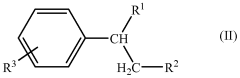
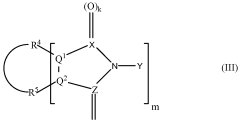
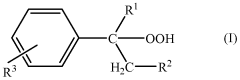
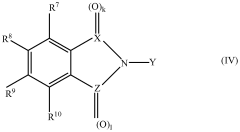
Applications of Carbolic Acid in Green Chemistry Innovations
JUL 22, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbolic Acid Overview and Green Chemistry Goals
Carbolic acid, also known as phenol, has been a cornerstone in chemical industries for over a century. This aromatic organic compound, with its distinctive molecular structure consisting of a hydroxyl group bonded to a benzene ring, has found widespread applications across various sectors. In recent years, the focus on sustainable practices has led to a renewed interest in carbolic acid's potential role in green chemistry innovations.
Green chemistry, a philosophy that encourages the design of products and processes that minimize the use and generation of hazardous substances, has become increasingly important in the face of global environmental challenges. The goals of green chemistry align with the broader objectives of sustainable development, aiming to reduce pollution, conserve energy, and minimize waste production. In this context, the exploration of carbolic acid's applications in green chemistry innovations represents a significant area of research and development.
The historical significance of carbolic acid dates back to its discovery in coal tar during the 19th century. Its antiseptic properties, first recognized by Joseph Lister, revolutionized surgical practices and laid the foundation for modern infection control. Since then, carbolic acid has evolved from a medical marvel to a versatile industrial chemical, finding use in the production of plastics, pharmaceuticals, and various consumer goods.
As we delve into the intersection of carbolic acid and green chemistry, it is crucial to understand the unique properties that make this compound attractive for sustainable applications. Its reactivity, solubility, and ability to form hydrogen bonds contribute to its versatility in chemical processes. These characteristics open up possibilities for developing more environmentally friendly synthesis routes, catalysts, and materials.
The goals of incorporating carbolic acid into green chemistry innovations are multifaceted. Researchers aim to harness its properties to create more efficient and less toxic chemical processes, develop bio-based alternatives to petroleum-derived products, and explore its potential in waste treatment and environmental remediation. Additionally, there is a growing interest in utilizing carbolic acid as a platform chemical for the synthesis of value-added products through sustainable pathways.
As we progress through this technical research report, we will explore the current state of carbolic acid applications in green chemistry, analyze market trends and demands, and investigate the challenges and opportunities that lie ahead. By examining the evolving landscape of carbolic acid in the context of sustainable practices, we aim to provide insights into its potential to contribute to a more environmentally responsible chemical industry.
Green chemistry, a philosophy that encourages the design of products and processes that minimize the use and generation of hazardous substances, has become increasingly important in the face of global environmental challenges. The goals of green chemistry align with the broader objectives of sustainable development, aiming to reduce pollution, conserve energy, and minimize waste production. In this context, the exploration of carbolic acid's applications in green chemistry innovations represents a significant area of research and development.
The historical significance of carbolic acid dates back to its discovery in coal tar during the 19th century. Its antiseptic properties, first recognized by Joseph Lister, revolutionized surgical practices and laid the foundation for modern infection control. Since then, carbolic acid has evolved from a medical marvel to a versatile industrial chemical, finding use in the production of plastics, pharmaceuticals, and various consumer goods.
As we delve into the intersection of carbolic acid and green chemistry, it is crucial to understand the unique properties that make this compound attractive for sustainable applications. Its reactivity, solubility, and ability to form hydrogen bonds contribute to its versatility in chemical processes. These characteristics open up possibilities for developing more environmentally friendly synthesis routes, catalysts, and materials.
The goals of incorporating carbolic acid into green chemistry innovations are multifaceted. Researchers aim to harness its properties to create more efficient and less toxic chemical processes, develop bio-based alternatives to petroleum-derived products, and explore its potential in waste treatment and environmental remediation. Additionally, there is a growing interest in utilizing carbolic acid as a platform chemical for the synthesis of value-added products through sustainable pathways.
