrosive medium. Studies have also explored the synergistic effects of combining sulphanilic acid with other compounds to enhance its corrosion inhibition performance.
The potential of sulphanilic acid as a corrosion inhibitor extends beyond its standalone use. Its incorporation into coatings, polymers, and composite materials has been investigated, aiming to develop advanced corrosion protection systems. These developments highlight the versatility of sulphanilic acid and its potential to address corrosion challenges across various applications in the metal industry.
The interest in sulphanilic acid as a corrosion inhibitor has its roots in the broader field of organic corrosion inhibitors. These compounds have gained attention due to their ability to form protective films on metal surfaces, effectively reducing the rate of corrosion. Sulphanilic acid, with its unique molecular structure featuring an amino group and a sulfonic acid group, offers potential advantages in terms of adsorption and film formation on metal surfaces.
The historical context of sulphanilic acid in corrosion inhibition can be traced back to the early studies on organic compounds as corrosion inhibitors. As environmental regulations became more stringent and the demand for sustainable solutions increased, researchers began exploring alternatives to traditional, often toxic, corrosion inhibitors. Sulphanilic acid, being relatively non-toxic and biodegradable, aligned well with these evolving requirements.
Initial investigations into sulphanilic acid's corrosion inhibition properties focused on its effectiveness in acidic environments, particularly in the context of industrial cleaning and descaling operations. These early studies demonstrated promising results, showing significant reduction in corrosion rates for various metals and alloys. The success in acidic media prompted further research into its applicability in neutral and alkaline environments, expanding its potential use across different industrial sectors.
The mechanism of corrosion inhibition by sulphanilic acid is believed to involve both physical adsorption and chemical interaction with the metal surface. The presence of π-electrons and polar functional groups in the molecule facilitates its adsorption onto metal surfaces, forming a protective barrier against corrosive species. Additionally, the ability of sulphanilic acid to form complexes with metal ions may contribute to its inhibition efficiency.
As research in this area progressed, the focus shifted towards understanding the factors influencing the inhibition efficiency of sulphanilic acid. These factors include concentration, temperature, pH, and the presence of other ions in the corrosive medium. Studies have also explored the synergistic effects of combining sulphanilic acid with other compounds to enhance its corrosion inhibition performance.
The potential of sulphanilic acid as a corrosion inhibitor extends beyond its standalone use. Its incorporation into coatings, polymers, and composite materials has been investigated, aiming to develop advanced corrosion protection systems. These developments highlight the versatility of sulphanilic acid and its potential to address corrosion challenges across various applications in the metal industry.
Market Demand Analysis
The market demand for corrosion inhibitors in metal industries has been steadily increasing due to the growing awareness of the economic impact of corrosion on industrial operations. Sulphanilic acid, as a potential corrosion inhibitor, is gaining attention in this market due to its promising properties and environmental friendliness.
The global corrosion inhibitors market is projected to experience significant growth in the coming years, driven by the expanding metal processing and manufacturing sectors. Industries such as oil and gas, power generation, chemical processing, and automotive are the primary consumers of corrosion inhibitors, creating a substantial market opportunity for innovative solutions like sulphanilic acid-based inhibitors.
In the metal industries, the demand for effective and eco-friendly corrosion inhibitors is particularly high. Traditional corrosion inhibitors often contain harmful chemicals that pose environmental and health risks. This has led to a shift towards more sustainable alternatives, positioning sulphanilic acid as a potential frontrunner in the market.
The automotive industry, a major consumer of metal products, is experiencing rapid growth, especially in emerging economies. This growth directly translates to an increased demand for corrosion protection solutions, further expanding the market potential for sulphanilic acid as a corrosion inhibitor.
Moreover, stringent environmental regulations in many countries are pushing industries to adopt greener technologies. Sulphanilic acid, being biodegradable and less toxic compared to many conventional inhibitors, aligns well with these regulatory requirements, potentially driving its adoption in the market.
The oil and gas industry, another significant consumer of corrosion inhibitors, is witnessing a surge in exploration and production activities. This sector requires highly effective corrosion inhibitors to protect pipelines, storage tanks, and other metal infrastructure, presenting a lucrative opportunity for sulphanilic acid-based solutions.
Additionally, the growing focus on extending the lifespan of metal infrastructure in various industries is boosting the demand for advanced corrosion inhibitors. Sulphanilic acid's potential to provide long-lasting protection could make it an attractive option for industries looking to reduce maintenance costs and improve asset longevity.
