Antifreeze's Contributions to High-Performance Material Science
JUL 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Antifreeze Evolution
The evolution of antifreeze in high-performance material science has been marked by significant advancements and breakthroughs over the past few decades. Initially developed to prevent ice formation in automotive cooling systems, antifreeze technology has since expanded its applications to various fields of material science, contributing to the development of novel high-performance materials.
In the early stages, antifreeze solutions primarily consisted of ethylene glycol or propylene glycol mixed with water. These solutions effectively lowered the freezing point of water, preventing damage to engine components in cold temperatures. However, as research progressed, scientists began to explore the potential of antifreeze compounds beyond their traditional automotive applications.
A major milestone in antifreeze evolution came with the discovery of antifreeze proteins (AFPs) in Arctic and Antarctic fish species. These naturally occurring proteins prevent ice crystal formation in the blood and tissues of organisms living in sub-zero environments. This discovery sparked intense research into biomimetic materials that could replicate the ice-inhibiting properties of AFPs.
The development of synthetic antifreeze polymers marked another significant leap forward. These polymers, designed to mimic the structure and function of natural AFPs, demonstrated superior ice-inhibiting capabilities compared to traditional glycol-based solutions. This breakthrough opened up new possibilities for creating materials with enhanced cold resistance and improved performance in extreme environments.
Recent advancements in nanotechnology have further revolutionized antifreeze materials. Researchers have successfully developed nanoparticle-based antifreeze additives that exhibit remarkable ice-inhibiting properties at extremely low concentrations. These nanoparticles can be incorporated into various materials, including coatings, textiles, and structural composites, significantly enhancing their cold weather performance.
The integration of antifreeze technology with smart materials represents the latest frontier in this field. Scientists are now developing materials that can dynamically respond to temperature changes, adjusting their antifreeze properties as needed. This adaptive approach promises to create a new generation of high-performance materials capable of withstanding extreme temperature fluctuations while maintaining optimal functionality.
As antifreeze technology continues to evolve, its impact on high-performance material science is becoming increasingly profound. From aerospace applications to biomedical engineering, the principles derived from antifreeze research are driving innovation across multiple industries, pushing the boundaries of what is possible in material design and performance.
In the early stages, antifreeze solutions primarily consisted of ethylene glycol or propylene glycol mixed with water. These solutions effectively lowered the freezing point of water, preventing damage to engine components in cold temperatures. However, as research progressed, scientists began to explore the potential of antifreeze compounds beyond their traditional automotive applications.
A major milestone in antifreeze evolution came with the discovery of antifreeze proteins (AFPs) in Arctic and Antarctic fish species. These naturally occurring proteins prevent ice crystal formation in the blood and tissues of organisms living in sub-zero environments. This discovery sparked intense research into biomimetic materials that could replicate the ice-inhibiting properties of AFPs.
The development of synthetic antifreeze polymers marked another significant leap forward. These polymers, designed to mimic the structure and function of natural AFPs, demonstrated superior ice-inhibiting capabilities compared to traditional glycol-based solutions. This breakthrough opened up new possibilities for creating materials with enhanced cold resistance and improved performance in extreme environments.
Recent advancements in nanotechnology have further revolutionized antifreeze materials. Researchers have successfully developed nanoparticle-based antifreeze additives that exhibit remarkable ice-inhibiting properties at extremely low concentrations. These nanoparticles can be incorporated into various materials, including coatings, textiles, and structural composites, significantly enhancing their cold weather performance.
The integration of antifreeze technology with smart materials represents the latest frontier in this field. Scientists are now developing materials that can dynamically respond to temperature changes, adjusting their antifreeze properties as needed. This adaptive approach promises to create a new generation of high-performance materials capable of withstanding extreme temperature fluctuations while maintaining optimal functionality.
As antifreeze technology continues to evolve, its impact on high-performance material science is becoming increasingly profound. From aerospace applications to biomedical engineering, the principles derived from antifreeze research are driving innovation across multiple industries, pushing the boundaries of what is possible in material design and performance.
Market Demand Analysis
The market demand for antifreeze technologies in high-performance materials science has been steadily growing, driven by the increasing need for materials that can withstand extreme temperatures and harsh environments. This demand spans across various industries, including aerospace, automotive, construction, and energy sectors.
