Photocatalytic Degradation of Pollutants Using Sulphanilic Acid-Derived Catalysts
JUL 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Photocatalysis Background
Photocatalysis has emerged as a promising technology for environmental remediation, particularly in the field of water and air purification. This process harnesses the power of light to activate catalysts, which in turn facilitate the degradation of pollutants into less harmful or completely harmless substances. The concept of photocatalysis was first discovered in the 1970s when researchers observed the splitting of water on titanium dioxide electrodes under ultraviolet light.
Since its inception, photocatalysis has gained significant attention due to its potential for sustainable and eco-friendly pollutant treatment. The process relies on the generation of highly reactive species, such as hydroxyl radicals and superoxide anions, which can effectively break down a wide range of organic and inorganic contaminants. This versatility has made photocatalysis an attractive option for addressing various environmental challenges, including water treatment, air purification, and self-cleaning surfaces.
The development of photocatalytic materials has been a key focus in this field, with titanium dioxide (TiO2) being the most widely studied and utilized photocatalyst. However, the search for more efficient and visible light-responsive catalysts has led to the exploration of various other materials, including metal oxides, sulfides, and composite structures. In recent years, there has been growing interest in the development of novel photocatalysts derived from organic compounds, such as sulphanilic acid.
Sulphanilic acid-derived catalysts represent an innovative approach to photocatalysis, offering potential advantages in terms of synthesis, tunability, and performance. These catalysts typically incorporate the sulphanilic acid moiety into their structure, which can enhance their light absorption properties and catalytic activity. The use of organic-based photocatalysts also opens up new possibilities for tailoring the material's properties through molecular design and functionalization.
The application of photocatalysis in environmental remediation has seen significant progress over the past decades. From initial laboratory-scale experiments, the technology has advanced to pilot and commercial-scale applications in various sectors. Water treatment plants have begun incorporating photocatalytic processes for the removal of persistent organic pollutants, while air purification systems utilizing photocatalytic materials have been implemented in both indoor and outdoor settings.
As environmental concerns continue to grow globally, the importance of efficient and sustainable pollution control technologies becomes increasingly apparent. Photocatalysis, with its ability to harness renewable solar energy and operate under ambient conditions, aligns well with the principles of green chemistry and sustainable development. The ongoing research in this field, including the development of sulphanilic acid-derived catalysts, aims to address current limitations and expand the applicability of photocatalysis in addressing pressing environmental challenges.
Since its inception, photocatalysis has gained significant attention due to its potential for sustainable and eco-friendly pollutant treatment. The process relies on the generation of highly reactive species, such as hydroxyl radicals and superoxide anions, which can effectively break down a wide range of organic and inorganic contaminants. This versatility has made photocatalysis an attractive option for addressing various environmental challenges, including water treatment, air purification, and self-cleaning surfaces.
The development of photocatalytic materials has been a key focus in this field, with titanium dioxide (TiO2) being the most widely studied and utilized photocatalyst. However, the search for more efficient and visible light-responsive catalysts has led to the exploration of various other materials, including metal oxides, sulfides, and composite structures. In recent years, there has been growing interest in the development of novel photocatalysts derived from organic compounds, such as sulphanilic acid.
Sulphanilic acid-derived catalysts represent an innovative approach to photocatalysis, offering potential advantages in terms of synthesis, tunability, and performance. These catalysts typically incorporate the sulphanilic acid moiety into their structure, which can enhance their light absorption properties and catalytic activity. The use of organic-based photocatalysts also opens up new possibilities for tailoring the material's properties through molecular design and functionalization.
The application of photocatalysis in environmental remediation has seen significant progress over the past decades. From initial laboratory-scale experiments, the technology has advanced to pilot and commercial-scale applications in various sectors. Water treatment plants have begun incorporating photocatalytic processes for the removal of persistent organic pollutants, while air purification systems utilizing photocatalytic materials have been implemented in both indoor and outdoor settings.
