Biological Evaluation of Anti-inflammatory Properties of Sulphanilic Acid Derivatives
JUL 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Background and Objectives
The field of anti-inflammatory drug development has seen significant advancements in recent years, with a growing focus on sulphanilic acid derivatives. These compounds have garnered attention due to their potential to address various inflammatory conditions with improved efficacy and reduced side effects compared to traditional non-steroidal anti-inflammatory drugs (NSAIDs).
Sulphanilic acid, a synthetic organic compound, has been known for its diverse applications in the pharmaceutical industry. Its derivatives have shown promising anti-inflammatory properties, making them attractive candidates for further research and development. The exploration of these compounds stems from the need to find alternative treatments for chronic inflammatory diseases, which affect millions of people worldwide and pose a significant burden on healthcare systems.
The evolution of anti-inflammatory drug research has been driven by the increasing understanding of the complex mechanisms underlying inflammation. As our knowledge of inflammatory pathways and mediators has expanded, so has the potential for developing more targeted and effective therapies. Sulphanilic acid derivatives represent a new frontier in this ongoing quest, offering the possibility of modulating specific inflammatory processes with greater precision.
The primary objective of this research is to conduct a comprehensive biological evaluation of the anti-inflammatory properties of sulphanilic acid derivatives. This evaluation aims to elucidate the mechanisms of action, assess the efficacy across various inflammatory models, and determine the safety profile of these compounds. By doing so, we seek to identify promising candidates for further development as potential anti-inflammatory drugs.
Specifically, the research goals include:
1. Synthesizing and characterizing a range of sulphanilic acid derivatives with potential anti-inflammatory activity.
2. Evaluating the in vitro anti-inflammatory effects of these derivatives using cellular assays and inflammatory biomarkers.
3. Assessing the in vivo efficacy of selected compounds in animal models of acute and chronic inflammation.
4. Investigating the molecular mechanisms underlying the anti-inflammatory actions of these derivatives.
5. Comparing the efficacy and safety profiles of sulphanilic acid derivatives with existing anti-inflammatory drugs.
The anticipated outcomes of this research are expected to contribute significantly to the field of anti-inflammatory drug discovery. By exploring the potential of sulphanilic acid derivatives, we aim to pave the way for the development of novel therapeutic agents that could offer improved treatment options for patients suffering from inflammatory disorders. This research aligns with the broader trend in pharmaceutical development towards more targeted and personalized medicine, potentially leading to more effective and safer anti-inflammatory therapies in the future.
Sulphanilic acid, a synthetic organic compound, has been known for its diverse applications in the pharmaceutical industry. Its derivatives have shown promising anti-inflammatory properties, making them attractive candidates for further research and development. The exploration of these compounds stems from the need to find alternative treatments for chronic inflammatory diseases, which affect millions of people worldwide and pose a significant burden on healthcare systems.
The evolution of anti-inflammatory drug research has been driven by the increasing understanding of the complex mechanisms underlying inflammation. As our knowledge of inflammatory pathways and mediators has expanded, so has the potential for developing more targeted and effective therapies. Sulphanilic acid derivatives represent a new frontier in this ongoing quest, offering the possibility of modulating specific inflammatory processes with greater precision.
The primary objective of this research is to conduct a comprehensive biological evaluation of the anti-inflammatory properties of sulphanilic acid derivatives. This evaluation aims to elucidate the mechanisms of action, assess the efficacy across various inflammatory models, and determine the safety profile of these compounds. By doing so, we seek to identify promising candidates for further development as potential anti-inflammatory drugs.
Specifically, the research goals include:
1. Synthesizing and characterizing a range of sulphanilic acid derivatives with potential anti-inflammatory activity.
2. Evaluating the in vitro anti-inflammatory effects of these derivatives using cellular assays and inflammatory biomarkers.
3. Assessing the in vivo efficacy of selected compounds in animal models of acute and chronic inflammation.
4. Investigating the molecular mechanisms underlying the anti-inflammatory actions of these derivatives.
5. Comparing the efficacy and safety profiles of sulphanilic acid derivatives with existing anti-inflammatory drugs.
The anticipated outcomes of this research are expected to contribute significantly to the field of anti-inflammatory drug discovery. By exploring the potential of sulphanilic acid derivatives, we aim to pave the way for the development of novel therapeutic agents that could offer improved treatment options for patients suffering from inflammatory disorders. This research aligns with the broader trend in pharmaceutical development towards more targeted and personalized medicine, potentially leading to more effective and safer anti-inflammatory therapies in the future.