In the aerospace industry, there is a significant push for materials that can maintain their structural integrity and performance in the extreme cold of high altitudes and space. Antifreeze technologies are crucial in developing lightweight, durable materials for aircraft and spacecraft components that can resist ice formation and maintain flexibility at sub-zero temperatures.
The automotive sector is another major driver of demand for antifreeze-enhanced materials. With the rise of electric vehicles and the need for more efficient thermal management systems, there is a growing market for advanced coolants and materials that can improve battery performance and longevity in cold climates. Additionally, the development of self-healing materials incorporating antifreeze properties is gaining traction for use in automotive exteriors and components.
In the construction industry, there is an increasing demand for concrete and other building materials that can resist freeze-thaw cycles, particularly in regions with harsh winters. Antifreeze admixtures and surface treatments that can improve the durability and lifespan of infrastructure are seeing growing adoption.
The energy sector, particularly in renewable energy, is also contributing to the market demand for antifreeze technologies. Wind turbines operating in cold climates require materials that can prevent ice accumulation on blades and maintain structural integrity in freezing conditions. Similarly, solar panels benefit from antifreeze coatings that can prevent snow and ice buildup, ensuring consistent energy production year-round.
The global market for antifreeze chemicals and materials is projected to experience substantial growth in the coming years. This growth is fueled by the expansion of end-use industries and the continuous development of new applications for antifreeze technologies in high-performance materials.
Research and development efforts are increasingly focused on bio-based and environmentally friendly antifreeze solutions, responding to the growing demand for sustainable materials across industries. This trend is likely to open up new market opportunities and drive innovation in the field of antifreeze technologies for high-performance materials.
In the aerospace industry, there is a significant push for materials that can maintain their structural integrity and performance in the extreme cold of high altitudes and space. Antifreeze technologies are crucial in developing lightweight, durable materials for aircraft and spacecraft components that can resist ice formation and maintain flexibility at sub-zero temperatures.
The automotive sector is another major driver of demand for antifreeze-enhanced materials. With the rise of electric vehicles and the need for more efficient thermal management systems, there is a growing market for advanced coolants and materials that can improve battery performance and longevity in cold climates. Additionally, the development of self-healing materials incorporating antifreeze properties is gaining traction for use in automotive exteriors and components.
In the construction industry, there is an increasing demand for concrete and other building materials that can resist freeze-thaw cycles, particularly in regions with harsh winters. Antifreeze admixtures and surface treatments that can improve the durability and lifespan of infrastructure are seeing growing adoption.
The energy sector, particularly in renewable energy, is also contributing to the market demand for antifreeze technologies. Wind turbines operating in cold climates require materials that can prevent ice accumulation on blades and maintain structural integrity in freezing conditions. Similarly, solar panels benefit from antifreeze coatings that can prevent snow and ice buildup, ensuring consistent energy production year-round.
The global market for antifreeze chemicals and materials is projected to experience substantial growth in the coming years. This growth is fueled by the expansion of end-use industries and the continuous development of new applications for antifreeze technologies in high-performance materials.
Research and development efforts are increasingly focused on bio-based and environmentally friendly antifreeze solutions, responding to the growing demand for sustainable materials across industries. This trend is likely to open up new market opportunities and drive innovation in the field of antifreeze technologies for high-performance materials.
Technical Challenges
The development of antifreeze proteins and their applications in high-performance material science face several significant technical challenges. One of the primary obstacles is the complexity of protein structure and function. Antifreeze proteins exhibit a diverse range of structures and mechanisms, making it difficult to fully understand and replicate their ice-binding properties. This complexity hinders the design and synthesis of artificial antifreeze proteins with tailored properties for specific applications.
Another major challenge lies in the scalability of antifreeze protein production. While natural sources like fish and insects produce these proteins, extracting them in large quantities is not economically viable for industrial applications. Recombinant protein production techniques have shown promise, but achieving high yields and maintaining protein functionality during large-scale production remains problematic. This limitation significantly impacts the potential for widespread use of antifreeze proteins in material science applications.
The stability of antifreeze proteins under various environmental conditions poses another technical hurdle. Many antifreeze proteins are sensitive to temperature changes and may lose their ice-binding capabilities at elevated temperatures. This instability limits their use in materials that may be exposed to fluctuating temperatures or harsh environmental conditions. Developing methods to enhance the thermal stability of antifreeze proteins without compromising their functionality is crucial for expanding their applications in high-performance materials.