As environmental concerns continue to grow globally, the importance of efficient and sustainable pollution control technologies becomes increasingly apparent. Photocatalysis, with its ability to harness renewable solar energy and operate under ambient conditions, aligns well with the principles of green chemistry and sustainable development. The ongoing research in this field, including the development of sulphanilic acid-derived catalysts, aims to address current limitations and expand the applicability of photocatalysis in addressing pressing environmental challenges.
Market Analysis
The market for photocatalytic degradation of pollutants using sulphanilic acid-derived catalysts is experiencing significant growth, driven by increasing environmental concerns and stringent regulations on water and air quality. This technology offers a promising solution for the removal of various organic pollutants, including dyes, pharmaceuticals, and industrial chemicals from wastewater and contaminated air.
The global water treatment market, which includes photocatalytic technologies, is projected to reach substantial value in the coming years. Factors contributing to this growth include rapid industrialization, urbanization, and the growing awareness of the need for sustainable water management practices. Developing countries, particularly in Asia-Pacific and Latin America, are expected to be key drivers of market expansion due to their increasing industrial activities and environmental challenges.
In the air purification sector, the demand for photocatalytic solutions is also on the rise. The global air purifier market is experiencing steady growth, with photocatalytic technologies gaining traction due to their ability to effectively degrade volatile organic compounds (VOCs) and other airborne pollutants. This trend is particularly evident in regions with high levels of air pollution, such as major urban centers in China and India.
The textile industry represents a significant market opportunity for sulphanilic acid-derived catalysts. With the increasing focus on sustainable manufacturing practices, there is a growing demand for efficient methods to treat textile wastewater containing various dyes and chemicals. Photocatalytic degradation offers a cost-effective and environmentally friendly solution, making it attractive to textile manufacturers seeking to comply with environmental regulations.
The pharmaceutical industry is another key market segment for this technology. As concerns over pharmaceutical residues in water bodies grow, there is an increasing need for advanced treatment methods. Photocatalytic degradation using sulphanilic acid-derived catalysts shows promise in effectively removing these contaminants, presenting opportunities for market growth in this sector.
Despite the positive market outlook, challenges remain. The high initial costs associated with implementing photocatalytic systems and the need for further research to improve catalyst efficiency and durability may hinder widespread adoption. However, ongoing technological advancements and increasing environmental pressures are expected to drive continued market expansion in the coming years.
The global water treatment market, which includes photocatalytic technologies, is projected to reach substantial value in the coming years. Factors contributing to this growth include rapid industrialization, urbanization, and the growing awareness of the need for sustainable water management practices. Developing countries, particularly in Asia-Pacific and Latin America, are expected to be key drivers of market expansion due to their increasing industrial activities and environmental challenges.
In the air purification sector, the demand for photocatalytic solutions is also on the rise. The global air purifier market is experiencing steady growth, with photocatalytic technologies gaining traction due to their ability to effectively degrade volatile organic compounds (VOCs) and other airborne pollutants. This trend is particularly evident in regions with high levels of air pollution, such as major urban centers in China and India.
The textile industry represents a significant market opportunity for sulphanilic acid-derived catalysts. With the increasing focus on sustainable manufacturing practices, there is a growing demand for efficient methods to treat textile wastewater containing various dyes and chemicals. Photocatalytic degradation offers a cost-effective and environmentally friendly solution, making it attractive to textile manufacturers seeking to comply with environmental regulations.
The pharmaceutical industry is another key market segment for this technology. As concerns over pharmaceutical residues in water bodies grow, there is an increasing need for advanced treatment methods. Photocatalytic degradation using sulphanilic acid-derived catalysts shows promise in effectively removing these contaminants, presenting opportunities for market growth in this sector.
Despite the positive market outlook, challenges remain. The high initial costs associated with implementing photocatalytic systems and the need for further research to improve catalyst efficiency and durability may hinder widespread adoption. However, ongoing technological advancements and increasing environmental pressures are expected to drive continued market expansion in the coming years.