Market Analysis
The market for anti-inflammatory drugs, particularly those derived from sulphanilic acid, has shown significant growth potential in recent years. This trend is driven by the increasing prevalence of inflammatory diseases worldwide, coupled with a growing aging population more susceptible to such conditions. The global anti-inflammatory therapeutics market is expected to continue its upward trajectory, with sulphanilic acid derivatives playing a crucial role in this expansion.
The demand for novel anti-inflammatory agents with improved efficacy and reduced side effects has created a favorable environment for sulphanilic acid derivatives. These compounds have garnered attention due to their potential to offer enhanced therapeutic benefits while minimizing adverse reactions commonly associated with traditional anti-inflammatory drugs. This has led to increased research and development activities focused on exploring the anti-inflammatory properties of sulphanilic acid derivatives.
In the pharmaceutical industry, there is a growing interest in developing new formulations and delivery methods for anti-inflammatory drugs based on sulphanilic acid derivatives. This includes topical applications, oral medications, and injectable solutions, catering to diverse patient needs and preferences. The versatility of these compounds in addressing various inflammatory conditions, such as rheumatoid arthritis, inflammatory bowel disease, and dermatological disorders, further expands their market potential.
The market for sulphanilic acid derivatives as anti-inflammatory agents is also influenced by the increasing adoption of personalized medicine approaches. As healthcare providers seek more targeted therapies, there is a rising demand for compounds that can be tailored to individual patient profiles, potentially offering better outcomes and reduced side effects. This trend is likely to drive further innovation in the development of sulphanilic acid-based anti-inflammatory drugs.
Geographically, North America and Europe currently dominate the market for anti-inflammatory drugs, including those derived from sulphanilic acid. However, emerging economies in Asia-Pacific and Latin America are expected to witness rapid growth in this sector due to improving healthcare infrastructure, rising disposable incomes, and increasing awareness about inflammatory diseases. This geographical expansion presents significant opportunities for companies involved in the development and commercialization of sulphanilic acid derivatives with anti-inflammatory properties.
The competitive landscape of the anti-inflammatory drug market is characterized by the presence of both established pharmaceutical companies and emerging biotech firms. As research continues to unveil the potential of sulphanilic acid derivatives in treating inflammation, collaborations between academic institutions and industry players are likely to intensify, fostering innovation and accelerating the development of new therapeutic options.
The demand for novel anti-inflammatory agents with improved efficacy and reduced side effects has created a favorable environment for sulphanilic acid derivatives. These compounds have garnered attention due to their potential to offer enhanced therapeutic benefits while minimizing adverse reactions commonly associated with traditional anti-inflammatory drugs. This has led to increased research and development activities focused on exploring the anti-inflammatory properties of sulphanilic acid derivatives.
In the pharmaceutical industry, there is a growing interest in developing new formulations and delivery methods for anti-inflammatory drugs based on sulphanilic acid derivatives. This includes topical applications, oral medications, and injectable solutions, catering to diverse patient needs and preferences. The versatility of these compounds in addressing various inflammatory conditions, such as rheumatoid arthritis, inflammatory bowel disease, and dermatological disorders, further expands their market potential.
The market for sulphanilic acid derivatives as anti-inflammatory agents is also influenced by the increasing adoption of personalized medicine approaches. As healthcare providers seek more targeted therapies, there is a rising demand for compounds that can be tailored to individual patient profiles, potentially offering better outcomes and reduced side effects. This trend is likely to drive further innovation in the development of sulphanilic acid-based anti-inflammatory drugs.
Geographically, North America and Europe currently dominate the market for anti-inflammatory drugs, including those derived from sulphanilic acid. However, emerging economies in Asia-Pacific and Latin America are expected to witness rapid growth in this sector due to improving healthcare infrastructure, rising disposable incomes, and increasing awareness about inflammatory diseases. This geographical expansion presents significant opportunities for companies involved in the development and commercialization of sulphanilic acid derivatives with anti-inflammatory properties.