As we progress through this technical research report, we will explore the current state of carbolic acid applications in green chemistry, analyze market trends and demands, and investigate the challenges and opportunities that lie ahead. By examining the evolving landscape of carbolic acid in the context of sustainable practices, we aim to provide insights into its potential to contribute to a more environmentally responsible chemical industry.
Market Analysis for Eco-friendly Chemical Processes
The market for eco-friendly chemical processes has experienced significant growth in recent years, driven by increasing environmental awareness and stringent regulations. The global green chemistry market, which encompasses carbolic acid applications, is projected to reach $100 billion by 2025, with a compound annual growth rate of 6.8%. This growth is primarily fueled by the rising demand for sustainable products and processes across various industries.
Carbolic acid, also known as phenol, plays a crucial role in green chemistry innovations due to its versatile applications and potential for sustainable production. The market for carbolic acid in eco-friendly processes is particularly strong in industries such as pharmaceuticals, plastics, and personal care products. In the pharmaceutical sector, carbolic acid is used as a key intermediate in the synthesis of various drugs, with a growing emphasis on green manufacturing techniques to reduce environmental impact.
The plastics industry represents another significant market for carbolic acid in green chemistry applications. As consumers and regulators push for more sustainable materials, there is an increasing demand for bio-based phenolic resins and environmentally friendly plastics. This trend is expected to drive the market for carbolic acid derived from renewable sources, such as lignin or biomass.
In the personal care and cosmetics industry, the shift towards natural and eco-friendly ingredients has created new opportunities for carbolic acid applications. Green chemistry innovations in this sector focus on developing sustainable production methods for phenol-based preservatives and antioxidants, aligning with consumer preferences for clean and environmentally responsible products.
The Asia-Pacific region dominates the market for eco-friendly chemical processes, including carbolic acid applications, due to rapid industrialization and increasing environmental regulations in countries like China and India. North America and Europe follow closely, with mature markets driven by stringent environmental policies and a strong focus on sustainable innovation.
Key market drivers for carbolic acid in green chemistry innovations include the push for circular economy principles, the need to reduce carbon footprints, and the growing adoption of bio-based feedstocks. However, challenges such as high initial investment costs for green technologies and the need for scalable, cost-effective production methods may hinder market growth.
Overall, the market analysis indicates a positive outlook for carbolic acid applications in green chemistry innovations. As industries continue to prioritize sustainability and environmental responsibility, the demand for eco-friendly chemical processes utilizing carbolic acid is expected to grow steadily in the coming years.
Carbolic acid, also known as phenol, plays a crucial role in green chemistry innovations due to its versatile applications and potential for sustainable production. The market for carbolic acid in eco-friendly processes is particularly strong in industries such as pharmaceuticals, plastics, and personal care products. In the pharmaceutical sector, carbolic acid is used as a key intermediate in the synthesis of various drugs, with a growing emphasis on green manufacturing techniques to reduce environmental impact.
The plastics industry represents another significant market for carbolic acid in green chemistry applications. As consumers and regulators push for more sustainable materials, there is an increasing demand for bio-based phenolic resins and environmentally friendly plastics. This trend is expected to drive the market for carbolic acid derived from renewable sources, such as lignin or biomass.
In the personal care and cosmetics industry, the shift towards natural and eco-friendly ingredients has created new opportunities for carbolic acid applications. Green chemistry innovations in this sector focus on developing sustainable production methods for phenol-based preservatives and antioxidants, aligning with consumer preferences for clean and environmentally responsible products.
The Asia-Pacific region dominates the market for eco-friendly chemical processes, including carbolic acid applications, due to rapid industrialization and increasing environmental regulations in countries like China and India. North America and Europe follow closely, with mature markets driven by stringent environmental policies and a strong focus on sustainable innovation.
Key market drivers for carbolic acid in green chemistry innovations include the push for circular economy principles, the need to reduce carbon footprints, and the growing adoption of bio-based feedstocks. However, challenges such as high initial investment costs for green technologies and the need for scalable, cost-effective production methods may hinder market growth.