Integrating antifreeze proteins into various materials and maintaining their activity presents another set of challenges. The proteins must be incorporated in a way that preserves their three-dimensional structure and ice-binding surfaces. This integration process can be particularly challenging when working with synthetic polymers or composite materials. Ensuring that the proteins remain active and accessible within the material matrix while maintaining the desired material properties is a complex balancing act.
Furthermore, the long-term stability and performance of materials incorporating antifreeze proteins remain uncertain. There is limited data on how these proteins behave over extended periods when integrated into materials. Questions about their resistance to degradation, potential for leaching, and long-term ice inhibition efficacy need to be addressed to ensure the reliability and safety of antifreeze protein-enhanced materials.
Lastly, the regulatory landscape surrounding the use of biological molecules in materials presents additional challenges. As antifreeze proteins are derived from living organisms, their use in consumer products may face scrutiny from regulatory bodies. Addressing safety concerns, potential allergenicity, and environmental impact will be crucial for the widespread adoption of antifreeze protein-based materials in various industries.
Another major challenge lies in the scalability of antifreeze protein production. While natural sources like fish and insects produce these proteins, extracting them in large quantities is not economically viable for industrial applications. Recombinant protein production techniques have shown promise, but achieving high yields and maintaining protein functionality during large-scale production remains problematic. This limitation significantly impacts the potential for widespread use of antifreeze proteins in material science applications.
The stability of antifreeze proteins under various environmental conditions poses another technical hurdle. Many antifreeze proteins are sensitive to temperature changes and may lose their ice-binding capabilities at elevated temperatures. This instability limits their use in materials that may be exposed to fluctuating temperatures or harsh environmental conditions. Developing methods to enhance the thermal stability of antifreeze proteins without compromising their functionality is crucial for expanding their applications in high-performance materials.
Integrating antifreeze proteins into various materials and maintaining their activity presents another set of challenges. The proteins must be incorporated in a way that preserves their three-dimensional structure and ice-binding surfaces. This integration process can be particularly challenging when working with synthetic polymers or composite materials. Ensuring that the proteins remain active and accessible within the material matrix while maintaining the desired material properties is a complex balancing act.
Furthermore, the long-term stability and performance of materials incorporating antifreeze proteins remain uncertain. There is limited data on how these proteins behave over extended periods when integrated into materials. Questions about their resistance to degradation, potential for leaching, and long-term ice inhibition efficacy need to be addressed to ensure the reliability and safety of antifreeze protein-enhanced materials.
Lastly, the regulatory landscape surrounding the use of biological molecules in materials presents additional challenges. As antifreeze proteins are derived from living organisms, their use in consumer products may face scrutiny from regulatory bodies. Addressing safety concerns, potential allergenicity, and environmental impact will be crucial for the widespread adoption of antifreeze protein-based materials in various industries.
Current Solutions
01 Composition of antifreeze solutions
Antifreeze solutions typically consist of a mixture of water and chemical compounds such as ethylene glycol or propylene glycol. These solutions are designed to lower the freezing point of water and prevent engine damage in cold temperatures. Additives may be included to enhance corrosion protection and improve heat transfer properties.- Composition of antifreeze solutions: Antifreeze solutions typically consist of a mixture of water and chemical compounds such as ethylene glycol or propylene glycol. These solutions are designed to lower the freezing point of water and prevent it from freezing in vehicle cooling systems and other applications. The composition may also include additives to enhance performance and protect against corrosion.
- Recycling and purification of used antifreeze: Methods for recycling and purifying used antifreeze have been developed to reduce waste and environmental impact. These processes often involve filtration, distillation, or chemical treatment to remove contaminants and restore the antifreeze to its original effectiveness. Recycled antifreeze can be reused in vehicles or other applications, reducing the need for new production.
- Antifreeze applications in renewable energy systems: Antifreeze solutions play a crucial role in renewable energy systems, particularly in solar thermal and geothermal applications. These solutions help maintain system efficiency by preventing freezing in cold climates and enabling heat transfer. Specialized antifreeze formulations may be developed to meet the specific requirements of these renewable energy technologies.
- Environmentally friendly antifreeze alternatives: Research has focused on developing more environmentally friendly antifreeze alternatives to traditional ethylene glycol-based solutions. These may include bio-based or plant-derived compounds that offer similar freezing point depression and heat transfer properties while being less toxic and more biodegradable. Such alternatives aim to reduce environmental impact and improve safety in various applications.
