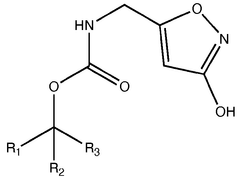
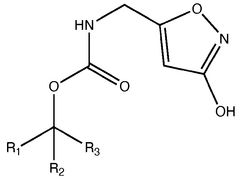
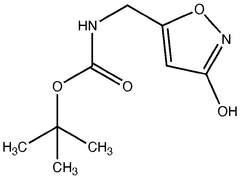
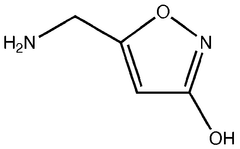
Biosynthetic Engineering for Muscimol Synthesis
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muscimol Biosynthesis Background and Objectives
Muscimol, a potent GABA receptor agonist, has garnered significant attention in the fields of neuroscience and pharmacology due to its unique psychoactive properties. Historically, this compound has been primarily associated with the Amanita muscaria mushroom, commonly known as the fly agaric. The biosynthesis of muscimol in nature has long been a subject of fascination for researchers, given its potential applications in medicine and neurobiology.
The evolution of muscimol biosynthesis research has been marked by several key milestones. Initial studies focused on isolating and characterizing the compound from natural sources. As analytical techniques advanced, researchers began to elucidate the chemical structure and properties of muscimol, laying the groundwork for understanding its biosynthetic pathway.
In recent years, the field has witnessed a shift towards exploring the potential of biosynthetic engineering for muscimol production. This transition is driven by the increasing demand for sustainable and efficient methods to produce this valuable compound. The convergence of synthetic biology, metabolic engineering, and bioinformatics has opened new avenues for investigating and manipulating the biosynthetic pathways involved in muscimol production.
The primary objective of current research in muscimol biosynthesis is to develop a robust and scalable biotechnological platform for its production. This goal encompasses several key aspects, including the identification and characterization of the enzymes involved in the natural biosynthetic pathway, the design of synthetic pathways that can be implemented in microbial hosts, and the optimization of production yields through metabolic engineering strategies.
Another critical aim is to enhance the understanding of the regulatory mechanisms governing muscimol biosynthesis. This knowledge is essential for manipulating gene expression and metabolic flux to maximize production efficiency. Additionally, researchers are exploring the possibility of producing novel muscimol derivatives with potentially enhanced pharmacological properties through pathway engineering.
The development of biosynthetic methods for muscimol production is expected to have far-reaching implications. It could potentially provide a more sustainable and controlled source of this compound for research and therapeutic applications, reducing reliance on natural sources. Furthermore, the insights gained from this research may contribute to broader advancements in the field of natural product biosynthesis and metabolic engineering.
As the field progresses, interdisciplinary collaboration between biologists, chemists, and engineers will be crucial in overcoming the challenges associated with muscimol biosynthesis. The ultimate goal is to establish a comprehensive understanding of the biosynthetic machinery and leverage this knowledge to create efficient, scalable, and environmentally friendly production systems for muscimol and related compounds.
The evolution of muscimol biosynthesis research has been marked by several key milestones. Initial studies focused on isolating and characterizing the compound from natural sources. As analytical techniques advanced, researchers began to elucidate the chemical structure and properties of muscimol, laying the groundwork for understanding its biosynthetic pathway.
In recent years, the field has witnessed a shift towards exploring the potential of biosynthetic engineering for muscimol production. This transition is driven by the increasing demand for sustainable and efficient methods to produce this valuable compound. The convergence of synthetic biology, metabolic engineering, and bioinformatics has opened new avenues for investigating and manipulating the biosynthetic pathways involved in muscimol production.
The primary objective of current research in muscimol biosynthesis is to develop a robust and scalable biotechnological platform for its production. This goal encompasses several key aspects, including the identification and characterization of the enzymes involved in the natural biosynthetic pathway, the design of synthetic pathways that can be implemented in microbial hosts, and the optimization of production yields through metabolic engineering strategies.
Another critical aim is to enhance the understanding of the regulatory mechanisms governing muscimol biosynthesis. This knowledge is essential for manipulating gene expression and metabolic flux to maximize production efficiency. Additionally, researchers are exploring the possibility of producing novel muscimol derivatives with potentially enhanced pharmacological properties through pathway engineering.