Overall, the market analysis indicates a positive outlook for carbolic acid applications in green chemistry innovations. As industries continue to prioritize sustainability and environmental responsibility, the demand for eco-friendly chemical processes utilizing carbolic acid is expected to grow steadily in the coming years.
Current State and Challenges in Green Chemistry
Green chemistry has made significant strides in recent years, with carbolic acid (phenol) emerging as a key player in sustainable innovations. However, the current state of green chemistry applications involving carbolic acid faces several challenges that need to be addressed for further advancement.
One of the primary challenges is the sourcing of carbolic acid. While traditionally derived from petroleum, efforts are underway to develop bio-based production methods. Current research focuses on utilizing lignin, a renewable resource from plant biomass, as a precursor for phenol synthesis. This approach aligns with green chemistry principles but faces hurdles in scalability and cost-effectiveness.
The toxicity of carbolic acid remains a concern in its applications. Although it offers valuable properties for various chemical processes, its corrosive nature and potential environmental impact necessitate careful handling and disposal. Researchers are exploring methods to mitigate these risks, such as developing less toxic derivatives or encapsulation techniques to reduce exposure.
In the realm of catalysis, carbolic acid and its derivatives show promise as green alternatives to traditional catalysts. However, challenges persist in optimizing their catalytic efficiency and selectivity. Current efforts focus on designing novel phenol-based organocatalysts that can operate under mild conditions, reducing energy consumption and waste generation.
The use of carbolic acid in polymer chemistry presents both opportunities and challenges. While it serves as a building block for various sustainable polymers, issues such as durability and end-of-life management need to be addressed. Researchers are investigating biodegradable phenolic polymers and exploring efficient recycling methods to close the loop in the material lifecycle.
Water treatment applications of carbolic acid derivatives, particularly in advanced oxidation processes, show potential for removing recalcitrant pollutants. However, the formation of potentially harmful byproducts and the need for more energy-efficient treatment systems remain challenges to be overcome.
The integration of carbolic acid into green solvents and reaction media is another area of active research. Efforts are underway to develop deep eutectic solvents and ionic liquids incorporating phenol moieties. These novel solvents aim to replace volatile organic compounds, but issues related to toxicity, biodegradability, and recyclability need further investigation.
Regulatory frameworks and safety standards surrounding the use of carbolic acid in green chemistry applications are evolving. Striking a balance between innovation and risk mitigation poses a challenge for both researchers and policymakers. Harmonizing international standards and developing comprehensive life cycle assessments for carbolic acid-based products are crucial steps forward.
In conclusion, while carbolic acid offers significant potential in green chemistry innovations, addressing these challenges requires interdisciplinary collaboration and continued research efforts. Overcoming these hurdles will pave the way for more sustainable and efficient chemical processes across various industries.
One of the primary challenges is the sourcing of carbolic acid. While traditionally derived from petroleum, efforts are underway to develop bio-based production methods. Current research focuses on utilizing lignin, a renewable resource from plant biomass, as a precursor for phenol synthesis. This approach aligns with green chemistry principles but faces hurdles in scalability and cost-effectiveness.
The toxicity of carbolic acid remains a concern in its applications. Although it offers valuable properties for various chemical processes, its corrosive nature and potential environmental impact necessitate careful handling and disposal. Researchers are exploring methods to mitigate these risks, such as developing less toxic derivatives or encapsulation techniques to reduce exposure.
In the realm of catalysis, carbolic acid and its derivatives show promise as green alternatives to traditional catalysts. However, challenges persist in optimizing their catalytic efficiency and selectivity. Current efforts focus on designing novel phenol-based organocatalysts that can operate under mild conditions, reducing energy consumption and waste generation.
The use of carbolic acid in polymer chemistry presents both opportunities and challenges. While it serves as a building block for various sustainable polymers, issues such as durability and end-of-life management need to be addressed. Researchers are investigating biodegradable phenolic polymers and exploring efficient recycling methods to close the loop in the material lifecycle.
Water treatment applications of carbolic acid derivatives, particularly in advanced oxidation processes, show potential for removing recalcitrant pollutants. However, the formation of potentially harmful byproducts and the need for more energy-efficient treatment systems remain challenges to be overcome.