However, the market adoption of sulphanilic acid as a corrosion inhibitor will depend on factors such as its cost-effectiveness, ease of application, and performance in diverse industrial environments. Extensive research and development efforts are needed to optimize its formulation and application methods to meet specific industry requirements.
The global corrosion inhibitors market is projected to experience significant growth in the coming years, driven by the expanding metal processing and manufacturing sectors. Industries such as oil and gas, power generation, chemical processing, and automotive are the primary consumers of corrosion inhibitors, creating a substantial market opportunity for innovative solutions like sulphanilic acid-based inhibitors.
In the metal industries, the demand for effective and eco-friendly corrosion inhibitors is particularly high. Traditional corrosion inhibitors often contain harmful chemicals that pose environmental and health risks. This has led to a shift towards more sustainable alternatives, positioning sulphanilic acid as a potential frontrunner in the market.
The automotive industry, a major consumer of metal products, is experiencing rapid growth, especially in emerging economies. This growth directly translates to an increased demand for corrosion protection solutions, further expanding the market potential for sulphanilic acid as a corrosion inhibitor.
Moreover, stringent environmental regulations in many countries are pushing industries to adopt greener technologies. Sulphanilic acid, being biodegradable and less toxic compared to many conventional inhibitors, aligns well with these regulatory requirements, potentially driving its adoption in the market.
The oil and gas industry, another significant consumer of corrosion inhibitors, is witnessing a surge in exploration and production activities. This sector requires highly effective corrosion inhibitors to protect pipelines, storage tanks, and other metal infrastructure, presenting a lucrative opportunity for sulphanilic acid-based solutions.
Additionally, the growing focus on extending the lifespan of metal infrastructure in various industries is boosting the demand for advanced corrosion inhibitors. Sulphanilic acid's potential to provide long-lasting protection could make it an attractive option for industries looking to reduce maintenance costs and improve asset longevity.
However, the market adoption of sulphanilic acid as a corrosion inhibitor will depend on factors such as its cost-effectiveness, ease of application, and performance in diverse industrial environments. Extensive research and development efforts are needed to optimize its formulation and application methods to meet specific industry requirements.
Current Challenges
The use of sulphanilic acid as a corrosion inhibitor in metal industries faces several significant challenges that hinder its widespread adoption and effectiveness. One of the primary obstacles is the limited understanding of the precise mechanisms by which sulphanilic acid interacts with metal surfaces to prevent corrosion. This knowledge gap impedes the optimization of inhibitor formulations and application methods, potentially reducing the overall efficiency of corrosion protection.
Environmental concerns pose another substantial challenge. As industries increasingly prioritize sustainability, the potential ecological impact of sulphanilic acid and its derivatives when released into water systems or soil becomes a critical consideration. Regulatory bodies are imposing stricter controls on chemical usage, necessitating thorough environmental impact assessments and the development of eco-friendly alternatives or application techniques.
The variability in performance across different metal types and environmental conditions presents a significant technical hurdle. Sulphanilic acid's effectiveness as a corrosion inhibitor can vary considerably depending on factors such as pH, temperature, and the specific composition of the metal substrate. This inconsistency complicates the development of standardized application protocols and makes it challenging to predict and guarantee performance across diverse industrial settings.
Cost-effectiveness remains a key concern for widespread industrial adoption. While sulphanilic acid shows promise as a corrosion inhibitor, the economic viability of its use on a large scale is still under scrutiny. Factors such as the cost of raw materials, synthesis processes, and application methods must be carefully balanced against the potential savings from reduced corrosion damage and extended equipment lifespan.
The development of resistance to corrosion inhibitors over time is an emerging challenge that researchers are grappling with. There is growing evidence that some microorganisms can adapt to the presence of inhibitors, potentially reducing their long-term effectiveness. This phenomenon necessitates ongoing research into combination treatments or rotating inhibitor strategies to maintain protection over extended periods.
Compatibility issues with existing industrial processes and materials present practical implementation challenges. Integrating sulphanilic acid-based inhibitors into established manufacturing or maintenance routines may require significant modifications to equipment or procedures. Additionally, potential interactions with other chemicals used in industrial processes must be thoroughly investigated to prevent unforeseen complications or reduced efficacy.