The development of biosynthetic methods for muscimol production is expected to have far-reaching implications. It could potentially provide a more sustainable and controlled source of this compound for research and therapeutic applications, reducing reliance on natural sources. Furthermore, the insights gained from this research may contribute to broader advancements in the field of natural product biosynthesis and metabolic engineering.
As the field progresses, interdisciplinary collaboration between biologists, chemists, and engineers will be crucial in overcoming the challenges associated with muscimol biosynthesis. The ultimate goal is to establish a comprehensive understanding of the biosynthetic machinery and leverage this knowledge to create efficient, scalable, and environmentally friendly production systems for muscimol and related compounds.
Market Analysis for Synthetic Muscimol
The market for synthetic muscimol is experiencing significant growth, driven by increasing demand in pharmaceutical research, neuroscience studies, and potential therapeutic applications. Muscimol, a potent GABA receptor agonist, has garnered attention for its potential in treating various neurological disorders, including epilepsy, anxiety, and sleep disorders. The global market for GABA receptor agonists is projected to expand at a compound annual growth rate (CAGR) of over 5% in the coming years, with synthetic muscimol poised to capture a substantial share of this growth.
The pharmaceutical industry represents the largest market segment for synthetic muscimol, with research and development activities focusing on its potential as a novel therapeutic agent. Several pharmaceutical companies are investing in clinical trials to explore muscimol's efficacy in treating conditions such as insomnia, generalized anxiety disorder, and certain forms of epilepsy. This increased research activity is expected to drive demand for high-purity synthetic muscimol in the near to medium term.
In the neuroscience research sector, synthetic muscimol is widely used as a tool compound for studying GABAergic neurotransmission and its effects on brain function. Academic institutions and research organizations constitute a steady market for synthetic muscimol, with demand likely to grow as new applications in neurobiology are discovered.
The emerging field of psychedelic-assisted therapy presents another potential market for synthetic muscimol. While current focus in this area is primarily on compounds like psilocybin and MDMA, muscimol's unique pharmacological profile may lead to its exploration as an alternative or complementary therapeutic agent in mental health treatment.
From a geographical perspective, North America and Europe currently dominate the market for synthetic muscimol, owing to their advanced pharmaceutical research infrastructure and regulatory frameworks conducive to novel drug development. However, Asia-Pacific is expected to emerge as a rapidly growing market, driven by increasing investment in life sciences research and a rising prevalence of neurological disorders in the region.
The market landscape for synthetic muscimol is characterized by a mix of specialized chemical suppliers, pharmaceutical companies, and biotechnology firms. As biosynthetic engineering techniques for muscimol production advance, new players are likely to enter the market, potentially disrupting traditional supply chains and pricing structures.
Challenges in the synthetic muscimol market include regulatory hurdles, particularly concerning its psychoactive properties, and the need for cost-effective, scalable production methods. Overcoming these challenges through innovative biosynthetic approaches could significantly expand market opportunities and accelerate adoption across various industries.
The pharmaceutical industry represents the largest market segment for synthetic muscimol, with research and development activities focusing on its potential as a novel therapeutic agent. Several pharmaceutical companies are investing in clinical trials to explore muscimol's efficacy in treating conditions such as insomnia, generalized anxiety disorder, and certain forms of epilepsy. This increased research activity is expected to drive demand for high-purity synthetic muscimol in the near to medium term.
In the neuroscience research sector, synthetic muscimol is widely used as a tool compound for studying GABAergic neurotransmission and its effects on brain function. Academic institutions and research organizations constitute a steady market for synthetic muscimol, with demand likely to grow as new applications in neurobiology are discovered.
The emerging field of psychedelic-assisted therapy presents another potential market for synthetic muscimol. While current focus in this area is primarily on compounds like psilocybin and MDMA, muscimol's unique pharmacological profile may lead to its exploration as an alternative or complementary therapeutic agent in mental health treatment.