The integration of carbolic acid into green solvents and reaction media is another area of active research. Efforts are underway to develop deep eutectic solvents and ionic liquids incorporating phenol moieties. These novel solvents aim to replace volatile organic compounds, but issues related to toxicity, biodegradability, and recyclability need further investigation.
Regulatory frameworks and safety standards surrounding the use of carbolic acid in green chemistry applications are evolving. Striking a balance between innovation and risk mitigation poses a challenge for both researchers and policymakers. Harmonizing international standards and developing comprehensive life cycle assessments for carbolic acid-based products are crucial steps forward.
In conclusion, while carbolic acid offers significant potential in green chemistry innovations, addressing these challenges requires interdisciplinary collaboration and continued research efforts. Overcoming these hurdles will pave the way for more sustainable and efficient chemical processes across various industries.
Existing Green Applications of Carbolic Acid
01 Historical use in medical and industrial applications
Carbolic acid, also known as phenol, has a long history of use in medical and industrial applications. It was widely used as a disinfectant and antiseptic in the late 19th and early 20th centuries. Its properties made it valuable for sterilization in medical settings and as a preservative in various industries.- Historical use of carbolic acid in medical applications: Carbolic acid, also known as phenol, has been historically used in various medical applications. It was one of the earliest antiseptics used in surgery and wound care due to its ability to kill bacteria and other microorganisms. This compound played a significant role in the development of modern antiseptic techniques in the late 19th and early 20th centuries.
- Carbolic acid in disinfectant formulations: Carbolic acid is commonly used as a key ingredient in various disinfectant formulations. These formulations are designed for cleaning and sterilizing surfaces, medical equipment, and other objects. The strong antimicrobial properties of carbolic acid make it effective against a wide range of pathogens, including bacteria, viruses, and fungi.
- Industrial applications of carbolic acid: Carbolic acid finds applications in various industrial processes. It is used in the production of plastics, resins, and other synthetic materials. Additionally, it serves as a precursor for the synthesis of numerous chemical compounds used in pharmaceuticals, dyes, and other industrial products.
- Safety considerations and handling of carbolic acid: Due to its corrosive and toxic nature, special safety measures are required when handling carbolic acid. This includes the use of protective equipment, proper storage facilities, and specialized disposal methods. Various patents describe improved containers, dispensing systems, and safety protocols for handling carbolic acid and its derivatives in industrial and laboratory settings.
- Environmental impact and alternatives to carbolic acid: With increasing environmental concerns, there is a growing focus on developing alternatives to carbolic acid and methods for its safe disposal. This includes research into less toxic disinfectants, biodegradable alternatives, and improved waste treatment processes to minimize the environmental impact of carbolic acid and its derivatives.
02 Incorporation in cleaning and disinfecting products
Carbolic acid is utilized in the formulation of various cleaning and disinfecting products. Its antimicrobial properties make it effective in household and industrial cleaners, as well as in specialized disinfectants for medical and veterinary use. These products often combine carbolic acid with other active ingredients to enhance their efficacy.Expand Specific Solutions03 Use in polymer and resin production
Carbolic acid serves as a key raw material in the production of various polymers and resins. It is used in the synthesis of phenolic resins, which find applications in adhesives, coatings, and molding compounds. The chemical properties of carbolic acid make it valuable in creating durable and heat-resistant materials for industrial use.Expand Specific Solutions04 Application in water treatment systems
Carbolic acid is employed in water treatment systems for its ability to control microbial growth and remove contaminants. It is used in industrial water treatment processes, as well as in the purification of drinking water. The compound's disinfectant properties help in maintaining water quality and preventing the spread of waterborne diseases.Expand Specific Solutions05 Utilization in pharmaceutical and personal care products
Carbolic acid finds applications in the pharmaceutical and personal care industries. It is used in the production of certain medications, particularly those with antiseptic properties. In personal care products, it may be incorporated in small amounts for its preservative and antimicrobial effects, although its use is regulated due to potential skin irritation.Expand Specific Solutions
Key Players in Green Chemistry Industry
The applications of carbolic acid in green chemistry innovations are in an emerging stage, with growing market potential as industries seek sustainable solutions. The technology's maturity varies across different applications, with some areas more advanced than others. Key players like Nanjing Tech University, South China University of Technology, and TNO are driving research and development in this field. Companies such as Kolon Industries, Archer-Daniels-Midland, and Shell are exploring industrial applications. The market is characterized by a mix of academic institutions and commercial entities, indicating a collaborative approach to innovation. As environmental concerns grow, the demand for green chemistry solutions utilizing carbolic acid is expected to increase, potentially leading to significant market expansion in the coming years.