- Antifreeze testing and quality control methods: Various testing and quality control methods have been developed to ensure the effectiveness and safety of antifreeze solutions. These may include techniques for measuring freezing point, boiling point, pH levels, and the presence of contaminants. Advanced testing methods can help identify the composition of antifreeze mixtures and detect any degradation or contamination in used antifreeze.
02 Recycling and purification of used antifreeze
Methods for recycling and purifying used antifreeze involve removing contaminants and restoring the solution's properties. This process may include filtration, distillation, or chemical treatment to remove impurities and adjust the concentration of active ingredients. Recycling antifreeze helps reduce environmental impact and conserve resources.Expand Specific Solutions03 Antifreeze applications in renewable energy systems
Antifreeze solutions are used in renewable energy systems such as solar thermal collectors and geothermal heat pumps. These applications require specialized formulations that can withstand high temperatures and provide long-term stability. The antifreeze helps prevent freezing and maintains efficient heat transfer in these systems.Expand Specific Solutions04 Environmentally friendly antifreeze alternatives
Development of eco-friendly antifreeze solutions focuses on using less toxic and more biodegradable ingredients. These alternatives may include plant-based glycols, organic acid technology, or other natural compounds that provide similar freezing point depression and heat transfer properties while reducing environmental impact.Expand Specific Solutions05 Testing and quality control of antifreeze
Methods for testing and ensuring the quality of antifreeze solutions involve measuring various parameters such as freezing point, boiling point, pH, and corrosion inhibition properties. Advanced techniques may include spectroscopic analysis or chromatography to determine the composition and detect contaminants. Regular testing helps maintain the effectiveness and safety of antifreeze products.Expand Specific Solutions
Industry Leaders
The antifreeze market in high-performance material science is in a mature growth phase, with a global market size expected to reach $7.7 billion by 2027. The technology's maturity is evident in the diverse applications across automotive, industrial, and aerospace sectors. Key players like BASF Corp., Clariant Produkte, and Chevron U.S.A. are driving innovation through advanced formulations and eco-friendly solutions. Emerging companies such as Arteco NV and Kukdong Jeyen Co. are focusing on specialized applications, while research institutions like Zhejiang University and Korea University Research & Business Foundation are contributing to technological advancements. The competitive landscape is characterized by a mix of established chemical giants and niche players, with increasing emphasis on sustainable and high-performance antifreeze solutions.
BASF Corp.
Technical Solution: BASF has developed advanced antifreeze formulations that contribute significantly to high-performance material science. Their innovative approach includes the use of organic acid technology (OAT) in their Glysantin® line of products. This technology provides superior corrosion protection for various metals in cooling systems, extending the life of engine components[1]. BASF's antifreeze solutions also incorporate nano-additive technology, which enhances heat transfer efficiency and improves overall thermal management in high-performance applications[2]. The company has further developed bio-based antifreeze solutions, utilizing renewable resources to create more sustainable and environmentally friendly products without compromising on performance[3].
Strengths: Extensive research capabilities, global market presence, and a diverse product portfolio. Weaknesses: Higher production costs for advanced formulations may impact pricing competitiveness.
Clariant Produkte (Deutschland) GmbH
Technical Solution: Clariant has made significant contributions to antifreeze technology in high-performance material science through their development of next-generation coolants. Their approach focuses on multifunctional additives that not only prevent freezing but also enhance the overall performance of cooling systems. Clariant's antifreeze solutions incorporate advanced polymer technology, which provides improved thermal stability and reduces deposit formation in high-temperature environments[4]. The company has also developed low-toxicity formulations using propylene glycol-based solutions, addressing environmental and safety concerns while maintaining high performance standards[5]. Additionally, Clariant's research has led to the creation of antifreeze products with extended service life, reducing maintenance requirements and improving long-term efficiency in industrial applications[6].
Strengths: Strong focus on sustainability and eco-friendly solutions, innovative additive technologies. Weaknesses: May face challenges in market penetration against established competitors.
Key Innovations
Antifreeze
PatentInactiveEP3476903A1
Innovation
- A combination of succinic acid, benzotriazole, and potassium hydroxide (KOH) with a pH range of 10.4 to 10.8, or succinic acid, cinnamic acid, benzotriazole, and KOH with a pH range of 8.5 to 10.8, which synergistically provides excellent frost protection and corrosion protection for all common metals, including solder, while reducing the need for high benzotriazole concentrations.