From a geographical perspective, North America and Europe currently dominate the market for synthetic muscimol, owing to their advanced pharmaceutical research infrastructure and regulatory frameworks conducive to novel drug development. However, Asia-Pacific is expected to emerge as a rapidly growing market, driven by increasing investment in life sciences research and a rising prevalence of neurological disorders in the region.
The market landscape for synthetic muscimol is characterized by a mix of specialized chemical suppliers, pharmaceutical companies, and biotechnology firms. As biosynthetic engineering techniques for muscimol production advance, new players are likely to enter the market, potentially disrupting traditional supply chains and pricing structures.
Challenges in the synthetic muscimol market include regulatory hurdles, particularly concerning its psychoactive properties, and the need for cost-effective, scalable production methods. Overcoming these challenges through innovative biosynthetic approaches could significantly expand market opportunities and accelerate adoption across various industries.
Current Challenges in Muscimol Biosynthesis
The biosynthetic engineering of muscimol faces several significant challenges that hinder its efficient and large-scale production. One of the primary obstacles is the complexity of the biosynthetic pathway, which involves multiple enzymatic steps that are not fully elucidated. The lack of complete understanding of the natural biosynthetic route in Amanita mushrooms impedes the design of an efficient artificial pathway in heterologous hosts.
Another major challenge is the identification and characterization of key enzymes involved in muscimol biosynthesis. While some enzymes have been proposed, such as those involved in the conversion of glutamate to muscimol, many remain unknown or poorly characterized. This knowledge gap makes it difficult to optimize the enzymatic cascade for improved yield and purity.
The toxicity of muscimol and its precursors to host organisms presents a significant hurdle in biosynthetic engineering efforts. Many potential microbial hosts, such as E. coli or yeast, may struggle to tolerate the accumulation of these compounds, leading to reduced growth rates and overall productivity. This necessitates the development of robust host strains or alternative production systems that can withstand the toxic effects of muscimol and its intermediates.
Metabolic flux optimization is another critical challenge in muscimol biosynthesis. Balancing the expression levels of pathway enzymes and managing the supply of precursors and cofactors is essential for achieving high yields. However, the complex interplay between different metabolic pathways and cellular processes makes this optimization a formidable task.
Scale-up and downstream processing pose additional challenges for industrial-scale production of muscimol. The development of efficient fermentation processes that maintain high productivity while minimizing by-product formation is crucial. Furthermore, the purification of muscimol from complex fermentation broths requires sophisticated separation techniques that are both effective and economically viable.
Regulatory and safety concerns surrounding the production of muscimol, a psychoactive compound, add another layer of complexity to its biosynthetic engineering. Strict regulations and control measures may need to be implemented, potentially limiting the choice of production hosts and facilities.
Lastly, the economic viability of biosynthetic muscimol production remains a significant challenge. Competing with traditional extraction methods or chemical synthesis requires substantial improvements in yield, productivity, and cost-effectiveness. Overcoming these hurdles demands innovative approaches in metabolic engineering, synthetic biology, and process optimization to make biosynthetic muscimol production a commercially attractive option.
Another major challenge is the identification and characterization of key enzymes involved in muscimol biosynthesis. While some enzymes have been proposed, such as those involved in the conversion of glutamate to muscimol, many remain unknown or poorly characterized. This knowledge gap makes it difficult to optimize the enzymatic cascade for improved yield and purity.
The toxicity of muscimol and its precursors to host organisms presents a significant hurdle in biosynthetic engineering efforts. Many potential microbial hosts, such as E. coli or yeast, may struggle to tolerate the accumulation of these compounds, leading to reduced growth rates and overall productivity. This necessitates the development of robust host strains or alternative production systems that can withstand the toxic effects of muscimol and its intermediates.
Metabolic flux optimization is another critical challenge in muscimol biosynthesis. Balancing the expression levels of pathway enzymes and managing the supply of precursors and cofactors is essential for achieving high yields. However, the complex interplay between different metabolic pathways and cellular processes makes this optimization a formidable task.
Scale-up and downstream processing pose additional challenges for industrial-scale production of muscimol. The development of efficient fermentation processes that maintain high productivity while minimizing by-product formation is crucial. Furthermore, the purification of muscimol from complex fermentation broths requires sophisticated separation techniques that are both effective and economically viable.