Technical Challenges
The photocatalytic degradation of pollutants using sulphanilic acid-derived catalysts faces several technical challenges that hinder its widespread application and efficiency. One of the primary obstacles is the limited light absorption range of these catalysts. Most sulphanilic acid-derived photocatalysts exhibit optimal activity under UV light, which constitutes only a small fraction of the solar spectrum. This limitation significantly reduces the overall efficiency of the process in real-world applications, especially when considering the use of natural sunlight as the primary energy source.
Another critical challenge is the rapid recombination of photogenerated electron-hole pairs within the catalyst. This phenomenon drastically reduces the quantum yield of the photocatalytic process, as the charge carriers recombine before they can participate in the degradation reactions. The development of strategies to effectively separate and prolong the lifetime of these charge carriers remains a key area of research in improving the performance of sulphanilic acid-derived catalysts.
The stability and reusability of the catalysts present additional hurdles. Many sulphanilic acid-derived photocatalysts suffer from photocorrosion or structural degradation during prolonged use, leading to a decrease in catalytic activity over time. This not only affects the long-term performance of the system but also raises concerns about the potential release of secondary pollutants into the environment.
Furthermore, the selectivity of the degradation process poses a significant challenge. In complex pollutant mixtures, which are common in real environmental samples, the catalysts may not effectively degrade all types of contaminants equally. Some pollutants may be more resistant to photocatalytic degradation, while others may form intermediate products that are potentially more harmful than the original compounds.
The scalability of the photocatalytic systems using sulphanilic acid-derived catalysts is another major technical challenge. While laboratory-scale experiments often show promising results, translating these findings into large-scale, industrially viable processes remains difficult. Issues such as uniform light distribution, mass transfer limitations, and catalyst recovery in large reactors need to be addressed to make the technology feasible for real-world applications.
Additionally, the synthesis of high-performance sulphanilic acid-derived catalysts often involves complex procedures and expensive precursors. This presents challenges in terms of cost-effectiveness and large-scale production. Developing simpler, more economical synthesis routes without compromising the catalytic activity is crucial for the practical implementation of these materials in environmental remediation.
Another critical challenge is the rapid recombination of photogenerated electron-hole pairs within the catalyst. This phenomenon drastically reduces the quantum yield of the photocatalytic process, as the charge carriers recombine before they can participate in the degradation reactions. The development of strategies to effectively separate and prolong the lifetime of these charge carriers remains a key area of research in improving the performance of sulphanilic acid-derived catalysts.
The stability and reusability of the catalysts present additional hurdles. Many sulphanilic acid-derived photocatalysts suffer from photocorrosion or structural degradation during prolonged use, leading to a decrease in catalytic activity over time. This not only affects the long-term performance of the system but also raises concerns about the potential release of secondary pollutants into the environment.
Furthermore, the selectivity of the degradation process poses a significant challenge. In complex pollutant mixtures, which are common in real environmental samples, the catalysts may not effectively degrade all types of contaminants equally. Some pollutants may be more resistant to photocatalytic degradation, while others may form intermediate products that are potentially more harmful than the original compounds.
The scalability of the photocatalytic systems using sulphanilic acid-derived catalysts is another major technical challenge. While laboratory-scale experiments often show promising results, translating these findings into large-scale, industrially viable processes remains difficult. Issues such as uniform light distribution, mass transfer limitations, and catalyst recovery in large reactors need to be addressed to make the technology feasible for real-world applications.
Additionally, the synthesis of high-performance sulphanilic acid-derived catalysts often involves complex procedures and expensive precursors. This presents challenges in terms of cost-effectiveness and large-scale production. Developing simpler, more economical synthesis routes without compromising the catalytic activity is crucial for the practical implementation of these materials in environmental remediation.