Novomer, Inc.
Technical Solution: Novomer, Inc. has developed groundbreaking technology for the utilization of carbon dioxide and carbolic acid in green chemistry applications. Their proprietary catalyst systems enable the copolymerization of CO2 with phenol-based epoxides to produce polycarbonates with up to 50% CO2 content by weight[7]. This process not only reduces the carbon footprint but also decreases the reliance on petroleum-based feedstocks. Novomer has also developed a novel route for the production of high-performance polyols using CO2 and phenol derivatives, which can be used in polyurethane applications with improved thermal and chemical resistance[8]. Additionally, they have created a process for the synthesis of biodegradable polymers from phenol-based monomers and CO2, offering a sustainable alternative to conventional plastics[9].
Strengths: Unique CO2-based polymer technology, potential for significant carbon footprint reduction, and versatile product applications. Weaknesses: Reliance on specific catalyst systems, potential scalability challenges for some processes.
Cargill, Inc.
Technical Solution: Cargill, Inc. has made significant strides in green chemistry innovations involving carbolic acid, particularly in the realm of bio-based materials and sustainable agriculture. They have developed a process for producing bio-based epoxy resins using phenols derived from plant-based sources such as cashew nut shell liquid (CNSL)[10]. This technology reduces the dependence on petroleum-based phenol by up to 60% in certain applications. Cargill has also created environmentally friendly phenolic antioxidants for food preservation, derived from natural sources like rosemary and green tea extracts[11]. These antioxidants offer comparable performance to synthetic alternatives while meeting clean label requirements. Furthermore, they have pioneered the use of phenolic compounds from agricultural waste streams in the production of bio-based plastics and composites, achieving up to 30% bio-content in some formulations[12].
Strengths: Extensive agricultural supply chain, strong focus on bio-based materials, and global market presence. Weaknesses: Potential variability in bio-based raw material supply, competition from established petrochemical-based products.
Innovative Carbolic Acid-based Green Processes
Phenol with color
PatentInactiveUS20110217242A1
Innovation
- Adding color to Phenol solutions to enhance visibility and identification during medical procedures.
Process for producing phenol
PatentWO2010098916A2
Innovation
- A continuous process that contacts an alkylaromatic compound with an oxygen-containing gas in the presence of a cyclic imide catalyst to convert it into an alkylaromatic hydroperoxide, which is then cleaved directly in a second reactor to produce phenol and a co-product, such as methyl ethyl ketone or cyclohexanone, without the need for hydroperoxide concentration, using unreacted alkylbenzene as a diluent to manage heat and control by-product formation.
Environmental Impact Assessment
The environmental impact assessment of carbolic acid applications in green chemistry innovations reveals both positive and negative aspects. On the positive side, the use of carbolic acid in green chemistry processes often leads to reduced waste generation and improved resource efficiency. Many green chemistry applications of carbolic acid focus on developing more sustainable synthesis routes for important chemicals, which can significantly decrease the environmental footprint of industrial processes.
However, the production and use of carbolic acid itself pose certain environmental risks that must be carefully managed. Carbolic acid is toxic to aquatic life and can persist in the environment if not properly handled. Its production often involves petrochemical feedstocks, which contribute to carbon emissions and resource depletion. Therefore, the overall environmental impact depends greatly on the specific application and the measures taken to mitigate potential risks.