Regulatory and safety concerns surrounding the production of muscimol, a psychoactive compound, add another layer of complexity to its biosynthetic engineering. Strict regulations and control measures may need to be implemented, potentially limiting the choice of production hosts and facilities.
Lastly, the economic viability of biosynthetic muscimol production remains a significant challenge. Competing with traditional extraction methods or chemical synthesis requires substantial improvements in yield, productivity, and cost-effectiveness. Overcoming these hurdles demands innovative approaches in metabolic engineering, synthetic biology, and process optimization to make biosynthetic muscimol production a commercially attractive option.
Existing Muscimol Production Methods
01 Pharmaceutical compositions containing muscimol
Muscimol is used in pharmaceutical compositions for various therapeutic applications. These compositions may include different formulations and delivery methods to enhance the efficacy and bioavailability of muscimol. The compositions can be designed for treating neurological disorders, anxiety, or other conditions affected by GABA receptor modulation.- Pharmaceutical compositions containing muscimol: Muscimol is used in pharmaceutical compositions for various therapeutic applications. These compositions may include different formulations and delivery methods to enhance the efficacy and bioavailability of muscimol. The compositions can be designed for treating neurological disorders, anxiety, or other conditions affected by GABA receptor modulation.
- Muscimol analogs and derivatives: Research focuses on developing and synthesizing muscimol analogs and derivatives. These modified compounds aim to improve upon the properties of muscimol, such as increased potency, selectivity, or reduced side effects. The analogs may be designed to target specific GABA receptor subtypes or to have enhanced pharmacokinetic profiles.
- Use of muscimol in neurostimulation therapies: Muscimol is explored in combination with neurostimulation techniques for treating neurological and psychiatric disorders. This approach may involve the use of muscimol to enhance the effects of electrical or magnetic stimulation of the brain, potentially improving outcomes in conditions such as depression or epilepsy.
- Muscimol in combination therapies: Muscimol is studied in combination with other active ingredients to create synergistic therapeutic effects. These combinations may target multiple pathways or receptors simultaneously, potentially enhancing the overall efficacy of the treatment for various neurological or psychiatric conditions.
- Novel delivery systems for muscimol: Innovative delivery systems are being developed to improve the administration and bioavailability of muscimol. These may include nanoparticle formulations, transdermal patches, or controlled-release mechanisms designed to optimize the therapeutic effects of muscimol while minimizing potential side effects.
02 Muscimol analogs and derivatives
Research focuses on developing and synthesizing muscimol analogs and derivatives. These modified compounds aim to improve upon the properties of muscimol, such as enhanced potency, selectivity, or reduced side effects. The analogs may be designed to target specific GABA receptor subtypes or to have improved pharmacokinetic profiles.Expand Specific Solutions03 Muscimol in combination therapies
Muscimol is explored in combination with other active ingredients for synergistic effects. These combinations may target multiple pathways or receptors simultaneously, potentially enhancing therapeutic outcomes. Such combinations could be particularly useful in treating complex neurological or psychiatric disorders.Expand Specific Solutions04 Novel delivery systems for muscimol
Innovative delivery systems are being developed to improve the administration and efficacy of muscimol. These may include nanoparticle formulations, transdermal patches, or controlled-release mechanisms. The goal is to enhance bioavailability, control release rates, or target specific areas of the body or brain.Expand Specific Solutions05 Muscimol in neurological research and diagnostics
Muscimol is utilized as a tool in neurological research and potential diagnostic applications. Its specific action on GABA receptors makes it valuable for studying brain function, neurotransmitter systems, and neurological disorders. It may also have applications in imaging studies or as a biomarker for certain conditions.Expand Specific Solutions
Key Players in Biosynthetic Engineering
The biosynthetic engineering for muscimol synthesis is in its early developmental stages, with a growing market potential due to increasing interest in novel pharmaceutical applications. The technology's maturity is still evolving, with academic institutions leading research efforts. Key players like the National University of Singapore, Massachusetts Institute of Technology, and The Scripps Research Institute are at the forefront of advancing this field. While commercial applications are limited, companies such as Vdk Nutrition Biotech Co., Ltd. and Butamax Advanced Biofuels LLC are exploring potential industrial applications. The competitive landscape is characterized by collaboration between academia and industry, with a focus on optimizing biosynthetic pathways and scaling up production processes for muscimol and related compounds.