The competitive landscape of the anti-inflammatory drug market is characterized by the presence of both established pharmaceutical companies and emerging biotech firms. As research continues to unveil the potential of sulphanilic acid derivatives in treating inflammation, collaborations between academic institutions and industry players are likely to intensify, fostering innovation and accelerating the development of new therapeutic options.
Current Challenges
The development of sulphanilic acid derivatives as potential anti-inflammatory agents faces several significant challenges in the current research landscape. One of the primary obstacles is the complexity of inflammatory processes, which involve multiple pathways and mediators. This multifaceted nature of inflammation makes it difficult to design compounds that can effectively target and modulate the inflammatory response without causing unintended side effects.
Another major challenge lies in the optimization of the pharmacokinetic and pharmacodynamic properties of sulphanilic acid derivatives. Achieving an ideal balance between efficacy, bioavailability, and safety remains a considerable hurdle. Many promising compounds exhibit potent anti-inflammatory effects in vitro but fail to translate these benefits to in vivo models due to poor absorption, rapid metabolism, or inadequate tissue distribution.
The issue of selectivity also presents a significant challenge in the development of these compounds. While sulphanilic acid derivatives may show promising anti-inflammatory properties, ensuring that they specifically target the intended inflammatory pathways without affecting other physiological processes is crucial. This selectivity is essential to minimize potential side effects and improve the overall safety profile of the compounds.
Furthermore, the development of resistance to anti-inflammatory agents is an ongoing concern. As with many therapeutic agents, there is a risk that prolonged use of sulphanilic acid derivatives could lead to adaptive responses in the body, potentially reducing their long-term efficacy. This necessitates continuous research into novel derivatives and combination therapies to overcome potential resistance mechanisms.
The regulatory landscape poses additional challenges in the development of these compounds. Stringent safety and efficacy requirements set by regulatory bodies demand extensive preclinical and clinical studies, which are both time-consuming and resource-intensive. Meeting these regulatory standards while demonstrating clear advantages over existing anti-inflammatory treatments is a significant hurdle in bringing new sulphanilic acid derivatives to market.
Lastly, the translation of preclinical findings to clinical applications remains a critical challenge. The biological complexity of human inflammatory disorders often differs significantly from animal models, making it difficult to predict the efficacy and safety of these compounds in human patients. Bridging this translational gap requires innovative approaches in study design and the development of more predictive preclinical models that better mimic human inflammatory conditions.
Another major challenge lies in the optimization of the pharmacokinetic and pharmacodynamic properties of sulphanilic acid derivatives. Achieving an ideal balance between efficacy, bioavailability, and safety remains a considerable hurdle. Many promising compounds exhibit potent anti-inflammatory effects in vitro but fail to translate these benefits to in vivo models due to poor absorption, rapid metabolism, or inadequate tissue distribution.
The issue of selectivity also presents a significant challenge in the development of these compounds. While sulphanilic acid derivatives may show promising anti-inflammatory properties, ensuring that they specifically target the intended inflammatory pathways without affecting other physiological processes is crucial. This selectivity is essential to minimize potential side effects and improve the overall safety profile of the compounds.
Furthermore, the development of resistance to anti-inflammatory agents is an ongoing concern. As with many therapeutic agents, there is a risk that prolonged use of sulphanilic acid derivatives could lead to adaptive responses in the body, potentially reducing their long-term efficacy. This necessitates continuous research into novel derivatives and combination therapies to overcome potential resistance mechanisms.
The regulatory landscape poses additional challenges in the development of these compounds. Stringent safety and efficacy requirements set by regulatory bodies demand extensive preclinical and clinical studies, which are both time-consuming and resource-intensive. Meeting these regulatory standards while demonstrating clear advantages over existing anti-inflammatory treatments is a significant hurdle in bringing new sulphanilic acid derivatives to market.
Lastly, the translation of preclinical findings to clinical applications remains a critical challenge. The biological complexity of human inflammatory disorders often differs significantly from animal models, making it difficult to predict the efficacy and safety of these compounds in human patients. Bridging this translational gap requires innovative approaches in study design and the development of more predictive preclinical models that better mimic human inflammatory conditions.