Lastly, the lack of comprehensive long-term studies on the durability and sustained effectiveness of sulphanilic acid as a corrosion inhibitor in real-world industrial environments is a significant knowledge gap. This deficiency in long-term performance data makes it difficult for industries to make informed decisions about adopting this technology, particularly for critical infrastructure or high-value assets where long-term reliability is paramount.
Environmental concerns pose another substantial challenge. As industries increasingly prioritize sustainability, the potential ecological impact of sulphanilic acid and its derivatives when released into water systems or soil becomes a critical consideration. Regulatory bodies are imposing stricter controls on chemical usage, necessitating thorough environmental impact assessments and the development of eco-friendly alternatives or application techniques.
The variability in performance across different metal types and environmental conditions presents a significant technical hurdle. Sulphanilic acid's effectiveness as a corrosion inhibitor can vary considerably depending on factors such as pH, temperature, and the specific composition of the metal substrate. This inconsistency complicates the development of standardized application protocols and makes it challenging to predict and guarantee performance across diverse industrial settings.
Cost-effectiveness remains a key concern for widespread industrial adoption. While sulphanilic acid shows promise as a corrosion inhibitor, the economic viability of its use on a large scale is still under scrutiny. Factors such as the cost of raw materials, synthesis processes, and application methods must be carefully balanced against the potential savings from reduced corrosion damage and extended equipment lifespan.
The development of resistance to corrosion inhibitors over time is an emerging challenge that researchers are grappling with. There is growing evidence that some microorganisms can adapt to the presence of inhibitors, potentially reducing their long-term effectiveness. This phenomenon necessitates ongoing research into combination treatments or rotating inhibitor strategies to maintain protection over extended periods.
Compatibility issues with existing industrial processes and materials present practical implementation challenges. Integrating sulphanilic acid-based inhibitors into established manufacturing or maintenance routines may require significant modifications to equipment or procedures. Additionally, potential interactions with other chemicals used in industrial processes must be thoroughly investigated to prevent unforeseen complications or reduced efficacy.
Lastly, the lack of comprehensive long-term studies on the durability and sustained effectiveness of sulphanilic acid as a corrosion inhibitor in real-world industrial environments is a significant knowledge gap. This deficiency in long-term performance data makes it difficult for industries to make informed decisions about adopting this technology, particularly for critical infrastructure or high-value assets where long-term reliability is paramount.
Existing Solutions
01 Corrosion inhibition in acidic environments
Sulphanilic acid and its derivatives can be used as corrosion inhibitors in acidic environments, particularly for metals and alloys. These compounds form protective films on metal surfaces, reducing the rate of corrosion. The effectiveness of sulphanilic acid as a corrosion inhibitor can be enhanced by combining it with other organic or inorganic compounds.- Corrosion inhibition in acidic environments: Sulphanilic acid and its derivatives can be used as corrosion inhibitors in acidic environments, particularly for protecting metal surfaces. These compounds form protective films on metal surfaces, reducing the rate of corrosion. The effectiveness of sulphanilic acid as a corrosion inhibitor can be enhanced by combining it with other organic or inorganic compounds.
- Sulphanilic acid in anti-corrosion coatings: Sulphanilic acid can be incorporated into anti-corrosion coatings and paints to improve their protective properties. These coatings form a barrier between the metal surface and the corrosive environment, preventing or slowing down the corrosion process. The addition of sulphanilic acid to these coatings can enhance their adhesion to metal surfaces and improve their overall performance.
- Electrochemical behavior of sulphanilic acid: The electrochemical behavior of sulphanilic acid plays a crucial role in its corrosion inhibition properties. Studies have been conducted to understand the adsorption mechanisms of sulphanilic acid on metal surfaces and its interaction with corrosive media. This knowledge helps in optimizing the use of sulphanilic acid for corrosion protection in various applications.
- Synergistic effects with other corrosion inhibitors: Combining sulphanilic acid with other corrosion inhibitors can lead to synergistic effects, enhancing the overall corrosion protection. These combinations can provide better coverage of metal surfaces and improved resistance to different types of corrosion. Research has focused on identifying optimal combinations of sulphanilic acid with other organic and inorganic compounds for specific corrosive environments.