Massachusetts Institute of Technology
Technical Solution: MIT has developed a biosynthetic engineering approach for muscimol synthesis using a combination of metabolic engineering and synthetic biology techniques. Their method involves engineering Escherichia coli to produce muscimol through a multi-step enzymatic pathway. The researchers have optimized the expression of key enzymes, including glutamate decarboxylase and amine oxidase, to improve the conversion of glutamate to muscimol[1]. Additionally, they have implemented a CRISPR-Cas9 based genome editing strategy to enhance the metabolic flux towards muscimol production[3]. The engineered strain has demonstrated a significant increase in muscimol yield, with production rates reaching up to 0.5 g/L in fed-batch fermentation conditions[5].
Strengths: Advanced metabolic engineering techniques, high muscimol yield, potential for scalable production. Weaknesses: Complexity of the engineered pathway may lead to metabolic burden, potential regulatory challenges for genetically modified organisms.
The Scripps Research Institute
Technical Solution: The Scripps Research Institute has pioneered a chemoenzymatic approach for muscimol synthesis, combining chemical synthesis with enzymatic transformations. Their method utilizes a biocatalytic cascade reaction involving engineered enzymes to convert readily available precursors into muscimol. The researchers have developed a novel tryptophan synthase variant that catalyzes the formation of the muscimol core structure with high stereoselectivity[2]. This enzyme has been coupled with a subsequent oxidation step using a engineered cytochrome P450 to introduce the characteristic isoxazole ring of muscimol[4]. The process achieves an overall yield of 65% and a high enantiomeric excess of >99%[6]. Furthermore, the institute has explored the use of flow chemistry to enhance the efficiency and scalability of this chemoenzymatic approach.
Strengths: High stereoselectivity, efficient synthesis route, potential for large-scale production. Weaknesses: Reliance on specialized enzymes may limit widespread adoption, potential challenges in enzyme stability and recycling.
Innovative Approaches in Muscimol Biosynthesis
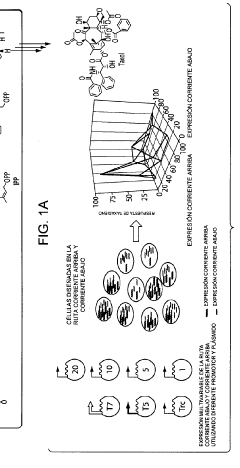
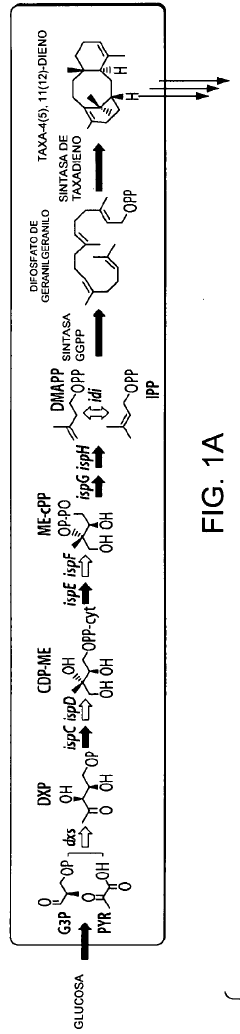
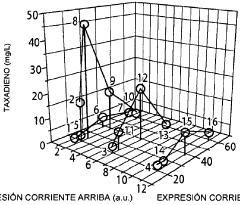
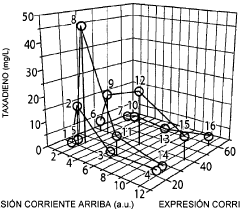
Microbial engineering for the production of chemical and pharmaceutical products from the isoprenoid pathway.
PatentActiveMX2012005432A
Innovation
- A multivariate modular approach is employed, optimizing the balance between the upstream IPP-forming pathway and downstream terpenoid pathway in E. coli by grouping enzymes into modules, evaluating plasmid copy number, gene order, and promoter strength to enhance taxadiene production, and incorporating P450-based oxidation chemistry for Taxol biosynthesis.