Current Solutions
01 Synthesis and modification of sulphanilic acid-derived catalysts
Various methods for synthesizing and modifying sulphanilic acid-derived catalysts are explored. These processes aim to improve catalyst performance and stability, potentially reducing degradation issues. Techniques may include chemical modifications, surface treatments, or incorporation of additional components to enhance catalyst properties.- Synthesis and modification of sulphanilic acid-derived catalysts: Various methods for synthesizing and modifying sulphanilic acid-derived catalysts are described. These processes aim to improve catalyst performance and stability, including techniques for altering the chemical structure and surface properties of the catalysts.
- Degradation mechanisms of sulphanilic acid-derived catalysts: Studies on the degradation mechanisms of sulphanilic acid-derived catalysts, including thermal decomposition, chemical leaching, and structural changes during catalytic reactions. Understanding these mechanisms is crucial for developing more stable and long-lasting catalysts.
- Stabilization techniques for sulphanilic acid-derived catalysts: Various methods to enhance the stability of sulphanilic acid-derived catalysts are explored. These include surface modifications, addition of stabilizing agents, and development of support materials that can prevent or slow down catalyst degradation.
- Regeneration and recycling of degraded catalysts: Techniques for regenerating and recycling degraded sulphanilic acid-derived catalysts are discussed. These methods aim to extend the lifespan of catalysts and reduce waste in industrial processes, including chemical and thermal treatments to restore catalytic activity.
- Analytical methods for studying catalyst degradation: Advanced analytical techniques for studying the degradation of sulphanilic acid-derived catalysts are presented. These methods include spectroscopic analysis, microscopy, and chromatography, which help in understanding the structural and chemical changes occurring during catalyst degradation.
02 Degradation mechanisms of sulphanilic acid-derived catalysts
Research focuses on understanding the degradation mechanisms of sulphanilic acid-derived catalysts. This includes investigating factors such as thermal stability, chemical resistance, and structural changes during catalytic processes. Identifying these mechanisms is crucial for developing strategies to mitigate catalyst degradation.Expand Specific Solutions03 Stabilization techniques for sulphanilic acid-derived catalysts
Various stabilization techniques are employed to enhance the longevity and performance of sulphanilic acid-derived catalysts. These may include the use of support materials, additives, or protective coatings to prevent or slow down degradation processes. The goal is to maintain catalyst activity over extended periods of use.Expand Specific Solutions04 Regeneration and recovery of degraded catalysts
Methods for regenerating and recovering degraded sulphanilic acid-derived catalysts are developed. These processes aim to restore catalyst activity and extend their useful life. Techniques may include chemical treatments, thermal processes, or physical methods to remove contaminants and restore catalyst structure.Expand Specific Solutions05 Analysis and characterization of catalyst degradation
Advanced analytical techniques are employed to characterize the degradation of sulphanilic acid-derived catalysts. These methods help in understanding the changes in catalyst structure, composition, and surface properties over time. Such analyses are crucial for developing improved catalyst formulations and degradation prevention strategies.Expand Specific Solutions
Industry Players
The photocatalytic degradation of pollutants using sulphanilic acid-derived catalysts is an emerging field in environmental remediation. The market is in its early growth stage, with increasing research focus and potential for commercial applications. The global market for advanced oxidation technologies, including photocatalysis, is projected to grow significantly in the coming years. Key players in this field include academic institutions like Nanjing University of Information Science & Technology, Shoolini University, and West Pomeranian University of Technology Szczecin, as well as companies like IFP Energies Nouvelles and Nanjing Aosai Environmental Protection Technology Co., Ltd. The technology is still evolving, with ongoing research to improve catalyst efficiency, stability, and scalability for practical environmental applications.
Nanjing University of Information Science & Technology
Technical Solution: Nanjing University of Information Science & Technology has developed a novel approach for photocatalytic degradation of pollutants using sulphanilic acid-derived catalysts. Their research focuses on synthesizing highly efficient photocatalysts by modifying titanium dioxide (TiO2) with sulphanilic acid. The modified TiO2 nanoparticles exhibit enhanced visible light absorption and improved charge separation, leading to superior photocatalytic activity[1]. The university's team has successfully demonstrated the degradation of various organic pollutants, including dyes and pharmaceutical compounds, under both UV and visible light irradiation[2]. Their catalyst design incorporates a hierarchical structure that maximizes the surface area and provides abundant active sites for pollutant adsorption and degradation[3].