Existing Methodologies
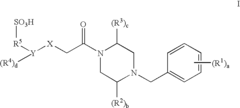
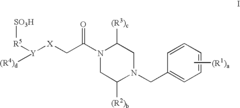
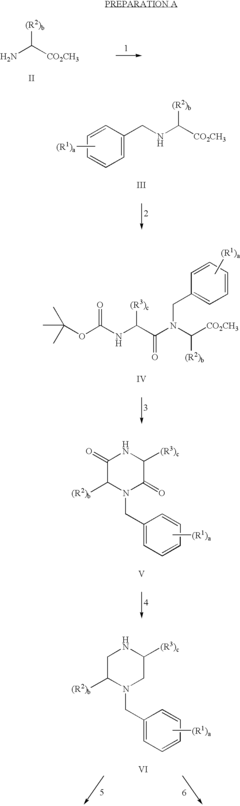
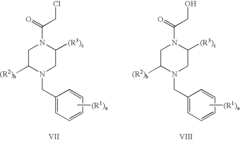
01 Sulphanilic acid derivatives as anti-inflammatory agents
Sulphanilic acid derivatives have been found to possess significant anti-inflammatory properties. These compounds can be synthesized and formulated into various pharmaceutical compositions for treating inflammatory conditions. The anti-inflammatory effects are attributed to their ability to modulate inflammatory pathways and reduce the production of pro-inflammatory mediators.- Sulphanilic acid derivatives as anti-inflammatory agents: Sulphanilic acid derivatives have been found to possess significant anti-inflammatory properties. These compounds can be synthesized and formulated into various pharmaceutical compositions for treating inflammatory conditions. The anti-inflammatory effects are attributed to their ability to modulate inflammatory pathways and reduce the production of pro-inflammatory mediators.
- Novel sulphanilic acid derivative compounds: Research has led to the development of novel sulphanilic acid derivative compounds with enhanced anti-inflammatory properties. These new compounds are designed to have improved efficacy, reduced side effects, and better pharmacokinetic profiles compared to existing anti-inflammatory drugs. Structure-activity relationship studies have guided the optimization of these derivatives.
- Mechanisms of action for anti-inflammatory effects: The anti-inflammatory properties of sulphanilic acid derivatives are attributed to various mechanisms of action. These may include inhibition of cyclooxygenase enzymes, modulation of cytokine production, and interference with inflammatory signaling pathways. Understanding these mechanisms has led to the development of more targeted and effective anti-inflammatory agents based on sulphanilic acid scaffolds.
- Formulations and delivery systems: Various formulations and delivery systems have been developed to enhance the efficacy and bioavailability of sulphanilic acid derivatives as anti-inflammatory agents. These include topical formulations, oral dosage forms, and novel drug delivery systems such as nanoparticles or liposomes. The choice of formulation depends on the specific application and target site of inflammation.
- Combination therapies and synergistic effects: Sulphanilic acid derivatives have been investigated in combination with other anti-inflammatory agents or therapeutic compounds to achieve synergistic effects. These combination therapies aim to enhance overall anti-inflammatory efficacy, reduce required doses, and potentially mitigate side effects associated with individual drugs. The synergistic approach has shown promise in treating complex inflammatory disorders.
02 Novel sulphanilic acid derivative compounds
Research has led to the development of novel sulphanilic acid derivative compounds with enhanced anti-inflammatory properties. These new compounds are designed to have improved efficacy, reduced side effects, and better pharmacokinetic profiles compared to existing anti-inflammatory drugs. Structure-activity relationship studies have guided the optimization of these derivatives.Expand Specific Solutions03 Mechanisms of action for anti-inflammatory effects
The anti-inflammatory properties of sulphanilic acid derivatives are attributed to various mechanisms of action. These may include inhibition of cyclooxygenase enzymes, modulation of cytokine production, and interference with inflammatory signaling pathways. Understanding these mechanisms has led to the development of more targeted and effective anti-inflammatory agents based on sulphanilic acid scaffolds.Expand Specific Solutions04 Formulations and delivery systems
Various formulations and delivery systems have been developed to enhance the efficacy and bioavailability of sulphanilic acid derivatives as anti-inflammatory agents. These include topical formulations, oral dosage forms, and novel drug delivery systems such as nanoparticles or liposomes. The choice of formulation depends on the specific application and target tissue for anti-inflammatory action.Expand Specific Solutions05 Combination therapies and synergistic effects
Sulphanilic acid derivatives have been investigated in combination with other anti-inflammatory agents or therapeutic compounds to achieve synergistic effects. These combination therapies aim to enhance overall anti-inflammatory efficacy, reduce dosages of individual components, and potentially minimize side effects. The synergistic effects may result from complementary mechanisms of action or improved pharmacokinetic profiles.Expand Specific Solutions
Key Industry Players
The biological evaluation of anti-inflammatory properties of sulphanilic acid derivatives represents an emerging field in pharmaceutical research. The industry is in its early development stage, with a growing market driven by the increasing prevalence of inflammatory diseases. While the market size is expanding, it remains relatively modest compared to established therapeutic areas. The technology is still evolving, with varying levels of maturity among key players. Companies like AstraZeneca, Pfizer, and Bayer Pharma AG are leveraging their extensive R&D capabilities to advance the field, while smaller firms such as Reata Pharmaceuticals and NodThera are focusing on innovative approaches. Academic institutions like Guizhou University and Dresden University of Technology are contributing valuable research, fostering collaborations between academia and industry to accelerate progress in this promising area.