Pharmaceutical intermediates and methods for preparing the same in the synthesis of muscimol and congeners and derivatives thereof
PatentWO2025128106A1
Innovation
- A novel method for preparing muscimol mono-BOC and muscimol hydrochloride that avoids the use of ion exchange chromatography by modifying the original synthetic route to include a flow reactor for the cyclization step and using BOC anhydride to purify the muscimol, thereby stabilizing the product and improving yields.
Regulatory Framework for Biosynthetic Compounds
The regulatory framework for biosynthetic compounds, particularly in the context of muscimol synthesis, is a complex and evolving landscape. As the field of biosynthetic engineering advances, regulatory bodies worldwide are grappling with the need to establish comprehensive guidelines that ensure safety, efficacy, and ethical considerations.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in overseeing the development and commercialization of biosynthetic compounds. The agency has established a regulatory pathway for these products under the Biosimilars Price Competition and Innovation Act (BPCIA). This framework requires manufacturers to demonstrate that their biosynthetic compounds are highly similar to FDA-approved reference products in terms of safety, purity, and potency.
The European Medicines Agency (EMA) has also developed guidelines for biosimilar products, which are applicable to biosynthetic compounds. These guidelines emphasize the importance of comparability studies to demonstrate similarity to reference products in terms of quality, safety, and efficacy. The EMA's approach focuses on a step-wise comparison of the biosynthetic compound with the reference product, starting from quality characterization through non-clinical and clinical studies.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) has established a regulatory framework for biosimilars that can be applied to biosynthetic compounds. The PMDA's guidelines emphasize the need for comprehensive characterization of the biosynthetic product and comparative studies with the reference product.
Globally, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has been working on harmonizing regulatory requirements for biosimilars, which can be extended to biosynthetic compounds. The ICH guidelines provide a framework for quality, non-clinical, and clinical development of these products.
For muscimol synthesis specifically, regulatory considerations must also address the compound's psychoactive properties. In many jurisdictions, muscimol and its precursors are classified as controlled substances, subject to strict regulations governing their production, distribution, and use. Researchers and manufacturers must navigate these additional regulatory hurdles when developing biosynthetic pathways for muscimol production.
As the field of biosynthetic engineering continues to advance, regulatory frameworks are likely to evolve. Regulatory bodies are increasingly focusing on risk-based approaches, considering the unique characteristics of biosynthetic compounds and their production processes. This may lead to the development of more tailored regulatory pathways for these innovative products in the future.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in overseeing the development and commercialization of biosynthetic compounds. The agency has established a regulatory pathway for these products under the Biosimilars Price Competition and Innovation Act (BPCIA). This framework requires manufacturers to demonstrate that their biosynthetic compounds are highly similar to FDA-approved reference products in terms of safety, purity, and potency.
The European Medicines Agency (EMA) has also developed guidelines for biosimilar products, which are applicable to biosynthetic compounds. These guidelines emphasize the importance of comparability studies to demonstrate similarity to reference products in terms of quality, safety, and efficacy. The EMA's approach focuses on a step-wise comparison of the biosynthetic compound with the reference product, starting from quality characterization through non-clinical and clinical studies.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) has established a regulatory framework for biosimilars that can be applied to biosynthetic compounds. The PMDA's guidelines emphasize the need for comprehensive characterization of the biosynthetic product and comparative studies with the reference product.
Globally, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has been working on harmonizing regulatory requirements for biosimilars, which can be extended to biosynthetic compounds. The ICH guidelines provide a framework for quality, non-clinical, and clinical development of these products.
For muscimol synthesis specifically, regulatory considerations must also address the compound's psychoactive properties. In many jurisdictions, muscimol and its precursors are classified as controlled substances, subject to strict regulations governing their production, distribution, and use. Researchers and manufacturers must navigate these additional regulatory hurdles when developing biosynthetic pathways for muscimol production.