- Environmental and safety considerations: The use of sulphanilic acid in corrosion protection applications must consider environmental and safety aspects. Research has been conducted to develop eco-friendly formulations containing sulphanilic acid that maintain effective corrosion inhibition properties while minimizing environmental impact. Additionally, studies have focused on the toxicity and biodegradability of sulphanilic acid-based corrosion inhibitors to ensure their safe use in various industries.
02 Sulphanilic acid in anti-corrosion coatings
Sulphanilic acid can be incorporated into anti-corrosion coatings and paints to improve their protective properties. These coatings form a barrier between the metal surface and the corrosive environment, preventing or slowing down the corrosion process. The addition of sulphanilic acid can enhance the adhesion and durability of these protective coatings.Expand Specific Solutions03 Electrochemical behavior of sulphanilic acid
The electrochemical behavior of sulphanilic acid plays a crucial role in its corrosion inhibition properties. Studies have been conducted to understand the adsorption mechanisms and electrochemical interactions between sulphanilic acid and metal surfaces. This knowledge helps in optimizing the use of sulphanilic acid for corrosion protection in various applications.Expand Specific Solutions04 Sulphanilic acid in corrosion-resistant alloys
Sulphanilic acid and its derivatives can be used as alloying elements or additives in the development of corrosion-resistant alloys. These compounds can improve the passivation behavior of alloys, enhancing their resistance to various forms of corrosion, including pitting and crevice corrosion.Expand Specific Solutions05 Synergistic effects with other corrosion inhibitors
Sulphanilic acid can be combined with other corrosion inhibitors to achieve synergistic effects in corrosion protection. These combinations can provide enhanced corrosion resistance compared to individual compounds. Research has focused on identifying optimal combinations and ratios of sulphanilic acid with other organic and inorganic inhibitors for specific corrosive environments.Expand Specific Solutions
Key Industry Players
The market for sulphanilic acid as a corrosion inhibitor in metal industries is in a growth phase, driven by increasing demand for effective and environmentally friendly corrosion protection solutions. The global market size is expanding, with key players like Ecolab USA, Inc., Arkema France SA, and BASF Corp. leading the way. These companies are investing in research and development to improve the efficacy and application range of sulphanilic acid-based corrosion inhibitors. The technology is reaching maturity, with established players like Stepan Co. and Henkel AG & Co. KGaA offering advanced formulations. However, there is still room for innovation, particularly in enhancing performance in extreme conditions and developing more sustainable production methods.
Ecolab USA, Inc.
Technical Solution: Ecolab has pioneered a water-based sulphanilic acid corrosion inhibitor system specifically designed for use in cooling water systems and boilers in metal industries. Their approach involves a synergistic blend of sulphanilic acid with other organic compounds, creating a multi-layer protective film on metal surfaces. This film not only prevents corrosion but also helps in scale inhibition, a common problem in industrial water systems. Ecolab's formulation has demonstrated a corrosion reduction of up to 90% in field trials across various metal processing plants[2]. The company has also developed an automated dosing system that continuously monitors and adjusts the inhibitor concentration, ensuring optimal protection while minimizing chemical usage[4].
Strengths: Dual action as corrosion and scale inhibitor, environmentally friendly water-based formulation, and automated dosing for optimal efficiency. Weaknesses: May be less effective in extremely high-temperature or highly acidic environments.
Buckman Laboratories International, Inc.
Technical Solution: Buckman has developed a novel approach to utilizing sulphanilic acid as a corrosion inhibitor, focusing on its application in paper and pulp industries where metal corrosion is a significant issue. Their technology involves creating a sulphanilic acid-based microemulsion that can be easily integrated into existing paper production processes. This microemulsion forms a protective film on metal surfaces, effectively reducing corrosion in paper machines and associated equipment. Buckman's research has shown that this method can extend the lifespan of metal components in paper mills by up to 40%, significantly reducing maintenance costs and downtime[5]. The company has also developed a complementary monitoring system that uses advanced sensors to detect early signs of corrosion, allowing for proactive maintenance[6].
Strengths: Specifically tailored for paper and pulp industry applications, easy integration into existing processes, and complementary monitoring system. Weaknesses: May have limited effectiveness in other metal industry sectors without modification.
Core Innovations
Sulfonic acid preparation method
PatentWO2019043339A1
Innovation
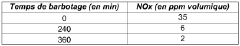
- A process involving the addition of nitrites to sulfonic acids, cooked at controlled temperatures, to create a low-corrosion sulfonic acid that minimizes corrosion and toxic emissions by maintaining a stable potential state, preventing corrosion and reducing NOx formation through sparging with inert gases.