Strengths: Enhanced visible light absorption, improved charge separation, and high surface area for efficient pollutant degradation. Weaknesses: Potential challenges in large-scale production and long-term stability of the catalyst under real-world conditions.
Hunan University
Technical Solution: Hunan University has developed an innovative approach to photocatalytic degradation of pollutants using sulphanilic acid-derived catalysts. Their research focuses on the design of hierarchical nanostructures that incorporate sulphanilic acid as a surface modifier and sensitizer. The university's team has successfully synthesized 3D flower-like TiO2 architectures functionalized with sulphanilic acid, which exhibit superior photocatalytic activity compared to conventional TiO2 nanoparticles[1]. These catalysts demonstrate enhanced visible light absorption and improved charge carrier separation, leading to efficient degradation of various organic pollutants, including textile dyes and pharmaceutical contaminants[2]. Additionally, Hunan University has explored the integration of graphene-based materials with sulphanilic acid-modified semiconductors to further enhance the photocatalytic performance through improved electron transport and increased adsorption capacity[3].
Strengths: Unique 3D nanostructures, enhanced visible light activity, and improved charge separation. Weaknesses: Potential challenges in maintaining the hierarchical structure during long-term use and scalability issues.
Key Innovations
A photocatalyst and a process for photocatalytic degradation of organic pollutants
PatentInactiveIN201641006074A
Innovation
- A photocatalyst comprising CuO-TiO2 supported on reduced graphene oxide, prepared through a process involving mesoporous TiO2 nanoparticles and copper loading on a graphene oxide substrate, is used in an aqueous medium under diffused sunlight and sonication to enhance degradation efficiency.
Method of photocatalytic degradation of contaminant in water using visible light source
PatentInactiveUS20150166367A1
Innovation
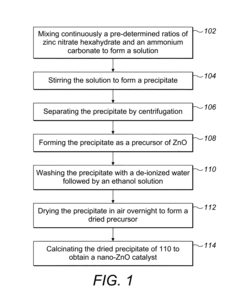
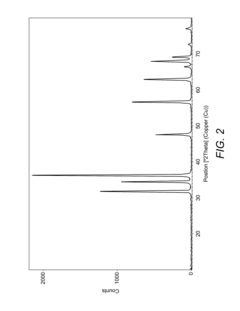
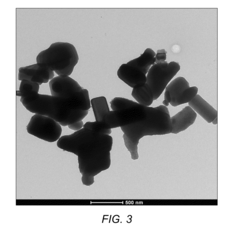
- A novel composition of a nano-zinc oxide (nano-ZnO) catalyst is synthesized using a precipitation method, calcined at 500°C for 6 hours, and used for photo-catalytic degradation of MTBE in water under visible light, eliminating the need for UV light and additional chemicals.
Environmental Impact
The environmental impact of photocatalytic degradation using sulphanilic acid-derived catalysts is a crucial aspect to consider in the development and application of this technology. These catalysts offer a promising solution for the removal of pollutants from water and air, potentially reducing the overall environmental burden of various industrial processes and human activities.
One of the primary benefits of using sulphanilic acid-derived catalysts in photocatalytic degradation is their ability to break down a wide range of organic pollutants into less harmful or completely harmless substances. This process can significantly reduce the concentration of toxic compounds in wastewater and air emissions, leading to improved water and air quality in affected areas. The degradation of persistent organic pollutants (POPs) is particularly noteworthy, as these substances are known for their long-term environmental persistence and bioaccumulation potential.
Furthermore, the use of photocatalytic processes powered by sunlight or artificial light sources can contribute to a reduction in energy consumption compared to traditional pollutant removal methods. This lower energy requirement translates to reduced greenhouse gas emissions associated with the treatment process, aligning with global efforts to combat climate change and promote sustainable technologies.