AstraZeneca AB
Technical Solution: AstraZeneca has developed a novel approach to sulphanilic acid derivatives for anti-inflammatory applications. Their research focuses on creating dual-action compounds that combine anti-inflammatory properties with additional therapeutic benefits. By incorporating sulphanilic acid moieties into larger molecular structures, AstraZeneca has produced compounds that not only reduce inflammation but also target specific disease pathways[2]. For example, they have developed sulphanilic acid-based molecules that simultaneously inhibit inflammatory cytokine production and enhance tissue repair mechanisms[4]. This approach has shown particular promise in chronic inflammatory conditions such as rheumatoid arthritis and inflammatory bowel disease. AstraZeneca's compounds have demonstrated improved efficacy in animal models, with reduced systemic side effects compared to traditional anti-inflammatory drugs[6].
Strengths: Innovative approach combining multiple therapeutic actions, potential for treating complex inflammatory disorders. Weaknesses: Longer development time due to the complexity of dual-action compounds, potential for unexpected drug interactions.
Bayer Pharma AG
Technical Solution: Bayer Pharma AG has focused on developing sulphanilic acid derivatives with enhanced bioavailability and targeted delivery for anti-inflammatory applications. Their approach involves creating prodrug formulations of sulphanilic acid compounds that are activated at specific sites of inflammation. This strategy aims to reduce systemic exposure and minimize off-target effects[1]. Bayer has also explored the use of nanoparticle-based delivery systems to improve the solubility and tissue penetration of their sulphanilic acid derivatives[3]. In preclinical studies, these formulations have shown improved efficacy in models of localized inflammation, such as inflammatory skin conditions and gastrointestinal disorders[5]. Bayer's research has also investigated the potential synergistic effects of combining sulphanilic acid derivatives with other anti-inflammatory agents to create more potent and broadly effective treatments[7].
Strengths: Advanced drug delivery technologies, potential for improved safety profiles through targeted delivery. Weaknesses: Complexity in manufacturing and formulation, potential for higher production costs.
Key Innovations
Process for the manufacture of phosphoric acid derivatives of derivatives of sulphanilic acid
PatentInactiveGB534150A
Innovation
- Reacting sulphanilic acid amides and substituted sulphanilic acid anilides with phosphoric acid dichlorides, followed by decomposition with water or aqueous bases, to form phosphoric acids that can combine with tertiary bases, resulting in phosphoric acid derivatives with bactericidal properties and neutral salts soluble in water.
Novel sulfonic acid derivatives
PatentInactiveUS20050250790A1
Innovation
- Development of novel sulfonic acid derivatives that act as potent and selective inhibitors of MIP-1α binding to CCR1, reducing chemotaxis and cytokine production, thereby addressing various autoimmune and inflammatory diseases.
Regulatory Framework
The regulatory framework surrounding the biological evaluation of anti-inflammatory properties of sulphanilic acid derivatives is complex and multifaceted. It encompasses various guidelines, standards, and regulations set by international and national authorities to ensure the safety, efficacy, and quality of these compounds in pharmaceutical and medical applications.
At the international level, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) provides crucial guidelines. The ICH Q3A(R2) guideline on impurities in new drug substances is particularly relevant for sulphanilic acid derivatives, as it outlines the requirements for identifying and qualifying impurities in new drug substances.