Antifreeze proteins from basidiomycetes
PatentInactiveEP1344827B1
Innovation
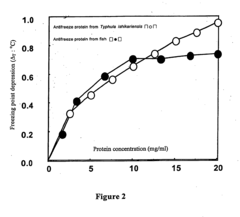
- Novel antifreeze proteins with high antifreeze activity are isolated and purified from basidiomycetes such as Typhula ishikariensis, which can be produced in large quantities at a low cost, using specific N-terminal amino acid sequences and a method involving culturing and purification techniques.
Environmental Impact
The environmental impact of antifreeze in high-performance material science is a critical consideration that extends beyond its primary applications. As antifreeze compounds find increasing use in advanced materials, their potential effects on ecosystems and human health have come under scrutiny.
One of the primary environmental concerns associated with antifreeze is its toxicity. Many traditional antifreeze formulations contain ethylene glycol, which can be harmful to wildlife and contaminate water sources if improperly disposed of. This has led to the development of more environmentally friendly alternatives, such as propylene glycol-based antifreeze, which exhibits lower toxicity levels while maintaining similar performance characteristics.
The production and disposal of antifreeze materials also contribute to their environmental footprint. Manufacturing processes often involve energy-intensive operations and the use of petrochemical-derived components, leading to greenhouse gas emissions and resource depletion. However, advancements in green chemistry and sustainable production methods are gradually mitigating these impacts, with some manufacturers adopting bio-based feedstocks and closed-loop recycling systems.
In the context of high-performance materials, the environmental impact of antifreeze extends to its role in enhancing material durability and longevity. By improving the resistance of materials to extreme temperatures and freeze-thaw cycles, antifreeze compounds can significantly extend the lifespan of products, potentially reducing waste and the need for frequent replacements. This indirect environmental benefit must be weighed against the immediate impacts of antifreeze production and use.
The incorporation of antifreeze properties into advanced materials has also led to innovations in energy efficiency. For instance, antifreeze-inspired coatings for heat exchangers and wind turbines can prevent ice formation, improving operational efficiency and reducing energy consumption. These applications demonstrate how antifreeze technology can contribute to broader environmental goals by enhancing the performance of renewable energy systems.
As research in this field progresses, there is a growing focus on developing biodegradable and non-toxic antifreeze compounds derived from natural sources. These eco-friendly alternatives aim to minimize environmental persistence and reduce the risk of bioaccumulation in food chains. Additionally, efforts are being made to improve the recyclability of antifreeze-containing materials, ensuring that end-of-life products can be safely and efficiently reprocessed.
The environmental impact of antifreeze in high-performance material science is a complex issue that requires ongoing assessment and mitigation strategies. As the field evolves, balancing the benefits of enhanced material performance with environmental stewardship remains a key challenge for researchers and industry professionals alike.
One of the primary environmental concerns associated with antifreeze is its toxicity. Many traditional antifreeze formulations contain ethylene glycol, which can be harmful to wildlife and contaminate water sources if improperly disposed of. This has led to the development of more environmentally friendly alternatives, such as propylene glycol-based antifreeze, which exhibits lower toxicity levels while maintaining similar performance characteristics.
The production and disposal of antifreeze materials also contribute to their environmental footprint. Manufacturing processes often involve energy-intensive operations and the use of petrochemical-derived components, leading to greenhouse gas emissions and resource depletion. However, advancements in green chemistry and sustainable production methods are gradually mitigating these impacts, with some manufacturers adopting bio-based feedstocks and closed-loop recycling systems.
In the context of high-performance materials, the environmental impact of antifreeze extends to its role in enhancing material durability and longevity. By improving the resistance of materials to extreme temperatures and freeze-thaw cycles, antifreeze compounds can significantly extend the lifespan of products, potentially reducing waste and the need for frequent replacements. This indirect environmental benefit must be weighed against the immediate impacts of antifreeze production and use.
The incorporation of antifreeze properties into advanced materials has also led to innovations in energy efficiency. For instance, antifreeze-inspired coatings for heat exchangers and wind turbines can prevent ice formation, improving operational efficiency and reducing energy consumption. These applications demonstrate how antifreeze technology can contribute to broader environmental goals by enhancing the performance of renewable energy systems.