However, it is essential to consider potential negative environmental impacts associated with the production and use of sulphanilic acid-derived catalysts. The synthesis of these catalysts may involve chemical processes that generate waste or require energy-intensive steps. A comprehensive life cycle assessment would be necessary to fully understand the net environmental impact of implementing this technology on a large scale.
Another important consideration is the fate of the degradation products resulting from the photocatalytic process. While the primary pollutants may be broken down, it is crucial to ensure that the resulting compounds do not pose new environmental risks. Ongoing research is needed to characterize these byproducts and assess their potential impacts on ecosystems and human health.
The scalability of photocatalytic degradation using sulphanilic acid-derived catalysts also plays a role in its overall environmental impact. As the technology is adopted more widely, there may be opportunities for economies of scale in catalyst production, potentially reducing the environmental footprint of manufacturing processes. Additionally, the development of more efficient and durable catalysts could further enhance the positive environmental outcomes of this technology.
In conclusion, while photocatalytic degradation using sulphanilic acid-derived catalysts shows promise for reducing environmental pollution, a holistic approach is necessary to fully evaluate its net environmental impact. This includes considering the entire life cycle of the catalysts, the efficiency of the degradation process, and the fate of resulting byproducts. Continued research and development in this field will be crucial for optimizing the environmental benefits while minimizing potential drawbacks.
One of the primary benefits of using sulphanilic acid-derived catalysts in photocatalytic degradation is their ability to break down a wide range of organic pollutants into less harmful or completely harmless substances. This process can significantly reduce the concentration of toxic compounds in wastewater and air emissions, leading to improved water and air quality in affected areas. The degradation of persistent organic pollutants (POPs) is particularly noteworthy, as these substances are known for their long-term environmental persistence and bioaccumulation potential.
Furthermore, the use of photocatalytic processes powered by sunlight or artificial light sources can contribute to a reduction in energy consumption compared to traditional pollutant removal methods. This lower energy requirement translates to reduced greenhouse gas emissions associated with the treatment process, aligning with global efforts to combat climate change and promote sustainable technologies.
However, it is essential to consider potential negative environmental impacts associated with the production and use of sulphanilic acid-derived catalysts. The synthesis of these catalysts may involve chemical processes that generate waste or require energy-intensive steps. A comprehensive life cycle assessment would be necessary to fully understand the net environmental impact of implementing this technology on a large scale.
Another important consideration is the fate of the degradation products resulting from the photocatalytic process. While the primary pollutants may be broken down, it is crucial to ensure that the resulting compounds do not pose new environmental risks. Ongoing research is needed to characterize these byproducts and assess their potential impacts on ecosystems and human health.
The scalability of photocatalytic degradation using sulphanilic acid-derived catalysts also plays a role in its overall environmental impact. As the technology is adopted more widely, there may be opportunities for economies of scale in catalyst production, potentially reducing the environmental footprint of manufacturing processes. Additionally, the development of more efficient and durable catalysts could further enhance the positive environmental outcomes of this technology.
In conclusion, while photocatalytic degradation using sulphanilic acid-derived catalysts shows promise for reducing environmental pollution, a holistic approach is necessary to fully evaluate its net environmental impact. This includes considering the entire life cycle of the catalysts, the efficiency of the degradation process, and the fate of resulting byproducts. Continued research and development in this field will be crucial for optimizing the environmental benefits while minimizing potential drawbacks.
Regulatory Framework
The regulatory framework surrounding photocatalytic degradation of pollutants using sulphanilic acid-derived catalysts is complex and multifaceted, involving various environmental protection agencies and legislative bodies at national and international levels. These regulations aim to ensure the safe and effective implementation of this technology while minimizing potential risks to human health and the environment.