The European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) have established specific regulations for the development and approval of anti-inflammatory drugs. These regulations include requirements for preclinical studies, clinical trials, and post-marketing surveillance. For sulphanilic acid derivatives, adherence to Good Laboratory Practice (GLP) and Good Manufacturing Practice (GMP) standards is essential throughout the research and development process.
In the context of biological evaluation, the Organisation for Economic Co-operation and Development (OECD) Guidelines for the Testing of Chemicals provide internationally accepted standards for assessing the safety of chemical substances, including potential anti-inflammatory compounds. These guidelines cover various aspects of toxicity testing, including acute toxicity, repeated dose toxicity, and genotoxicity studies.
Specific to anti-inflammatory properties, regulatory bodies often require extensive in vitro and in vivo studies to demonstrate efficacy and safety. These may include cell-based assays, animal models of inflammation, and eventually, human clinical trials. The design and conduct of these studies must comply with ethical guidelines, such as those outlined in the Declaration of Helsinki for human subjects research.
Environmental regulations also play a role in the development and use of sulphanilic acid derivatives. The European Union's Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation, for instance, requires manufacturers and importers to assess and manage the risks associated with the substances they produce or import, including potential environmental impacts.
As research in this field progresses, regulatory frameworks continue to evolve. Emerging technologies, such as in silico modeling and high-throughput screening methods, are increasingly being considered by regulatory agencies as complementary approaches to traditional testing methods. This shift towards alternative methods aims to reduce animal testing and accelerate the drug development process while maintaining high safety standards.
Researchers and pharmaceutical companies working on sulphanilic acid derivatives must navigate this complex regulatory landscape to ensure compliance at every stage of development. This includes obtaining necessary approvals for research protocols, adhering to reporting requirements, and maintaining comprehensive documentation of all studies and findings.
At the international level, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) provides crucial guidelines. The ICH Q3A(R2) guideline on impurities in new drug substances is particularly relevant for sulphanilic acid derivatives, as it outlines the requirements for identifying and qualifying impurities in new drug substances.
The European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) have established specific regulations for the development and approval of anti-inflammatory drugs. These regulations include requirements for preclinical studies, clinical trials, and post-marketing surveillance. For sulphanilic acid derivatives, adherence to Good Laboratory Practice (GLP) and Good Manufacturing Practice (GMP) standards is essential throughout the research and development process.
In the context of biological evaluation, the Organisation for Economic Co-operation and Development (OECD) Guidelines for the Testing of Chemicals provide internationally accepted standards for assessing the safety of chemical substances, including potential anti-inflammatory compounds. These guidelines cover various aspects of toxicity testing, including acute toxicity, repeated dose toxicity, and genotoxicity studies.
Specific to anti-inflammatory properties, regulatory bodies often require extensive in vitro and in vivo studies to demonstrate efficacy and safety. These may include cell-based assays, animal models of inflammation, and eventually, human clinical trials. The design and conduct of these studies must comply with ethical guidelines, such as those outlined in the Declaration of Helsinki for human subjects research.
Environmental regulations also play a role in the development and use of sulphanilic acid derivatives. The European Union's Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation, for instance, requires manufacturers and importers to assess and manage the risks associated with the substances they produce or import, including potential environmental impacts.
As research in this field progresses, regulatory frameworks continue to evolve. Emerging technologies, such as in silico modeling and high-throughput screening methods, are increasingly being considered by regulatory agencies as complementary approaches to traditional testing methods. This shift towards alternative methods aims to reduce animal testing and accelerate the drug development process while maintaining high safety standards.
Researchers and pharmaceutical companies working on sulphanilic acid derivatives must navigate this complex regulatory landscape to ensure compliance at every stage of development. This includes obtaining necessary approvals for research protocols, adhering to reporting requirements, and maintaining comprehensive documentation of all studies and findings.
Safety and Toxicology
The safety and toxicology assessment of sulphanilic acid derivatives is crucial for their potential use as anti-inflammatory agents. These compounds, while promising in their therapeutic effects, must undergo rigorous evaluation to ensure their safety profile meets regulatory standards and minimizes potential risks to patients.