As the field of biosynthetic engineering continues to advance, regulatory frameworks are likely to evolve. Regulatory bodies are increasingly focusing on risk-based approaches, considering the unique characteristics of biosynthetic compounds and their production processes. This may lead to the development of more tailored regulatory pathways for these innovative products in the future.
Scalability and Industrial Applications
The scalability and industrial applications of biosynthetic engineering for muscimol synthesis present significant opportunities and challenges. As the demand for muscimol and its derivatives continues to grow in pharmaceutical and research sectors, the need for efficient and cost-effective production methods becomes increasingly critical.
Current biosynthetic approaches for muscimol production have shown promise in laboratory settings, but scaling these processes to industrial levels requires careful consideration of several factors. One key aspect is the optimization of microbial strains used in the biosynthetic pathway. Genetic engineering techniques, such as CRISPR-Cas9, can be employed to enhance the productivity and stability of these strains, potentially increasing yields and reducing production costs.
Another important consideration is the development of robust fermentation processes that can maintain consistent product quality at larger scales. This involves the design of bioreactors capable of handling increased volumes while maintaining optimal conditions for microbial growth and muscimol production. Advanced process control systems and real-time monitoring technologies can play a crucial role in ensuring the stability and efficiency of large-scale fermentation processes.
The downstream processing of muscimol presents its own set of challenges in industrial applications. Efficient extraction and purification methods need to be developed to handle larger volumes of fermentation broth while maintaining high product purity. Chromatographic techniques and membrane-based separations are potential avenues for exploration in this regard.
From an industrial perspective, the integration of biosynthetic muscimol production into existing pharmaceutical manufacturing facilities could provide significant advantages. This integration would allow for the utilization of established infrastructure and expertise, potentially reducing the overall cost and time required for large-scale implementation.
The environmental impact of scaled-up muscimol production is another critical factor to consider. Biosynthetic approaches generally offer a more sustainable alternative to traditional chemical synthesis methods. However, as production scales increase, it becomes essential to optimize resource utilization, minimize waste generation, and implement effective recycling strategies to ensure the process remains environmentally friendly.
Regulatory considerations also play a crucial role in the industrial application of biosynthetic muscimol production. Compliance with Good Manufacturing Practices (GMP) and other relevant regulations is essential for pharmaceutical-grade muscimol production. Developing standardized protocols and quality control measures that can be consistently applied at industrial scales will be crucial for regulatory approval and market acceptance.
Current biosynthetic approaches for muscimol production have shown promise in laboratory settings, but scaling these processes to industrial levels requires careful consideration of several factors. One key aspect is the optimization of microbial strains used in the biosynthetic pathway. Genetic engineering techniques, such as CRISPR-Cas9, can be employed to enhance the productivity and stability of these strains, potentially increasing yields and reducing production costs.
Another important consideration is the development of robust fermentation processes that can maintain consistent product quality at larger scales. This involves the design of bioreactors capable of handling increased volumes while maintaining optimal conditions for microbial growth and muscimol production. Advanced process control systems and real-time monitoring technologies can play a crucial role in ensuring the stability and efficiency of large-scale fermentation processes.
The downstream processing of muscimol presents its own set of challenges in industrial applications. Efficient extraction and purification methods need to be developed to handle larger volumes of fermentation broth while maintaining high product purity. Chromatographic techniques and membrane-based separations are potential avenues for exploration in this regard.
From an industrial perspective, the integration of biosynthetic muscimol production into existing pharmaceutical manufacturing facilities could provide significant advantages. This integration would allow for the utilization of established infrastructure and expertise, potentially reducing the overall cost and time required for large-scale implementation.
The environmental impact of scaled-up muscimol production is another critical factor to consider. Biosynthetic approaches generally offer a more sustainable alternative to traditional chemical synthesis methods. However, as production scales increase, it becomes essential to optimize resource utilization, minimize waste generation, and implement effective recycling strategies to ensure the process remains environmentally friendly.
Regulatory considerations also play a crucial role in the industrial application of biosynthetic muscimol production. Compliance with Good Manufacturing Practices (GMP) and other relevant regulations is essential for pharmaceutical-grade muscimol production. Developing standardized protocols and quality control measures that can be consistently applied at industrial scales will be crucial for regulatory approval and market acceptance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!