As research in this field progresses, there is a growing focus on developing biodegradable and non-toxic antifreeze compounds derived from natural sources. These eco-friendly alternatives aim to minimize environmental persistence and reduce the risk of bioaccumulation in food chains. Additionally, efforts are being made to improve the recyclability of antifreeze-containing materials, ensuring that end-of-life products can be safely and efficiently reprocessed.
The environmental impact of antifreeze in high-performance material science is a complex issue that requires ongoing assessment and mitigation strategies. As the field evolves, balancing the benefits of enhanced material performance with environmental stewardship remains a key challenge for researchers and industry professionals alike.
Safety Regulations
The safety regulations surrounding antifreeze and its applications in high-performance material science have evolved significantly in recent years. As antifreeze compounds continue to play a crucial role in developing advanced materials, regulatory bodies have implemented stringent guidelines to ensure their safe handling, storage, and disposal. These regulations primarily focus on minimizing environmental impact and protecting human health.
One of the key areas of concern is the toxicity of traditional antifreeze chemicals, such as ethylene glycol. Regulatory agencies have mandated the use of less toxic alternatives, such as propylene glycol, in many applications. This shift has led to the development of new antifreeze formulations that maintain high performance while reducing potential harm to humans and wildlife.
Environmental protection agencies have also implemented strict guidelines for the disposal of antifreeze-containing materials. These regulations require proper treatment and recycling of antifreeze solutions to prevent contamination of soil and water sources. Companies working with antifreeze in material science applications must adhere to specific waste management protocols and maintain detailed records of their disposal practices.
Occupational safety regulations have been established to protect workers handling antifreeze compounds in research and manufacturing settings. These include requirements for personal protective equipment, proper ventilation systems, and emergency response procedures in case of spills or exposure. Regular safety training and health monitoring programs are also mandated for personnel working with these materials.
The transportation of antifreeze and related materials is subject to comprehensive regulations to prevent accidents and spills during transit. These guidelines cover packaging requirements, labeling standards, and specific handling procedures for different modes of transportation. Companies must ensure compliance with both domestic and international transportation regulations when shipping antifreeze-containing products or raw materials.
As the field of high-performance material science continues to advance, regulatory bodies are actively updating safety standards to keep pace with new developments. This includes addressing emerging concerns related to novel antifreeze compounds and their potential long-term effects on the environment and human health. Ongoing research and collaboration between industry, academia, and regulatory agencies are essential to maintain a balance between innovation and safety in this rapidly evolving field.
Compliance with these safety regulations is not only a legal requirement but also a critical factor in the commercial success of antifreeze-based materials. Companies that demonstrate a strong commitment to safety and environmental responsibility are better positioned to gain market acceptance and maintain long-term sustainability in the high-performance materials sector.
One of the key areas of concern is the toxicity of traditional antifreeze chemicals, such as ethylene glycol. Regulatory agencies have mandated the use of less toxic alternatives, such as propylene glycol, in many applications. This shift has led to the development of new antifreeze formulations that maintain high performance while reducing potential harm to humans and wildlife.
Environmental protection agencies have also implemented strict guidelines for the disposal of antifreeze-containing materials. These regulations require proper treatment and recycling of antifreeze solutions to prevent contamination of soil and water sources. Companies working with antifreeze in material science applications must adhere to specific waste management protocols and maintain detailed records of their disposal practices.
Occupational safety regulations have been established to protect workers handling antifreeze compounds in research and manufacturing settings. These include requirements for personal protective equipment, proper ventilation systems, and emergency response procedures in case of spills or exposure. Regular safety training and health monitoring programs are also mandated for personnel working with these materials.
The transportation of antifreeze and related materials is subject to comprehensive regulations to prevent accidents and spills during transit. These guidelines cover packaging requirements, labeling standards, and specific handling procedures for different modes of transportation. Companies must ensure compliance with both domestic and international transportation regulations when shipping antifreeze-containing products or raw materials.
As the field of high-performance material science continues to advance, regulatory bodies are actively updating safety standards to keep pace with new developments. This includes addressing emerging concerns related to novel antifreeze compounds and their potential long-term effects on the environment and human health. Ongoing research and collaboration between industry, academia, and regulatory agencies are essential to maintain a balance between innovation and safety in this rapidly evolving field.
Compliance with these safety regulations is not only a legal requirement but also a critical factor in the commercial success of antifreeze-based materials. Companies that demonstrate a strong commitment to safety and environmental responsibility are better positioned to gain market acceptance and maintain long-term sustainability in the high-performance materials sector.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!