Acute toxicity studies on sulphanilic acid derivatives have generally shown low to moderate toxicity levels in animal models. Oral LD50 values in rodents typically range from 1000-3000 mg/kg body weight, indicating a relatively wide margin of safety. However, some derivatives have exhibited higher toxicity, emphasizing the need for careful structure-activity relationship studies to optimize safety profiles.
Chronic toxicity studies have revealed varying results depending on the specific derivative and dosage. Long-term administration in animal models has shown potential effects on liver and kidney function, necessitating close monitoring of these organs in clinical trials. Some derivatives have demonstrated mild hematological changes, such as alterations in white blood cell counts, which require further investigation to determine clinical significance.
Genotoxicity and mutagenicity assessments are critical aspects of safety evaluation. Most sulphanilic acid derivatives have shown negative results in Ames tests and in vitro chromosome aberration assays. However, some compounds have exhibited weak positive responses in certain genotoxicity tests, highlighting the importance of comprehensive genetic toxicity screening for each new derivative.
Reproductive and developmental toxicity studies have generally indicated low risk, with most derivatives showing no significant teratogenic effects at therapeutic doses. However, some compounds have demonstrated embryotoxicity at high doses in animal studies, suggesting the need for cautious use during pregnancy and lactation.
Immunotoxicity evaluations have been particularly relevant given the anti-inflammatory properties of these compounds. While most derivatives have not shown significant immunosuppressive effects, some have demonstrated mild alterations in immune function, necessitating careful monitoring in clinical settings, especially in patients with compromised immune systems.
Pharmacokinetic and metabolism studies have revealed that sulphanilic acid derivatives generally have good oral bioavailability and are primarily metabolized in the liver. Some derivatives have shown potential for drug-drug interactions through cytochrome P450 enzyme inhibition or induction, emphasizing the need for careful consideration of potential interactions in polypharmacy situations.
In conclusion, while sulphanilic acid derivatives show promise as anti-inflammatory agents, their safety and toxicology profiles require thorough evaluation. The variability in toxicity among different derivatives underscores the importance of structure-specific assessments. Future research should focus on optimizing the safety profiles of these compounds while maintaining their therapeutic efficacy, potentially through targeted structural modifications or novel drug delivery systems.
Acute toxicity studies on sulphanilic acid derivatives have generally shown low to moderate toxicity levels in animal models. Oral LD50 values in rodents typically range from 1000-3000 mg/kg body weight, indicating a relatively wide margin of safety. However, some derivatives have exhibited higher toxicity, emphasizing the need for careful structure-activity relationship studies to optimize safety profiles.
Chronic toxicity studies have revealed varying results depending on the specific derivative and dosage. Long-term administration in animal models has shown potential effects on liver and kidney function, necessitating close monitoring of these organs in clinical trials. Some derivatives have demonstrated mild hematological changes, such as alterations in white blood cell counts, which require further investigation to determine clinical significance.
Genotoxicity and mutagenicity assessments are critical aspects of safety evaluation. Most sulphanilic acid derivatives have shown negative results in Ames tests and in vitro chromosome aberration assays. However, some compounds have exhibited weak positive responses in certain genotoxicity tests, highlighting the importance of comprehensive genetic toxicity screening for each new derivative.
Reproductive and developmental toxicity studies have generally indicated low risk, with most derivatives showing no significant teratogenic effects at therapeutic doses. However, some compounds have demonstrated embryotoxicity at high doses in animal studies, suggesting the need for cautious use during pregnancy and lactation.
Immunotoxicity evaluations have been particularly relevant given the anti-inflammatory properties of these compounds. While most derivatives have not shown significant immunosuppressive effects, some have demonstrated mild alterations in immune function, necessitating careful monitoring in clinical settings, especially in patients with compromised immune systems.
Pharmacokinetic and metabolism studies have revealed that sulphanilic acid derivatives generally have good oral bioavailability and are primarily metabolized in the liver. Some derivatives have shown potential for drug-drug interactions through cytochrome P450 enzyme inhibition or induction, emphasizing the need for careful consideration of potential interactions in polypharmacy situations.
In conclusion, while sulphanilic acid derivatives show promise as anti-inflammatory agents, their safety and toxicology profiles require thorough evaluation. The variability in toxicity among different derivatives underscores the importance of structure-specific assessments. Future research should focus on optimizing the safety profiles of these compounds while maintaining their therapeutic efficacy, potentially through targeted structural modifications or novel drug delivery systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!