In terms of air quality, the use of carbolic acid in green chemistry innovations generally results in lower volatile organic compound (VOC) emissions compared to traditional chemical processes. This can lead to improved air quality in industrial areas and reduced formation of ground-level ozone. However, proper ventilation and emission control systems are crucial to prevent the release of carbolic acid vapors during handling and processing.
Water pollution is a significant concern when using carbolic acid. While green chemistry applications aim to minimize water usage and contamination, accidental spills or improper disposal can lead to severe water pollution. Implementing robust wastewater treatment systems and following strict handling protocols are essential to protect aquatic ecosystems.
Soil contamination is another potential risk associated with carbolic acid use. However, green chemistry innovations often incorporate principles of atom economy and waste minimization, which can significantly reduce the likelihood of soil contamination compared to conventional chemical processes. Proper storage, handling, and disposal practices are crucial to prevent soil pollution.
The long-term environmental effects of carbolic acid applications in green chemistry are still being studied. While many innovations show promise in reducing overall environmental impact, continued research is needed to fully understand the potential consequences of widespread adoption. Life cycle assessments and environmental monitoring programs are essential tools for evaluating the true environmental impact of these applications.
In conclusion, the environmental impact of carbolic acid applications in green chemistry innovations is complex and multifaceted. While these innovations often aim to reduce overall environmental harm, careful consideration must be given to the potential risks associated with carbolic acid use. Implementing robust safety measures, optimizing processes for minimal environmental impact, and conducting thorough environmental assessments are crucial steps in ensuring that the benefits of these innovations outweigh the potential risks.
However, the production and use of carbolic acid itself pose certain environmental risks that must be carefully managed. Carbolic acid is toxic to aquatic life and can persist in the environment if not properly handled. Its production often involves petrochemical feedstocks, which contribute to carbon emissions and resource depletion. Therefore, the overall environmental impact depends greatly on the specific application and the measures taken to mitigate potential risks.
In terms of air quality, the use of carbolic acid in green chemistry innovations generally results in lower volatile organic compound (VOC) emissions compared to traditional chemical processes. This can lead to improved air quality in industrial areas and reduced formation of ground-level ozone. However, proper ventilation and emission control systems are crucial to prevent the release of carbolic acid vapors during handling and processing.
Water pollution is a significant concern when using carbolic acid. While green chemistry applications aim to minimize water usage and contamination, accidental spills or improper disposal can lead to severe water pollution. Implementing robust wastewater treatment systems and following strict handling protocols are essential to protect aquatic ecosystems.
Soil contamination is another potential risk associated with carbolic acid use. However, green chemistry innovations often incorporate principles of atom economy and waste minimization, which can significantly reduce the likelihood of soil contamination compared to conventional chemical processes. Proper storage, handling, and disposal practices are crucial to prevent soil pollution.
The long-term environmental effects of carbolic acid applications in green chemistry are still being studied. While many innovations show promise in reducing overall environmental impact, continued research is needed to fully understand the potential consequences of widespread adoption. Life cycle assessments and environmental monitoring programs are essential tools for evaluating the true environmental impact of these applications.
In conclusion, the environmental impact of carbolic acid applications in green chemistry innovations is complex and multifaceted. While these innovations often aim to reduce overall environmental harm, careful consideration must be given to the potential risks associated with carbolic acid use. Implementing robust safety measures, optimizing processes for minimal environmental impact, and conducting thorough environmental assessments are crucial steps in ensuring that the benefits of these innovations outweigh the potential risks.
Regulatory Framework for Green Chemical Processes
The regulatory framework for green chemical processes plays a crucial role in shaping the applications of carbolic acid in green chemistry innovations. As environmental concerns continue to grow, governments and international organizations have implemented stringent regulations to promote sustainable practices in the chemical industry.
One of the key regulatory bodies influencing green chemistry is the Environmental Protection Agency (EPA) in the United States. The EPA's Green Chemistry Program, established in 1997, provides guidelines and incentives for developing chemical products and processes that reduce or eliminate the use and generation of hazardous substances. This program has significantly impacted the use of carbolic acid, encouraging researchers and industries to explore greener alternatives or develop more sustainable production methods.