At the global level, the United Nations Environment Programme (UNEP) provides guidelines and recommendations for water treatment technologies, including photocatalytic processes. These guidelines often serve as a basis for national regulations and standards. The World Health Organization (WHO) also plays a crucial role in setting water quality standards that indirectly influence the development and application of photocatalytic technologies.
In the United States, the Environmental Protection Agency (EPA) is the primary regulatory body overseeing water treatment technologies. The EPA's Clean Water Act and Safe Drinking Water Act provide the legislative framework for regulating pollutant degradation methods, including photocatalytic processes. The agency sets Maximum Contaminant Levels (MCLs) for various pollutants and evaluates treatment technologies based on their ability to meet these standards.
The European Union has established the Water Framework Directive (WFD) and the Urban Waste Water Treatment Directive, which set comprehensive guidelines for water quality and treatment across member states. These directives influence the development and implementation of photocatalytic technologies for pollutant degradation. The European Chemicals Agency (ECHA) also plays a role in regulating the use of catalysts and their potential environmental impacts.
In Asia, countries like China and Japan have their own regulatory bodies and standards for water treatment technologies. China's Ministry of Ecology and Environment and Japan's Ministry of the Environment have established guidelines for the use of advanced oxidation processes, including photocatalytic degradation, in water treatment applications.
Regulatory frameworks also address the safety and handling of sulphanilic acid-derived catalysts. Occupational safety regulations, such as those set by the Occupational Safety and Health Administration (OSHA) in the United States, govern the use and handling of these materials in industrial settings. Additionally, transportation and disposal of these catalysts are subject to hazardous materials regulations in many jurisdictions.
As the field of photocatalytic degradation continues to evolve, regulatory frameworks are expected to adapt to new technological developments. This may include the establishment of specific standards for photocatalytic processes, guidelines for catalyst design and optimization, and protocols for assessing the long-term environmental impacts of these technologies. Ongoing collaboration between researchers, industry stakeholders, and regulatory bodies will be crucial in shaping an effective and balanced regulatory landscape for this promising pollution control technology.
At the global level, the United Nations Environment Programme (UNEP) provides guidelines and recommendations for water treatment technologies, including photocatalytic processes. These guidelines often serve as a basis for national regulations and standards. The World Health Organization (WHO) also plays a crucial role in setting water quality standards that indirectly influence the development and application of photocatalytic technologies.
In the United States, the Environmental Protection Agency (EPA) is the primary regulatory body overseeing water treatment technologies. The EPA's Clean Water Act and Safe Drinking Water Act provide the legislative framework for regulating pollutant degradation methods, including photocatalytic processes. The agency sets Maximum Contaminant Levels (MCLs) for various pollutants and evaluates treatment technologies based on their ability to meet these standards.
The European Union has established the Water Framework Directive (WFD) and the Urban Waste Water Treatment Directive, which set comprehensive guidelines for water quality and treatment across member states. These directives influence the development and implementation of photocatalytic technologies for pollutant degradation. The European Chemicals Agency (ECHA) also plays a role in regulating the use of catalysts and their potential environmental impacts.
In Asia, countries like China and Japan have their own regulatory bodies and standards for water treatment technologies. China's Ministry of Ecology and Environment and Japan's Ministry of the Environment have established guidelines for the use of advanced oxidation processes, including photocatalytic degradation, in water treatment applications.
Regulatory frameworks also address the safety and handling of sulphanilic acid-derived catalysts. Occupational safety regulations, such as those set by the Occupational Safety and Health Administration (OSHA) in the United States, govern the use and handling of these materials in industrial settings. Additionally, transportation and disposal of these catalysts are subject to hazardous materials regulations in many jurisdictions.
As the field of photocatalytic degradation continues to evolve, regulatory frameworks are expected to adapt to new technological developments. This may include the establishment of specific standards for photocatalytic processes, guidelines for catalyst design and optimization, and protocols for assessing the long-term environmental impacts of these technologies. Ongoing collaboration between researchers, industry stakeholders, and regulatory bodies will be crucial in shaping an effective and balanced regulatory landscape for this promising pollution control technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!