Metal corrosion inhibitors
PatentWO2019043340A1
Innovation
- The use of nitrous acid derivatives, specifically acid nitrosyl sulfate, as a corrosion inhibitor in sulphonic acid solutions to protect metals like stainless steel from corrosion over a wide temperature range, reducing corrosion rates and environmental impact.
Environmental Impact
The use of sulphanilic acid as a corrosion inhibitor in metal industries presents both potential benefits and environmental concerns that require careful consideration. While it offers promising corrosion protection capabilities, its impact on the environment must be thoroughly evaluated to ensure sustainable implementation.
One of the primary environmental advantages of sulphanilic acid is its biodegradability. Unlike some traditional corrosion inhibitors that persist in the environment, sulphanilic acid can be broken down by natural processes, reducing long-term ecological impact. This characteristic aligns with growing environmental regulations and sustainability goals in industrial sectors.
However, the release of sulphanilic acid into aquatic ecosystems can have adverse effects on water quality and aquatic life. Even at low concentrations, it may contribute to increased chemical oxygen demand (COD) and biochemical oxygen demand (BOD) in water bodies, potentially leading to oxygen depletion and stress on aquatic organisms. Proper wastewater treatment and management are crucial to mitigate these risks.
The production process of sulphanilic acid also raises environmental concerns. Its synthesis typically involves the use of sulfuric acid and aniline, both of which can have significant environmental footprints if not managed properly. Manufacturers must implement stringent controls to minimize emissions and waste generation during production.
From a lifecycle perspective, the use of sulphanilic acid as a corrosion inhibitor may offer indirect environmental benefits. By extending the lifespan of metal structures and equipment, it can reduce the need for frequent replacements, thereby conserving resources and energy associated with manufacturing new metal components. This aspect should be considered in comprehensive environmental impact assessments.
The potential for bioaccumulation of sulphanilic acid in the food chain is another area of environmental concern. While current research suggests limited bioaccumulation potential, long-term studies are needed to fully understand its behavior in complex ecosystems and its potential impact on higher trophic levels.
Regulatory compliance is a critical factor in the environmental evaluation of sulphanilic acid. As environmental regulations become increasingly stringent, industries must ensure that the use of this corrosion inhibitor meets all applicable standards for chemical use, discharge limits, and workplace safety. This may require ongoing monitoring and adaptation of usage practices.
In conclusion, while sulphanilic acid shows promise as an effective corrosion inhibitor, its widespread adoption in metal industries must be balanced with comprehensive environmental stewardship. Further research into its long-term ecological effects, coupled with the development of advanced treatment technologies and responsible usage protocols, will be essential to maximize its benefits while minimizing environmental risks.
One of the primary environmental advantages of sulphanilic acid is its biodegradability. Unlike some traditional corrosion inhibitors that persist in the environment, sulphanilic acid can be broken down by natural processes, reducing long-term ecological impact. This characteristic aligns with growing environmental regulations and sustainability goals in industrial sectors.
However, the release of sulphanilic acid into aquatic ecosystems can have adverse effects on water quality and aquatic life. Even at low concentrations, it may contribute to increased chemical oxygen demand (COD) and biochemical oxygen demand (BOD) in water bodies, potentially leading to oxygen depletion and stress on aquatic organisms. Proper wastewater treatment and management are crucial to mitigate these risks.
The production process of sulphanilic acid also raises environmental concerns. Its synthesis typically involves the use of sulfuric acid and aniline, both of which can have significant environmental footprints if not managed properly. Manufacturers must implement stringent controls to minimize emissions and waste generation during production.
From a lifecycle perspective, the use of sulphanilic acid as a corrosion inhibitor may offer indirect environmental benefits. By extending the lifespan of metal structures and equipment, it can reduce the need for frequent replacements, thereby conserving resources and energy associated with manufacturing new metal components. This aspect should be considered in comprehensive environmental impact assessments.
The potential for bioaccumulation of sulphanilic acid in the food chain is another area of environmental concern. While current research suggests limited bioaccumulation potential, long-term studies are needed to fully understand its behavior in complex ecosystems and its potential impact on higher trophic levels.
Regulatory compliance is a critical factor in the environmental evaluation of sulphanilic acid. As environmental regulations become increasingly stringent, industries must ensure that the use of this corrosion inhibitor meets all applicable standards for chemical use, discharge limits, and workplace safety. This may require ongoing monitoring and adaptation of usage practices.