In the European Union, the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation has been instrumental in promoting green chemistry practices. REACH requires companies to register chemical substances and provide safety information, which has led to increased scrutiny of carbolic acid usage and the development of safer alternatives.
The United Nations' Strategic Approach to International Chemicals Management (SAICM) has also influenced the regulatory landscape for green chemical processes globally. SAICM aims to achieve the sound management of chemicals throughout their lifecycle, promoting the principles of green chemistry and sustainable development.
Many countries have implemented their own regulatory frameworks to support green chemistry innovations. For instance, Japan's Chemical Substances Control Law (CSCL) and China's Measures for Environmental Management of New Chemical Substances have both been updated to align with global green chemistry initiatives.
These regulatory frameworks have led to the development of specific guidelines for the use of carbolic acid in green chemistry applications. For example, regulations often require the implementation of closed-loop systems to minimize emissions and waste generation during carbolic acid production and use. Additionally, there are strict requirements for the treatment and disposal of carbolic acid-containing waste to prevent environmental contamination.
The regulatory landscape also encourages the development of greener synthesis routes for carbolic acid production. Traditional methods often involve energy-intensive processes and the use of hazardous substances. Regulations now incentivize research into bio-based production methods and catalytic processes that reduce energy consumption and minimize the generation of harmful by-products.
Furthermore, regulations have spurred innovation in the development of safer alternatives to carbolic acid in various applications. This has led to the exploration of bio-based phenolic compounds and the design of novel molecules that maintain the desired functionality while reducing environmental impact.
As the field of green chemistry continues to evolve, regulatory frameworks are expected to become increasingly stringent, driving further innovations in the sustainable use and production of carbolic acid and related compounds. This ongoing regulatory pressure will likely result in more environmentally friendly processes and products, aligning with the broader goals of sustainable development and environmental protection.
One of the key regulatory bodies influencing green chemistry is the Environmental Protection Agency (EPA) in the United States. The EPA's Green Chemistry Program, established in 1997, provides guidelines and incentives for developing chemical products and processes that reduce or eliminate the use and generation of hazardous substances. This program has significantly impacted the use of carbolic acid, encouraging researchers and industries to explore greener alternatives or develop more sustainable production methods.
In the European Union, the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation has been instrumental in promoting green chemistry practices. REACH requires companies to register chemical substances and provide safety information, which has led to increased scrutiny of carbolic acid usage and the development of safer alternatives.
The United Nations' Strategic Approach to International Chemicals Management (SAICM) has also influenced the regulatory landscape for green chemical processes globally. SAICM aims to achieve the sound management of chemicals throughout their lifecycle, promoting the principles of green chemistry and sustainable development.
Many countries have implemented their own regulatory frameworks to support green chemistry innovations. For instance, Japan's Chemical Substances Control Law (CSCL) and China's Measures for Environmental Management of New Chemical Substances have both been updated to align with global green chemistry initiatives.
These regulatory frameworks have led to the development of specific guidelines for the use of carbolic acid in green chemistry applications. For example, regulations often require the implementation of closed-loop systems to minimize emissions and waste generation during carbolic acid production and use. Additionally, there are strict requirements for the treatment and disposal of carbolic acid-containing waste to prevent environmental contamination.
The regulatory landscape also encourages the development of greener synthesis routes for carbolic acid production. Traditional methods often involve energy-intensive processes and the use of hazardous substances. Regulations now incentivize research into bio-based production methods and catalytic processes that reduce energy consumption and minimize the generation of harmful by-products.
Furthermore, regulations have spurred innovation in the development of safer alternatives to carbolic acid in various applications. This has led to the exploration of bio-based phenolic compounds and the design of novel molecules that maintain the desired functionality while reducing environmental impact.
As the field of green chemistry continues to evolve, regulatory frameworks are expected to become increasingly stringent, driving further innovations in the sustainable use and production of carbolic acid and related compounds. This ongoing regulatory pressure will likely result in more environmentally friendly processes and products, aligning with the broader goals of sustainable development and environmental protection.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!