In conclusion, while sulphanilic acid shows promise as an effective corrosion inhibitor, its widespread adoption in metal industries must be balanced with comprehensive environmental stewardship. Further research into its long-term ecological effects, coupled with the development of advanced treatment technologies and responsible usage protocols, will be essential to maximize its benefits while minimizing environmental risks.
Cost-Benefit Analysis
The cost-benefit analysis of using sulphanilic acid as a corrosion inhibitor in metal industries reveals a promising economic potential. Initial investment costs for implementing sulphanilic acid-based corrosion protection systems are relatively low compared to traditional methods. The primary expenses include the procurement of sulphanilic acid, which is readily available and cost-effective due to its widespread use in various industries.
One of the significant benefits is the extended lifespan of metal equipment and structures. By effectively inhibiting corrosion, sulphanilic acid reduces the frequency of maintenance and replacement, leading to substantial long-term cost savings. This is particularly advantageous in industries with high-value metal assets, such as oil and gas, marine, and manufacturing sectors.
The application process of sulphanilic acid as a corrosion inhibitor is generally straightforward and does not require extensive modifications to existing systems. This translates to minimal disruption to operations during implementation, further enhancing its cost-effectiveness. Additionally, the low toxicity of sulphanilic acid compared to some traditional corrosion inhibitors reduces environmental compliance costs and potential health-related expenses.
However, it is important to consider the ongoing costs associated with the regular application of sulphanilic acid. While these costs are typically lower than those of conventional corrosion prevention methods, they still represent a recurring expense that must be factored into long-term budgeting.
The efficiency of sulphanilic acid in preventing corrosion can lead to improved productivity and reduced downtime. This indirect benefit can result in significant financial gains, especially in industries where equipment reliability is crucial for maintaining production schedules.
When comparing the costs and benefits, the use of sulphanilic acid as a corrosion inhibitor generally shows a favorable return on investment. The initial outlay and ongoing expenses are outweighed by the savings from reduced corrosion damage, decreased maintenance requirements, and extended equipment lifespans. This positive cost-benefit ratio makes sulphanilic acid an attractive option for metal industries seeking to optimize their corrosion prevention strategies.
It is worth noting that the exact cost-benefit ratio may vary depending on specific industry conditions, environmental factors, and the scale of implementation. Therefore, individual assessments are recommended for businesses considering the adoption of sulphanilic acid-based corrosion inhibition systems to ensure optimal economic outcomes tailored to their unique operational contexts.
One of the significant benefits is the extended lifespan of metal equipment and structures. By effectively inhibiting corrosion, sulphanilic acid reduces the frequency of maintenance and replacement, leading to substantial long-term cost savings. This is particularly advantageous in industries with high-value metal assets, such as oil and gas, marine, and manufacturing sectors.
The application process of sulphanilic acid as a corrosion inhibitor is generally straightforward and does not require extensive modifications to existing systems. This translates to minimal disruption to operations during implementation, further enhancing its cost-effectiveness. Additionally, the low toxicity of sulphanilic acid compared to some traditional corrosion inhibitors reduces environmental compliance costs and potential health-related expenses.
However, it is important to consider the ongoing costs associated with the regular application of sulphanilic acid. While these costs are typically lower than those of conventional corrosion prevention methods, they still represent a recurring expense that must be factored into long-term budgeting.
The efficiency of sulphanilic acid in preventing corrosion can lead to improved productivity and reduced downtime. This indirect benefit can result in significant financial gains, especially in industries where equipment reliability is crucial for maintaining production schedules.
When comparing the costs and benefits, the use of sulphanilic acid as a corrosion inhibitor generally shows a favorable return on investment. The initial outlay and ongoing expenses are outweighed by the savings from reduced corrosion damage, decreased maintenance requirements, and extended equipment lifespans. This positive cost-benefit ratio makes sulphanilic acid an attractive option for metal industries seeking to optimize their corrosion prevention strategies.
It is worth noting that the exact cost-benefit ratio may vary depending on specific industry conditions, environmental factors, and the scale of implementation. Therefore, individual assessments are recommended for businesses considering the adoption of sulphanilic acid-based corrosion inhibition systems to ensure optimal economic outcomes tailored to their unique operational contexts.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!